FIGURE 40-1. Pathophysiologic mechanisms and clinical presentations in type 1 diabetes mellitus. The clinical presentation in T1DM can be classical or emergency diabetic ketoacidosis (DKA), depending on the extent of insulin deficiency, effects of counteracting hormones, and other conditions. DKA can be viewed as an exaggerated compensatory physiologic mechanism.
Because T1DM is detected in the majority of patients after a relatively short period of symptoms, such as increased thirst, polyuria, and unexplained weight loss, the natural history of the disease has been poorly defined until recently. With the current ability to define individuals at high risk for developing T1DM based on high-risk HLA and autoantibody positivity, our understanding of the prediabetic period is improving. Current prospective studies of children with increased risk for T1DM (TEDDY, DIPP; DAISY, DiPiS, BABYDiab, PANDA)34,35 have revealed that autoantibodies to GAD65, IA-2, or insulin and decreased ability to release insulin in response to glucose36 may develop several years before the clinical diagnosis. The sequence of events preceding the diagnosis of overt T1DM would include the following: (1) genetic predisposition; (2) overt immunologic abnormalities with normal glucose levels; (3) development of β-cell dysfunction; (4) development of overt hyperglycemia with detectable C peptide; and (5) the final stage of insulin dependency, with disappearance of C peptide (Fig. 40-2). The fact that T1DM develops in persons of all ages must be taken into account when studying the natural history in children and adults. It is of significance that T1DM is often associated with other autoimmune diseases such as autoimmune thyroiditis (15% to 30%), Addison’s disease (0.5%), and celiac disease (4% to 9%). Additionally, the risk of autoimmune diseases is increased among relatives to T1DM patients.37
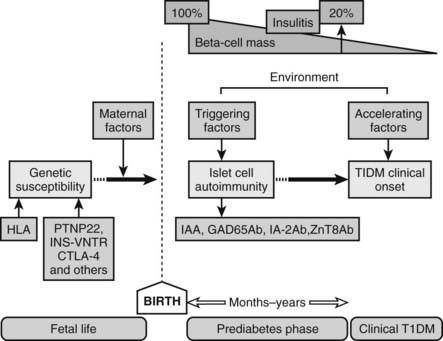
FIGURE 40-2. Natural history and etiology of type 1 diabetes mellitus. Several genetic and environmental factors are important in the etiology of T1DM. Autoimmune markers (IAA, GAD65Ab, IA-2Ab, ZnT8Ab) can be detected in the prediabetes phase long before clinical onset.
Diagnostic Criteria
The basis for distinction between T1DM and T2DM is islet autoimmunity and the patient’s dependence on insulin. Most patients have a history of polyuria, polydipsia, and unexplained weight loss (see Fig. 40-1). The American Diabetes Association (ADA)4 and the International Society for Pediatric and Adolescent Diabetes (ISPAD)28 have recommended diagnostic criteria for T1DM (Table 40-1).
Table 40-1. Criteria for the Diagnosis of Diabetes
| 1. | Fasting plasma glucose (FPG) ≥126 mg/dL (7.0 mmol/L). Fasting is defined as no caloric intake for at least 8 hr.* |
| OR | |
| 2. | Symptoms of hyperglycemia and a casual plasma glucose ≥ 200 mg/dL (11.1 mmol/L). Casual is defined as any time of day without regard to time since last meal. The classic symptoms of hyperglycemia include polyuria, polydipsia, and unexplained weight loss. |
| OR | |
| 3. | 2-hr plasma glucose ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT). The test should be performed as described by the World Health Organization, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water.* |
* In the absence of unequivocal hyperglycemia, these criteria should be confirmed by repeat testing on a different day.
From American Diabetes Association: Standards of medical care in diabetes–2008. Diabetes Care 31(Suppl. 1):S12–S54, 2008.
Evidence has been presented that adult-onset T2DM may progress to insulin dependence at a rate of 1% to 2% per year.3 This rate is increased in GAD65 autoantibody–positive patients classified with T2DM,38 and this form of T1DM has variably been termed type 1.5 diabetes, latent autoimmune diabetes in adults (LADA), or slowly progressive T1DM.39
Glucose tolerance tests, both oral and intravenous, are being used to evaluate diabetic states. The oral glucose tolerance test (OGTT) is a diagnostic criterion (see Table 40-1). Although glucose tolerance tests are more important in disorders of impaired glucose tolerance and T2DM, they may also find an increased use in the prediction and diagnosis of T1DM.40
Classification
T1DM can be clinically classified into two main types: immune-mediated, or type 1A, and the uncommon idiopathic, or type 1B (~10% of patients). Unlike immune-mediated T1ADM, T1BDM is not associated with islet-cell antibodies or HLA, although both forms can involve ketosis acidosis.4,5
Epidemiology
PREVALENCE
The prevalence of T1DM is low compared with that of T2DM. Among individuals aged 30 years or younger, the prevalence of T1DM does not usually exceed about 0.3%, compared with prevalence rates for T2DM of 4.2% worldwide and nearly 25% in certain high-risk populations.33 While current attention is directed toward the trends of the “epidemic” of T2DM, recent data suggest that there is a “parallel rise” in the incidence rates of both T1DM and T2DM.41 Both geographic and ethnic variations are seen in the prevalence rates. The International Diabetes Federation (IDF) estimates that in 2006 there were 440,000 cases of T1DM among children younger than 15 years of age, and that Southeast Asia contributes to a quarter of the prevalent cases, while Europe contributes with one fifth.33 It has been pointed out that the results of prevalence studies should be viewed with caution. Different age groups were studied,4,33 and although geographical variation may be related to variations in genetic predisposition, environmental risk factors, or both, it may also be due to some research methodological problems.33 In addition, it is unclear whether recently observed increases in incidence rates worldwide reflect a change in the age at onset of diabetes or a true increase in prevalence of T1DM.42
INCIDENCE
The incidence rate is the frequency with which new cases of T1DM are detected during a defined period. The rate is expressed as an annual number of cases per 100,000 age-corrected individuals. A determination of incidence rate therefore requires a precise knowledge of the total number of individuals in each age group and the number of new patients diagnosed in the particular area during 1 year. Determinations of secular trends constitute an important part of population-based epidemiologic studies. However, such analyses are rare because they require careful follow-up investigations during several subsequent years.
Globally, the current rise in incidence rates by 3% (2% to 5%) is expected to increase 40% higher in 2010 compared to figures from 199843,44 (Fig. 40-3). Currently it is estimated that more than 70,000 new patients are diagnosed each year worldwide.33 There are more suggestions that the increasing incidence rates and changing trends are seen in populations with high incidence and populations with previously lower incidence rates.27 Over the last 3 decades, T1DM has shown temporal trends in most of the regions of the world except Central America and the West Indies, while European data showed that the main recent rise in incidence rates is in Central-Eastern countries.43 Other studies such as EURODIAB44 and DiaMond45 have attempted to better define the incidence rates in various populations by using prospective, population-based, geographically defined registries. Overall, the average annual incidence rate varies widely, from 0.1 per 100,000 children in parts of Asia and South America43,46 to 64.2 per 100,000 in Finland.47 Worldwide epidemiologic investigations of T1DM as a noncommunicable disease indicate a 4% to 6% annual increase in incidence rate in Scandinavia,33,44 and similar data are being collected in other countries.48 Pooled data from all sites in the EURODIAB study demonstrated an annual rate of increase in incidence of 3.4%, with more rapid rates of increase occurring in certain regions.43,44 About 1% of all children born in Scandinavian countries will manifest T1DM during their lifetimes. The causes of this rapid rise are not understood, and further studies are needed to define to what extent the rise may be due to environmental factors, genetic admixture, or both.
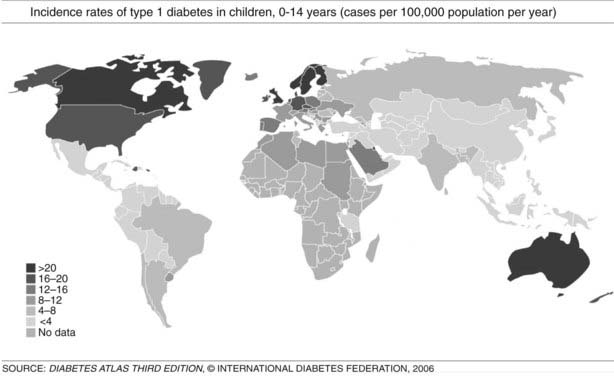
FIGURE 40-3. Incidence rates of type 1 diabetes mellitus (T1DM), 0 to 14 years: global trends in childhood T1DM. The incidence of T1DM varies widely, depending on genetic background and geographic location.
(From Diabetes Atlas, 3rd ed. Brussels: International Diabetes Federation, 2006, with permission.)
GEOGRAPHICAL DISTRIBUTION
T1DM can occur in any region of the world, and recently it was observed that the incidence is increasing in countries with both high and low incidence rates; in the latter group, the rise is steeper.43,48 However, it is suggested that the incidence of T1DM increases with increasing distance away from the equator (see Fig. 40-3). The annual incidence of T1DM is higher in northern Europe than in the Mediterranean area, with the exception of Sardinia. Furthermore, the incidence in Iceland is lower than that in Sweden or Finland.43,44 Surprisingly, the T1DM incidence rate in Estonia is about 25% of the rate in Finland, in spite of their close geographic proximity.49 The incidence rates within countries also show stable differences. The eastern and southern parts of Finland,50 as well as the central and southern parts of Sweden,51 have higher incidence rates than the northern parts of those countries.
The cause of geographic variation remains unknown, but it has been speculated to be related to genetic factors primarily associated with different HLA-DR or DQ genotypes of the major histocompatibility complex (MHC) on chromosome 6. HLA class II molecules from the DQ and DR loci that are necessary (but not sufficient) for disease vary greatly between countries and thereby affect disease incidence.49,52,53 In addition, environmental factors are important in understanding the pathogenesis of T1DM and may also help to explain differences in geographic distribution. This is supported by the observation that monozygotic twins show less than 20% to 30% concordance rates for T1DM.54
In the European region, incidence rates show correlation with the frequency of HLA susceptibility genes in the general population.52,55 In this region, there is also wide variation in incidence rates, ranging from 6 to 62 cases/100,000/year among children 0 to 14 years of age, with higher rates observed in Scandinavian countries, especially Finland and Sweden.44,47 In countries with populations of European origins, there is less variation: Canada reports 21/100,000/year, the United States 16/100,000/year, and New Zealand and Australia have incidence rates ranging from 16 to 20 cases/100,000/year.43 In the Middle East and North Africa, few reliable data are available. The incidence rates range from 1.0/100,000/year in Pakistan to 8 to 12 cases/100,000/year in Egypt, Sudan, and Libya; however, reports from Kuwait showed a rise in incidence rates from 15.4 in 1992 to 20.9 in 1997. There are few published data from the Southeast Asian region, and data from the African region are mostly lacking. In South and Central America, the incidence rates are low, except for Argentina, 6.8, and Uruguay, 8.3 cases/100,000/year. The Western Pacific countries have low incidence rates, especially China, which has one of the lowest incidence rates worldwide, ranging from 0.1 to 0.6 cases/100,000/year (see Fig. 40-3).43,48
It has been documented that migration of people from relatively low incidence countries into countries with a “diabetogenic” environment (i.e., with high incidence rates) increases the risk among the migrant groups. Migrants, within a short period of time, assume comparative risks of the native population, and several studies confirmed the importance of country of origin in the risk of T1DM.56,57 Improved epidemiology and better diagnostic criteria to distinguish different forms of diabetes are critical in obtaining reliable incidence rates for various countries and states.
VARIATION WITH AGE
Until recently, T1DM was thought to occur almost exclusively in children and adolescents (Table 40-2). Epidemiologic studies with rigorous diagnostic criteria4 suggest, however, that the clinical onset of T1DM may occur at any age. The incidence rate varies with both age and gender (Fig. 40-4). The peak for both girls and boys, age 11 to 14 years, has been discernible in most studies and seems to be present irrespective of the country or area studied.44,46,48 This peak is associated with puberty and the maximal velocity of pubertal growth, and it may be associated with reduced insulin sensitivity due to hormonal factors related to growth. Previous studies have suggested that the annual rate of increase in incidence is higher in younger age groups, with rates of 6.4% in 0- to 4-year-olds, 3.1% in 5- to 9-year-olds, and 2.4% in 10- to 14-year-olds.44 In children, minor incidence peaks occur at 4 to 6 years58 and 7 to 8 years,59,60 which have been associated with entrance into preschool or school programs.61 Recent data suggest that around 50% to 60% of cases of T1DM in Western countries occur before the 15th birthday, and that more than 90% of childhood and adolescent diabetes is T1DM.55 Epidemiologic investigation has indicated that the incidence rate of T1DM in patients older than 20 years is lower than that seen in children, except for a possible peak at around 50 to 65 years.62 In addition to adults having what is considered a more classic T1DM clinical picture, some adult patients initially classified and treated as having T2DM may require insulin after 1 to 5 years of therapy with diet, exercise, or oral hypoglycemic agents.38,39 As noted earlier, this type of diabetes is referred to as type 1.5 diabetes, latent autoimmune diabetes in the adult (LADA), or slowly progressive insulin-dependent diabetes mellitus (SPIDDM).39,63 These patients are positive for islet autoantibodies and have lower body mass index and higher frequencies of high-risk HLA types compared with other “type 2” patients.63 It is speculated that these patients in fact have T1DM in addition to genetic factors that may inhibit rapid progression of β-cell killing.62
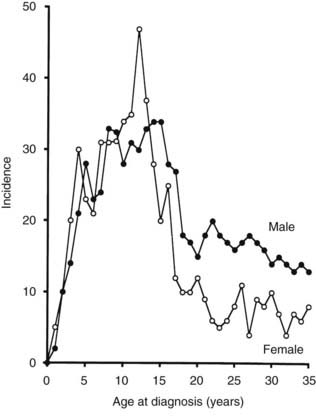
FIGURE 40-4. Age-adjusted incidence of insulin-dependent diabetes mellitus in relation to age at onset in male and female subjects.
(Data from Nystrom L, Dahlquist G, Ostman J et al: Risk of developing insulin-dependent diabetes mellitus [IDDM] before 35 years of age: indications of climatological determinants for age at onset. Int J Epidemiol 21:352–358, 1992, with permission.)
Although rare, some forms of diabetes mellitus can occur in the newborn period, at birth, or during the first months of life. Neonatal diabetes (NDM) describes one kind of diabetes that occurs in the first month (up to 6 months) of life at a rate of 1 in 500,000 live births. It is characterized by insulin-sensitive hyperglycemia. About half of cases of NDM are transient (TNDM), but the remaining progress into a permanent (PNDM) type, with lifelong dependence on exogenous insulin. Clinically the infant is thirsty, with excessive urination and hyperglycemia and/or glycosuria, but the condition may progress into ketoacidosis. PNDM usually proceeds after the second year, and differentiation between the two forms can only be done through genetic testing. TNDM occurs at birth up to 3 to 6 months and is correlated to defects in specific genes: ZAC/HYMAI (ZAC pleomorphic adenoma gene–like 1, or PLAG1, and hydatidiform mole–associated and imprinted transcript, HYMAI), or KCNJ11, the moderately activating mutation of the Kir6.2 gene, or hepatocyte nuclear factor 1β (HNF1β) and SUR1 (sulfonylurea receptor 1). The PNDM, on the other hand, is related to several gene defects, such as pancreatic aplasia, activating mutations of KCNJ11 of the Kir6.2 gene that encodes for ATP potassium channel (KATP), the ABCC8 gene of sulfonylurea receptor 1 (SUR1), complete deficiency of glucokinase IPF1 (PDX1) gene, which encodes insulin promoter factor1, PTF1A, which encodes the pancreas transcription factor 1A, and also mutations in forehead box p3 (FOXP3) gene (also known as T-cell regulatory gene).64
VARIATION WITH GENDER
It has been reported that the peak incidence in girls occurs earlier than in boys59 (see Fig. 40-4). If the clinical onset of T1DM is linked to pubertal growth, this difference in incidence rate can be explained by the fact that pubertal growth occurs earlier in girls. In children followed from birth because of T1DM genetic risk, the male-to-female ratio was 1.4 : 1.0 before 6 years of age at T1DM diagnosis. This ratio was increased (1.7 : 1.0) among children younger than 18 months of age.65 Prepubertal boys were found to be taller at the clinical onset of T1DM.60 In addition, newly diagnosed children of both genders showed advanced skeletal maturity.66 Even if boys tend to show an increased height compared with controls, their growth seems to cease about 35 weeks before the clinical onset of T1DM.60 It therefore is possible that processes affecting the pancreatic β-cell mass and the ability to produce insulin may have profound effects on body growth and function at a young age. Because these processes differ slightly between boys and girls, growth characteristics may offer a simple explanation for the differences seen in incidence rates between the genders.
A study that examined sex differences in T1DM incidence before the age of 15 showed a slight male preponderance in many but not all European countries, whereas a female preponderance was found in most African and Asian countries.67 Interestingly, the male excess was seen in all countries with the highest incidence rates (>20/105/yr), whereas a female excess was seen in all countries with low incidence rates (<4.5/l05/yr). Studies of older age groups have consistently shown a male preponderance for new cases of T1DM, with male/female ratios ranging from 1.3 to 2.5 : 1.68 One registry of T1DM among 15- to 34-year-olds (see Fig. 40-4) demonstrated that T1DM was 1.5 times more common among men than women.61 Further studies are necessary to document gender-dependent incidence rates and to explain their mechanisms.
SEASONAL VARIATIONS
T1DM shows cyclic, sinusoidal appearance of seasonal variation in date of diagnosis, with a peak in winter months.48 This variation is proposed to be related to the timing of occurrences of certain precipitating factors such as viral infection and cold climate.48 It is of interest that islet cell autoantibodies appearing during the prediabetic state follow similar seasonal variations, being more prevalent in colder months and rare in summer and spring. Additionally, the triggering of β-cell autoimmunity appears to have variable trends over subsequent years and does not equally affect genetically susceptible siblings.69 These observations suggest that triggering of immunologic markers may be related to factors (most likely viruses) that prevail mostly in colder months with variable frequency of occurrence.
Etiology
The absence of an unambiguous mode of inheritance, the presence of a period of subclinical islet autoimmunity preceding clinical onset of disease, human leukocyte antigen (HLA) genes that control the immune response, and age and seasonal variation must be taken into account in attempts to explain the cause of T1DM. A defined etiologic factor, endogenous or exogenous, capable of causing T1DM remains to be identified (see Table 40-5). Because evidence exists of genetic heterogeneity in T1DM, it is possible that different causative factors are responsible. In experimental animals (see Table 40-5), both viral and chemical agents have been used to induce diabetes reproducibly, and certain strains of animals are at higher risk for developing diabetes due to genetic factors. In addition, only indirect evidence suggests that environmental factors that are clearly diabetogenic in animals are involved in initiating T1DM in humans. The following is a brief summary of possible genetic or environmental factors that are associated with the appearance of T1DM.
INHERITANCE
The mode of inheritance is complex, and around 80% to 85% of cases of T1DM occur sporadically without familial aggregation.52 Among HLA-identical siblings of T1DM-affected patients, about 20% eventually manifest the disease. The overall lifetime risk for first-degree relatives has been estimated at about 8% for siblings—15 times higher than the general population—and 5% for children of parents with T1DM.70 The risk of T1DM among offspring ranges from 2% to 4% if the mother was affected, compared to 6% to 9% if the father was affected, and the risk tolls to 30% when both parents are affected.52,70
GENETIC FACTORS
T1DM is both genetically associated with and linked to certain HLA genetic factors of the major histocompatibility complex (MHC).71,72 By using DNA sequence information in the genetic analysis, it is found that more than 95% of all patients in whom T1DM onset occurred before age 30 years are positive for the chromosome 6 HLA haplotypes DRB1*04-DQAI*0301-BI*0302, DRBI*03-DQA1*0501-BI*0201, or both. Although some 40% to 50% of the background population carry these HLA factors, they represent necessary but insufficient prerequisites for the development of T1DM. It has been estimated that HLA contributes about 60% of T1DM risk among first-degree relatives to T1DM patients. Hence it is not surprising that less than 10% of genetically susceptible subjects develop the disease.68 Other genetic factors have indeed been identified,73,74 but none has shown a level of importance comparable to that of HLA.
The HLA haplotypes DRB1*04-DQAI*0301-BI*0302 and DRBI*03-DQA1*0501-BI*0201 are the two major-risk haplotypes71,75 (Table 40-3). The most important alleles are DQB1*0302 and DQB1*0201, along with DRB1*03. DRBI*04 is a large family of related molecules, and DRB*0401 confers an independent risk, whereas DRB1*0403 is negatively associated with T1DM and may protect or decelerate an ongoing disease process.75 DRB1*03 seems to be more important than DQB1*0201, as only DRBI*03-DQA1*0501-BI*0201, not DRBI*07-DQA1*0501-BI*0201, confers T1DM risk. DQB1*0401 and DQB1*0404 are susceptibility alleles on the DRB1*04-DQAI*0301-BI*0302 haplotype. DQB1*0604 and DQB1*0501 are susceptibility alleles, together with either DRBI*03-DQA1*0501-BI*0201 or DRB1*04-DQAI*0301-BI*0302. The genetic linkage and association between T1DM and HLA is remarkable in that certain HLA haplotypes are protective. Most prominently, DQA1*0102-B1*0602 and DQA1*0102-B1*0603 are protective before age 15 years (see Table 40-3). The detailed mechanisms by which HLA confers either risk or resistance is not fully understood.76 The function of these molecules is to display peptide antigens to be recognized by T-cell receptors (TCR). The disease association may therefore be related either to an inability to induce immunologic tolerance to certain autoantigens or to antigen presentation of an endogenous autoantigen. In the first case, the subject may be exposed to infectious agents that mimic autoantigens. Reactivity to the infectious agent sets off an immune response that cross-reacts with self. In the second case, the immune reaction may result in a direct attack on the individual’s own cells.
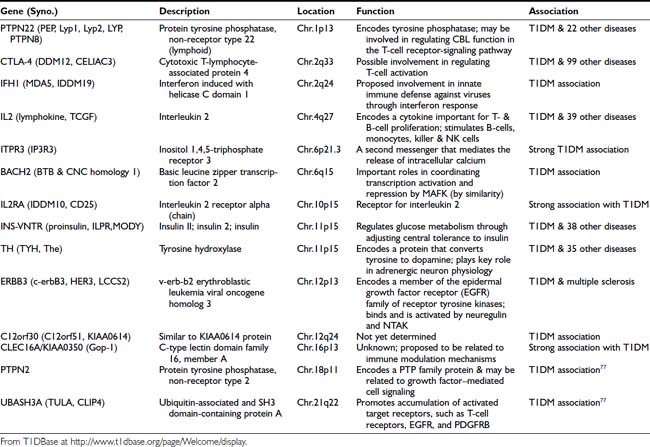
The genetics of T1DM is studied extensively because it represents a paradigm for genetically complex diseases. Genome screens and studies on candidate genes have provided evidence for genetic linkage between polymorphic DNA markers and more than 10 putative T1DM susceptibility genes (refer to T1DBase at http://www.t1dbase.org/page/Welcome/display). Currently, it has been shown that PTPN22 (chr.1p13), CTLA-4 (chr.2q33), IFH1 (chr.2q24), IL2 (chr.4q27), ITPR3 (chr.6p21), IL2RA (chr.10p15), INS-VNTR (chr.11p15), TH (chr.11p15), ERBB3 (chr.12p13), C12orf30 (chr.12q24), CLEC16A/KIAA0350 (chr.16p13), PTPN2 (chr.18p11) genes and two recently identified novel loci, BACH2 (chr.6q15) and UBASH3A (chr.21q22)77 are all associated with increased T1DM risk (Table 40-4). References to these individual genetic factors are found at the T1DBase website.
Taken together, however, these and other genetic factors have limited effects on the relative risk of T1DM. It needs to be determined if these genetic factors contribute to T1DM by being protective or if they have an additive or potentiating effect on the risk provided by HLA. The mechanisms by which these factors contribute to appearance of the disease remain to be clarified. It is noted that most of the genetic factors linked to T1DM risk are involved in the function of the immune system.
ENVIRONMENTAL FACTORS
T1DM is characterized by a multifactorial web of environmental factors (Table 40-5). The interaction between genetic and environmental factors and the aggressive islet autoimmune phenomena is complex. The fact that the disease has a relatively long latent period, in which autoimmunity is triggered long before the onset of the clinical syndrome (see Fig. 40-2), indicates a role of environmental factors as initiators or promoters in relation to genetic predisposition.68 Several environmental factors are incriminated in the etiology of T1DM, but linking is indirect, and a definite proof of confirmed association for each factor is yet to be achieved, since the majority of investigations have been carried out at the time of clinical diagnosis and not in relation to the time of islet autoimmunity.
Table 40-5. Viruses and Other Environmental Factors Implicated In or Able to Induce Type 1 (Insulin-Dependent) Diabetes
| Factor | Comment | |
|---|---|---|
| Host | Diabetes in Animals | |
| Viruses | ||
| Coxsackie (RNA) | Human | Mice |
| Rubella (RNA) | Human | Hamsters |
| Mumps (RNA) | Human | None |
| Cytomegalovirus (DNA) | Human | Rat CMV in rats |
| Echovirus (RNA) | Human | None |
| Ljunganvirus (RNA) | Human | Bank voles |
| Rotavirus (RNA) | Human | None |
| Diet and Nutrition | ||
| Cow’s milk protein/bovine insulin | Early introduction before 4 months of age | |
| Cereals/gluten | Early introduction before 4 months of age | |
| High-calorie carbohydrates | ||
| Nitrosamine-containing foods | ||
| Vitamin-D deficiency | ||
| Growth, Body Size, and Weight Gain | Excess stress on β cells (accelerator hypothesis)28 | |
| Psychological Stress | Possibly through changing the hypothalamic-pituitary-adrenal regulatory mechanism | |
| Hygiene Theory | Inadequate maturation of immune system and subsequent autoimmune reaction | |
| Toxins | ||
| Pyriminyl (Vacor) | ||
| Pentamidine isothiocyanate | ||
| N-3-Pyridylmethyl-N′p′-nitrophenylurea (PNU) | ||
Viral Infection
There are numerous reports of individual cases in which T1DM onset followed an acute viral infection, borne out by subsequent animal studies in which viruses have been shown to have diabetogenic activity.78 The first case linking T1DM to an acute viral infection was reported in the late 19th century; the onset of T1DM appeared to be precipitated by a mumps infection in a child.79 Many similar reports have followed since,78,80 and taken together, these reports suggest a relation between the clinical onset of T1DM and several viruses, including rubella, mumps, coxsackievirus B, rotavirus, cytomegalovirus, and Epstein-Barr virus, have been implicated.78 The true relation between these viral diseases and the clinical onset of T1DM remains conjectural.
Viruses are thought to increase the risk of T1DM through different proposed mechanisms. Whereas some viruses induce rapid and direct effect, causing β-cell destruction and subsequent insulin deficiency, other viruses induce slower, longstanding effect through activation of autoreactive T cells. Viruses may also inhibit insulin production by inducing interferons and HLA antigen expression, or they may mimic autoantigens of β cells.78,80
T1DM develops in individuals who are positive for HLA-DR3-DQ2, DR4-DQ8, or both (discussed earlier). The DQ6 haplotype containing DQB1*0602 confers resistance among children. Because these specificities are present in about half the population, a virus inducing either islet autoimmunity, clinical onset of T1DM, or both, may not be spread effectively enough to cause disease. In addition, variations in the annual incidence rate are often taken as evidence of an involvement of virus. An annual variation was found in a population of 15- to 34-year-olds, with lower numbers of new patients being identified during the summer months.48,69 In the group of children younger than 6 or 7 years who have T1DM, however, this annual variation is not always present.51,81
Earlier studies suggested that the congenital rubella syndrome was strongly associated with T1DM; approximately 10% to 20% of children develop autoimmune T1DM, and around 50% of them develop autoimmune antibodies.80 However, most countries with high incidence, such as Finland and Sweden, had successfully implemented vaccination programs against rubella virus. Vaccination practices have prevented rubella epidemics, but they have not affected the incidence rate of T1DM.
Maternal enteroviral infection during pregnancy also appears to be a risk factor for childhood T1DM.82 It is therefore possible that gestational infections by many types of viruses affect the maturation of the immune system, causing certain children to be more predisposed to autoimmunity and thereby increasing the risk for T1DM. Prior exposure to measles, mumps, and rubella, but not vaccination, decreased prevalence of pancreatic and thyroid autoantibodies.83 Maternal viral infections or reduced exposure to natural infections may be associated with an increase in T1DM.
Enteroviruses, in particular the coxsackie B serotype have been extensively studied at the time of clinical onset84 but rarely in relation to the appearance of islet autoimmunity.80 Coxsackie B4 virus was isolated from the pancreas of a child who died at presentation with T1DM, propagated in the in-vitro cultures of endocrine pancreatic cells, and then shown to have diabetic activity in certain mouse strains.85 Coxsackievirus infection in the mouse seems to be associated with virus replication in the β cells, followed by the formation of GAD65 antibodies.86,87 Two important hypotheses follow from these experiments. One hypothesis is that the coxsackievirus induces β-cell neoantigens, which initiate an (auto)immune reaction. This hypothesis can be tested by analyzing the appearance of such neoantigens. The neoantigen may initiate a devastating reaction if its structure mimics a self-protein. In line with this hypothesis, a sequence in GAD65 is identical to that in a coxsackievirus antigen.88,89 Another hypothesis is that coxsackie B virus replication in β cells results in β-cell necrosis and the formation of antibodies against β-cell constituents or “hidden antigens” not normally surveyed by the immune system.90 An autoimmune reaction is initiated that may escalate with time.
Other viruses incriminated in T1DM etiology include rotaviruses, which are common causes of childhood gastroenteritis. These viruses showed peptide sequences similar to T-cell epitopes in IA-2 and GAD antibodies, suggesting their role in T1DM.82 Cytomegalovirus is a DNA virus that is thought to induce autoimmunity through a molecular mimicry mechanism. Mumps virus was correlated to autoimmunity and even to overt T1DM. Human endogenous retroviruses (HERV) were also reported to correlate with T1DM.80
In experimental animals (see Table 40-4), several viruses are known to induce T1DM, either through direct effects on the β cells, causing rapid destruction (e.g., encephalomyocarditis virus EMC-D) or through the disruption of the normal immune regulatory mechanisms (e.g., Kilham rat virus [KRV], which causes autoimmune T1DM in diabetes-resistant BioBreeding [DR-BB] rats).78 Other viruses (e.g., reovirus) were found to affect primarily the immune system of the host to induce a polyclonal autoantibody response. The production of autoantibodies appeared to be closely related to the pathogenesis of disease in mice.86,87 Captured bank voles may develop T1DM with islet autoantibodies and signs of parechovirus infection, raising the question whether there are zoonotic virus–inducing islet autoimmunity, T1DM, or both.91
The virus-induced disease in mice depends on strain, because some mouse strains are resistant to the pathogenesis of a virus that induces T1DM in another strain. Similar strain dependency to the β-cytotoxic agent streptozotocin, followed by the inoculation of a diabetogenic virus, rendered otherwise virus-resistant mice diabetic.92 This observation may be significant to humans because it is possible that repeated injuries to the pancreatic β cells over several years of life may eventually induce T1DM. An additional role of HLA has been suggested, because it cannot be excluded that repeated injuries are particularly detrimental if the T1DM-associated HLA alleles are linked with a poor regenerative capacity of the pancreatic β cells.
Hygiene Hypothesis
The hygiene hypothesis proposes that as living environment is improved, children become less exposed to infectious agents, which leads to inadequate maturation of their immune systems. This hypothesis suggests that early exposure to pathogens may enhance the immune responses of those children, thereby suppressing autoimmune reactions involved in T1DM pathogenesis. The latter suggestion is based on increasing incidences of diseases like asthma or other atopic disorders, in addition to the fact that T1DM is more prevalent in developed societies. The hypothesis also suggests that younger children are more prone to infections, since they did not acquire antibodies against viruses (e.g., enterovirus antibodies from their mothers), resulting in an increased risk for T1DM or other autoimmune diseases.80 Indeed, other studies reported higher maternal enterovirus antibodies in countries with lower incidence of T1DM compared to countries with high incidence.82
Dietary Factors
The dietary hypothesis is based on earlier observations that breastfed children have a lesser risk of T1DM. It was also noted that early exposure of infants younger than 6 months to cow’s milk proteins may double the T1DM risk, particularly in HLA high-risk children. Other studies described an association between islet autoimmunity and shorter duration of breastfeeding, as well as early exposure of infants to bovine protein.93 The Diabetes Prediction and Prevention (DIPP) study of Finland reported a fivefold higher risk of multiple islet autoantibodies among children with HLA DQB1*0302 who received cow’s milk formula before the age of 4 months. Similar associations between islet autoimmunity or T1DM and intake of glutens, foods rich in protein, carbohydrates, and nitrosamine compounds were reported.94 Additionally, early exposure to bovine insulin in cow’s milk may lead to formation of autoantibodies against human insulin through cross-reaction.95 These findings are controversial. The Diabetes Auto-Immunity Study in the Young (DAISY) found no evidence correlating cow’s milk, enteroviral infection, or vaccinations with increased T1DM risk.96 Similarly, the EURODIAB Substudy-2 Group suggested that rapid growth, rather than cow’s milk or early introduction of solid food, would explain the increased incidence rate of T1DM.97 The islet autoimmune processes in the spontaneously diabetic NOD mouse and BB rat are easily inhibited by dietary manipulation.98 Studies in inbred and pathogen-free laboratory rodents underscore the possible importance of the gut immune system in T1DM.
Maternal Factors
Several maternal factors were reported to increase the risk of T1DM in children. Higher maternal age (and to a lesser extent, paternal age) at the time of delivery increased the risk of T1DM in the offspring. Exposure of the mother, as mentioned earlier, to enteroviral infection, colder season of delivery, ABO blood group incompatibility, maternal islet autoantibodies, or eclampsia all increased the risk of T1DM.99 An analysis of nondiabetic mothers with islet autoantibodies at delivery indicated that an association between HLA and islet autoimmunity may depend on environmental exposure during pregnancy.30,82 Follow-up of mothers and children will determine the risk of T1DM. However, mothers who developed T1DM after 8 years of age confer lower risk on their offspring compared to fathers with T1DM. On the other hand, birth order seems to have positive association with the risk of T1DM, since the first child carries the highest risk, and this risk declines by 15% with each new child.99 In the NOD mouse, it was reported that obliteration of the maternal transmission of antibodies inhibited the T-cell-mediated destruction of islet β cells in NOD mice.100 These and other investigations, in humans as well as in laboratory animals, underscore the importance of clarifying whether transmission of maternal autoantibodies affects the incidence of T1DM in susceptible offspring.
Birth Weight and Growth Rate
Several studies suggest a positive association between T1DM HLA risk and an increase in perinatal and postnatal growth. Children with higher weight-for-height or BMI during infancy or childhood have greater T1DM risk.101 It has also been suggested that high energy intake (particularly disaccharides such as sucrose) may be important to hyperinsulinemia resulting in rapid growth and T1DM susceptibility.102 The “accelerator hypothesis” suggests that factors like rapid growth, weight gain, and high energy intake would stress β cells, leading to acceleration (not triggering) of cell-mediated autoimmune destruction28 and that this may explain the increasing incidence of T1DM among young age groups.
Psychological Stress
Case-controlled studies reported that negative life events occurring during the first 2 years of life, difficult adaptation, or child behavioral deviances may increase the risk of T1DM.102 It has also been reported that psychological stress, measured as psychosocial strain during pregnancy, seems to be involved in the induction or progression of β-cell-related autoimmunity in cord blood and during the first year of life.103 Additionally, high parenting stress, experiences of serious life events, foreign origin of the mother, and low socioeconomic status were all associated with β-cell-related autoimmunity in young children.104 It was hypothesized that parent-infant relationship may be a “psychobiological regulator” and that early disruption of this relationship may cause stress and affect the hypothalamic-pituitary-adrenal axis. Such changes may affect the immature autonomic nervous system of the child and subsequently impair the regulation of autoimmunity.105
Toxic Substances
Alloxan and streptozotocin are widely used to induce β-cell destruction and diabetes in experimental animals.106 Some species including man are more resistant to these drugs than others. Streptozotocin is commonly used in treating certain gastroenteric tumors, including glucagonomas; however, T1DM rarely develops in these patients. A number of compounds structurally related to streptozotocin and alloxan have been implicated as possible environmental agents contributing to T1DM. The nitrosamine moiety of the streptozotocin molecule may be diabetogenic when present on molecules other than D-glucose.107 Pyriminil (Vacor), an effective rodenticide, is highly diabetogenic in humans.106 Individuals in whom diabetes developed after ingestion of Vacor had islet cell surface antibodies, indicating that β-cell-related autoimmunity may develop after β-cell destruction. The TEDDY study (The Environmental Determinants of Diabetes in the Young) is an international effort to substantiate the possibility that environmental chemicals are causative factors in the development of T1DM.108
In summary, virus and chemical agents have direct effects on pancreatic β cells and may therefore represent the causative factors that initiate the autoimmune process against these cells. The alternative hypothesis is that these agents potentiate a β-cell-destructive process that is genetically determined or initiated by environmental factors.
Pathology
Studies of the pancreas in newly diagnosed T1DM patients have indicated that the gland is diminished in size compared with that in matched controls.8,109 An atrophic pancreas with little or negligible residual insulin is typical of a patient with longstanding T1DM. The atrophy affects primarily the tail of the pancreas. In this part, the composition of the endocrine pancreas is dominated by β and α cells, whereas in the pancreatic head, β and pancreatic polypeptide (PP) cells predominate. In addition to insulin deficiency, the pancreatic atrophy appears to be reflected in lower serum levels of pancreatic trypsin and isoamylase.110,111 It is speculated that the pancreas atrophy is a result of an intrapancreatic insulin deficiency affecting the growth of the pancreas.
The insulin deficiency is the result of a specific loss of the β cells, which affects the total mass of the endocrine pancreas in T1DM (Table 40-6). The islets are small and appear pseudoatrophic.112,113 In contrast to the normal pancreas, in which the β cells predominate, the endocrine cells in the diabetic pancreas are primarily α, δ, and PP cells. Lobular variation has been described, and areas with β-cell-containing islets without pancreatic atrophy also have been detected, suggesting that parts of the pancreas may be spared112 in individuals with newly diagnosed T1DM. Sometimes β cells are seen in close proximity to duct cells, suggesting neoformation of cells. The understanding of the developing endocrine pancreas has improved, and a number of transcription factors controlling the process have been identified.114 However, one of the characteristics of the pancreas in many children with newly diagnosed T1DM is the presence of inflammatory cells adjacent to islets or cords of newly formed β cells.112
Table 40-6. Morphometric Analysis of the Endocrine Pancreas in Control Individuals and Patients with Type 1 Diabetes Mellitus
| Cell Type | Endocrine Pancreas (µg) | |
|---|---|---|
| Controls | T1DM | |
| β cells | 850 | 0 |
| α cells | 230 | 150 |
| δ cells | 125 | 97 |
| PP cells | 190 | 166 |
| All cells | 1395 | 413 |
PP, Pancreatic polypeptide.
Adapted from Rachier J, Goebbles RM, Henquin JC: Cellular composition of the human diabetic pancreas. Diabetologia 24:336–371, 1983, with permission.
The presence of inflammatory cells in the pancreas of diabetes patients with short-duration disease was initially demonstrated at the turn of the 20th century. The term insulitis was first introduced in 1940,7 but the phenomenon was not established until later quantitative investigations reported insulitis in 16 of 23 individuals with T1DM who died within 6 months of diagnosis.8 It is believed that insulitis is not detected in a greater percentage of new-onset T1DM patients because the total mass of β cells is already markedly reduced at the time of clinical diagnosis.13 Immunocytochemical investigation in rare specimens of pancreas from patients who died shortly after the clinical onset of T1DM indicates that all cell types considered part of the immune system populate the islets to form the insulitis115; T lymphocytes, B lymphocytes, macrophages, and occasional natural killer (NK) cells may be seen. In agreement with earlier studies, examination of pancreatic islets in laparoscopically obtained biopsy specimens from newly diagnosed T1DM patients116,117 demonstrated insulitis in about 60% of patients, with a T cell-predominant infiltration and increased expression of HLA class I.118 Notably, the presence of insulitis was strongly correlated with the presence of other autoimmune features, specifically positivity for GAD65 or IA-2 autoantibodies.118 In contrast, recent examination of specimens from 1507 pancreatic donors aged 25 to 60 years revealed 62 (4%) islet autoantibody–positive subjects. Insulitis was found in only 2 of the 62 (3%) subjects; the 2 subjects were positive for multiple islet autoantibodies and susceptible HLA. None of the 62 matched controls. These results suggest that positivity for islet autoantibodies does not immediately mean that insulitis is present. Islet autoantibodies may precede insulitis, which therefore may require the concurrent presence of multiple islet autoantibodies.119
Individuals at risk for development of T1DM have been found to have hyperproinsulinemia, a condition thought to reflect maximally stimulated or perhaps exhausted β cells.120,121 When compared with matched controls, HLA-identical siblings of patients with T1DM have been found to have evidence of both insulin resistance and impaired β-cell function.122 To estimate the residual mass of insulin-producing cells in humans, it is important to take into account such factors as the maximal rate of insulin release, extraction of insulin in the liver and peripheral tissues, and resistance to insulin action.123 It has been shown that intensive therapy for T1DM helps sustain endogenous insulin secretion, which in turn is associated with better metabolic control and lower risk for hypoglycemia and chronic complications.124,125
The role of immune cells in the process leading to the disappearance of β cells is well established in the NOD mouse and BB rat.26 It is possible to transfer diabetes adoptively with clonal T lymphocytes in both species. Similarly, transfer of T1DM between HLA-identical siblings has been achieved by bone marrow transplantation.126
Taken together, current observations support the hypothesis that T1DM is an immune-mediated disease and that immunocytes play a pivotal role in the β-cell killing. Although the total pancreatic insulin content in experimental animals appears to correlate well with the total β-cell mass, it cannot be excluded that a diminished β-cell mass alters the rate of insulin biosynthesis and secretion in remaining β cells, as well as insulin resistance in peripheral tissues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree