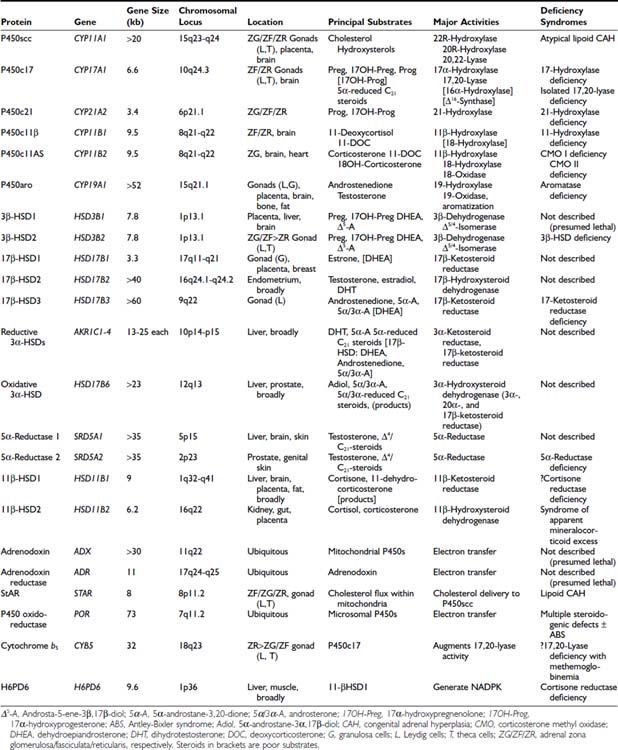
FIGURE 96-1. Cyclopentanoperhydrophenanthrene steroid nucleus. Steroid rings are identified with boxed capital letters, and carbon atoms are numbered. Substituents and hydrogens are labeled as α or β if they are positioned behind or in front of the plane of the page, respectively.
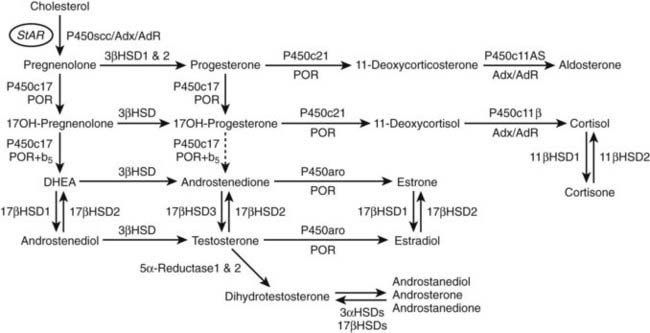
FIGURE 96-2. Major human steroidogenic pathways. Key enzymes and cofactor proteins are shown near arrows indicating chemical reactions. The StAR protein (oval) mobilizes cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane, where P450scc cleaves cholesterol to pregnenolone, the first committed intermediate in steroid biosynthesis. The steroids in the first column are Δ5-steroids, which constitute the preferred pathway to C19 steroids in human beings, and the dashed arrow indicates poor flux from 17α-hydroxyprogesterone to androstenedione. Steroids in the second column and farther right are Δ4-steroids, except the C18 estrogens (estrone and estradiol) and 5α-reduced steroids, including the potent androgen DHT and other androstanes (bottom row). Not all intermediate steroids, pathways, and enzymes are shown.
CYTOCHROME P450 ENZYMES
Mammalian cytochrome P450 enzymes fall into two broad classes, type 1 and type 2.3 Type 1 enzymes and their electron-transfer proteins reside in the mitochondria (Table 96-1) of eukaryotes; almost all bacterial P450s are also type 1 enzymes. Human type 1 P450 enzymes include the cholesterol side-chain cleavage enzyme P450scc; the two isozymes of 11-hydroxylase (i.e., P450c11β and P450c11AS); and two of the three principal enzymes in vitamin D metabolism (i.e., 1α-hydroxylase and 24-hydroxylase). Type 1 enzymes receive electrons from the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) via adrenodoxin, a small, soluble, iron-sulfur protein. Adrenodoxin does not oxidize NADPH directly, however, but receives the two electrons from NADPH via the flavoprotein adrenodoxin reductase (Fig. 96-3). Type 2 enzymes, in contrast, receive electrons from NADPH via the flavin adenine dinucleotide (FAD)-flavin mononucleotide (FMN) two-flavin protein, P450 oxidoreductase (POR). Type 2 enzymes are exclusively located in the smooth endoplasmic reticulum and constitute the majority of the human P450 enzymes.
Table 96-1. Intracellular Location of Steroidogenic Proteins
| Mitochondria | Cytoplasm | Endoplasmic Reticulum |
|---|---|---|
| P450scc | P450c17 | |
| P450c11β | P450c21 | |
| P450c11AS | P450aro | |
| Adrenodoxin reductase | P450-oxidoreductase | |
| Adrenodoxin | ||
| StAR | StAR | Cytochrome b5 |
| 3β-HSD1 and 2 | 3β-HSD1 and 2 | 3β-HSD1 and 2 |
| 17β-HSD1 | 17β-HSD1–3* | |
| Reductive 3α-HSDs includes 17β-HSD5 | Oxidative 3α-HSD 5α-Reductase 1 and 2 11β-HSD1 and 2 |
HSD, Hydroxysteroid dehydrogenase.
* 17β-HSD4 is located in peroxisomes.
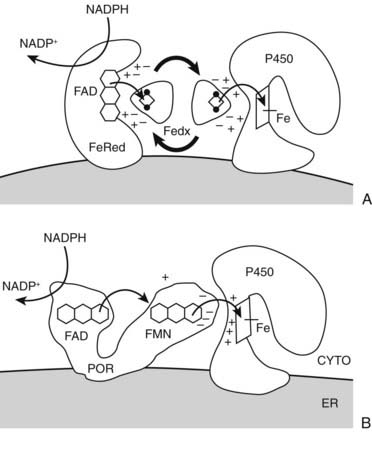
FIGURE 96-3. Electron transfer pathways for steroidogenic cytochrome P450 enzymes. A, In type 1 (mitochondrial) enzymes, the two electrons from the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) pass from the flavin (FAD) of ferredoxin (adrenodoxin) reductase (FeRed) to the iron-sulfur (Fe2S2, diamond with dots) cluster of ferredoxin (adrenodoxin, Fedx) and then to the heme of the P450 (square with iron atom [Fe]). Negatively-charged residues in Fedx (−) guide docking and electron transfer with positively-charged residues (+) in both Fedx and the P450. B, In type 2 (microsomal) enzymes, the flavoprotein P450-oxidoreductase (POR) receives electrons from NADPH to its FAD moiety, transfers electrons to its FMN moiety, and after a conformational rearrangement, directly transfers electrons from the FMN to the P450. Negative charges on POR (−) and positive charges (+) on the P450 guide the interaction as with the type 1 P450; phosphorylation and cytochrome b5 also regulate electron transfer and catalysis. Heme of P450 is indicated by square with iron atom (Fe).
P450 enzymes activate molecular oxygen using their heme center and electrons from NADPH. Substrate binding is required prior to heme reduction with one electron, which enables oxygen binding, the second one-electron transfer, and formation of the iron-oxygen complex which oxygenates the substrate. Thus, P450 reactions on steroids are limited to oxygen insertion (hydroxylation) reactions and, in a few notable cases, oxidative carbon-carbon bond cleavage reactions (Table 96-2).
HYDROXYSTEROID DEHYDROGENASES AND REDUCTASES
All hydroxysteroid dehydrogenases (HSDs) and related enzymes use nicotinamide cofactors either to reduce or to oxidize the steroid by two electrons through a hydride transfer mechanism.2 Most examples involve the conversion of a secondary alcohol to a ketone or vice versa, and in the case of the 3β-hydroxysteroid dehydrogenase/Δ5/4-isomerases, the dehydrogenation is accompanied by the isomerization of the adjacent carbon-carbon double bond from the Δ5 (between carbons 5 and 6) to the Δ4 positions (see Figs. 96-1 and 96-2). The human steroid 5α-reductases types 1 and 2, which are included with the HSDs for convenience, reduce olefinic carbon-carbon double bonds to the saturated state rather than acting on carbon centers bonded to oxygen.
The HSDs can be categorized according to either structural or functional classification schemes. Structurally, HSDs are members of either the short-chain dehydrogenase reductase (SDR) or aldo-keto reductase (AKR) families.4 The SDR enzymes are β-α-β proteins where up to seven parallel β-strands fan across the center of the molecule, forming the “Rossman fold” characteristic of oxidation/reduction enzymes that use nicotinamide cofactors. The AKR enzymes are soluble proteins that contain a beta-barrel or triosephosphate isomerase (TIM-barrel) motif in which eight parallel β-strands lie in a slanted circular distribution like the staves of a barrel. In both cases, the active site contains a critical tyrosine and lysine pair of residues involved in proton transfer from or to the steroid alcohol during catalysis. Functionally, HSDs act either as true dehydrogenases, using NAD+ as a cofactor to convert hydroxysteroids to ketosteroids, or as ketosteroid reductases, utilizing predominantly NADPH to reduce ketosteroids. Many HSDs catalyze either oxidation or reduction in vitro based on the pH and cofactor concentrations, but these enzymes, when expressed in intact mammalian cells, drive steroid flux primarily in one direction.4 These directional preferences derive primarily from the relative abundance of the oxidized and reduced form of cofactors and the relative affinity of each enzyme for NAD(H) versus NADP(H), because cofactor concentrations exceed steroid concentrations by many orders of magnitude.2,5 Consequently, the directional preference of some “reductive” enzymes can be reduced or reversed by depleting cells of NADPH or by mutations that impair NADPH binding.6
ACUTE REGULATION OF STEROIDOGENESIS
Every time that a pulse of corticotrophin (adrenocorticotropic hormone [ACTH]) reaches the adrenal cortex, or a pulse of luteinizing hormone (LH) reaches the gonad, a subsequent pulse of steroid hormone production is observed within minutes. Although it has long been known that the loss of trophic hormones from the pituitary gland leads to adrenal and gonadal atrophy, the action of ACTH and LH to promote organ survival and to maintain steroidogenic capacity occurs at three distinct levels. First, as seen in long-term exposure to ACTH (e.g., in Cushing’s disease), ACTH promotes adrenal growth. This growth occurs primarily by ACTH stimulating the production of cyclic adenosine monophosphate (cAMP), which in turn promotes the synthesis of insulin-like growth factor 2 (IGF-2),7,8 basic fibroblast growth factor,9 and epidermal growth factor.10 Together, these growth factors stimulate adrenal cellular hypertrophy and hyperplasia. Second, ACTH acts long term through cAMP, and angiotensin II acts through the calcium/calmodulin pathway to promote the transcription of genes encoding various steroidogenic enzymes and electron-donating cofactor proteins. Third, ACTH fosters the increased flow of cholesterol into mitochondria, where it becomes substrate for the first and rate-limiting enzyme, P450scc. This acute response occurs within minutes and is inhibited by inhibitors of protein synthesis (e.g., puromycin or cycloheximide), indicating that a short-lived protein species mediates this process. Although other proteins are involved in the chronic replenishment of mitochondrial cholesterol, abundant biochemical, clinical, and genetic evidence implicates the steroidogenic acute regulatory protein (StAR) as this labile protein mediator.11
StAR is a 37-kilodalton (kD) phosphoprotein that is cleaved to a 30-kD form when it enters the mitochondrion. Overexpression of mouse StAR in mouse Leydig MA-10 cells increased their basal steroidogenic rate,12 and cotransfection of expression vectors for both StAR and the P450scc system in nonsteroidogenic COS-1 cells augmented pregnenolone synthesis above that obtained with the P450scc system alone.13 Mutations in StAR cause the most common form of congenital lipoid adrenal hyperplasia,13,14 in which very little steroid is made, and targeted disruption of the Star gene in the mouse causes a similar phenotype.15
The mechanism of StAR’s action is not known in detail.16 StAR acts exclusively on the outer mitochondrial membrane (OMM),17,18 and its activity in promoting steroidogenesis is proportional to its residency time on the OMM.18 When expressed in cytoplasm or added to mitochondria in vitro, both the 37-kD “precursor” and the 30-kD “mature form” of StAR are equally active, but StAR is inactive in the mitochondrial intramembranous space or matrix.18 Thus, it is StAR’s cellular localization, not its cleavage, that determines whether or not it is active. StAR has a sterol-binding pocket that accommodates a single molecule of cholesterol.19 The interaction of StAR with the OMM involves conformational changes20,21 that are necessary for StAR to accept and discharge cholesterol molecules. Although StAR can transfer cholesterol between synthetic membranes in vitro,22 suggesting that other protein molecules are not needed for its action, this activity can also be seen with the inactive mutant R182L, which causes lipoid CAH.23 Thus, StAR’s action to promote steroidogenesis is distinct from its cholesterol-transfer activity. StAR appears to interact with the peripheral benzodiazepine receptor (PBR)24 voltage-dependent anion channel 1 (VDAC1) and phosphate carrier protein,25 all proteins found on the outer mitochondrial membrane. Each molecule of StAR appears to be recycled, moving hundreds of molecules of cholesterol before the cleavage/inactivation event.26 Although StAR is required for the acute steroidogenic response, steroidogenesis will persist in the absence of StAR at about 14% of the StAR-induced rate,27 accounting for the steroidogenic capacity of tissues that lack StAR (e.g., the placenta and the brain).
CHRONIC MAINTENANCE OF THE STEROIDOGENIC MACHINERY
While the acute regulation of steroidogenesis is determined by access of cholesterol to the P450scc enzyme, which is mediated by StAR, P450scc is the enzymatic rate-limiting step in steroidogenesis. Thus the chronic regulation of steroidogenesis is quantitatively (how much) determined by P450scc gene expression28 and qualitatively (which steroids) determined by the expression of downstream enzymes. The episodic bursts of cAMP resulting from the binding of ACTH and LH to their respective receptors are necessary but not sufficient for the continued expression of the steroidogenic enzymes and the production of steroids. Patients with inactivating mutations in the ACTH receptor29 or LH receptor30 make negligible steroids from the affected glands. Conversely, activating mutations of the Gsα protein, which couples receptor binding to cAMP generation, and activating mutations of the LH receptor cause hypersecretion of steroids.31 Indeed, cAMP-responsive elements have been identified in the genes for most of the human steroidogenic P450 enzymes, but this mechanism alone does not allow for the diversity of steroid production observed in the various zones of the adrenal cortex, the gonads of both sexes, the placenta, and the brain.
Other transcription factors (e.g., AP-2, SP-1, SP-3, NF1C, NR4A1, NR4A2, GATA4, and GATA6) aid in defining the basal- and cAMP-stimulated transcription of each gene, which is also regulated in a tissue-specific manner by the regulatory elements unique to each gene. Among these factors, steroidogenic factor-1 (SF-1, NR5A1), an orphan nuclear receptor, coordinates the expression of steroidogenic enzymes in adrenal and gonadal cells.32 By contrast, steroidogenesis in the brain33 and placenta34,35 is independent of SF-1. Targeted disruption of SF-1 in the mouse not only disrupts steroid biosynthesis but also blocks the development of the adrenal glands, gonads, and the ventromedial hypothalamus in homozygous animals.36 Furthermore, SF-1 does not act in isolation, but its action is modified by other transcription factors (e.g., WT-1 and DAX-137) or by sumoylation and phosphorylation.38 The development of steroidogenic organs is intimately related to the capacity to produce steroids, and multiple factors acting on the genes for steroidogenic enzymes yield both common features and diversity among the steroidogenic tissues.
Most steroidogenic enzymes derive from a single mRNA species. The most prominent exception to this paradigm is aromatase, whose gene has four different promoters that enable vastly different regulation of expression of the same aromatase protein in many different tissues.39 Although different transcripts of several genes (including 17β-HSDs types 1, 2, and 3) have been described, the encoded proteins derived from “exon skipping” are inactive if translated.40
Human Steroidogenic P450s
P450SCC
Encoded by the CYP11A1 gene, P450scc consumes three equivalents of NADPH and molecular oxygen during the conversion of cholesterol to pregnenolone. Although the enzyme is named for the cleavage of the cholesterol side chain, this process consists of three discrete steps: (1) the 22-hydroxylation of cholesterol; (2) the 20-hydroxylation of 22(R)-hydroxycholesterol; and (3) the oxidative scission of the C20-C22 bond of 20(R), 22(R)-dihydroxycholesterol—the side-chain cleavage event. The enzyme will utilize free hydroxysterol intermediates as substrates for the side-chain cleavage reaction, a tool that is used experimentally because the hydroxysterols are much more soluble than cholesterol and because their access to P450scc is independent of StAR.13 In vivo, however, little of these free intermediates probably accumulate because their kcat/Km ratios are much higher than for cholesterol,41 and the high Kd for pregnenolone (about 3000 nM) drives product dissociation. This complex process is the rate-limiting step in steroidogenesis, with turnover numbers of only about 20 molecules of cholesterol per molecule P450scc per minute.41 P450scc will also cleave the side chain of other hydroxysterols (e.g., 7-dehydrocholesterol), and 20- and 22-hydroxylates vitamin D.42
The single human gene for P450scc43 encodes an mRNA of 2 kb.44 A 39-amino-acid mitochondrial leader peptide that targets P450scc to the mitochondria is then proteolytically removed to yield a 482-amino-acid protein. Forms of P450scc targeted to the endoplasmic reticulum are inactive,45 demonstrating that the mitochondrial environment is required for activity. Expression of P450scc is induced in the adrenal zona fasciculata/reticularis,46 testis,47 and ovary by cAMP; and in the zona glomerulosa by intracellular calcium/protein kinase C.48,49 In contrast, placental P450scc expression is constitutive50 and is caused at least in part by the LBP family of transcription factors.35,51 Side-chain cleavage activity and pregnenolone biosynthesis have been demonstrated in the rat and human brain52; and abundant P450scc expression is found in the rodent brain, especially in fetal life. Deletion of the gene for P450scc has been described in rabbits53 and mice,54 abrogating all steroidogenesis and thus proving that P450scc is the only enzyme that can convert cholesterol to pregnenolone. While homozygous mutations in P450scc are expected to be embryonic lethal by eliminating placental progesterone synthesis, a small number of patients has been described having P450scc mutations that typically retain partial enzymatic activity.55,56
P450C17
For investigators studying the enzymology and genetics of the steroidogenic pathways, P450c17 is especially interesting. Clinical observations showed that adrenal 17α-hydroxylase activity (reflected by serum cortisol concentrations) was fairly constant throughout life, whereas adrenal 17,20-lyase activity (reflected by serum DHEA and DHEAS concentrations) was low in early childhood but rose abruptly during adrenarche at ages 8 to 10 years.57,58 This dissociation between adrenal secretion of 17α-hydroxylase products (cortisol) and 17,20-lyase products (DHEA) suggested that distinct enzymes performed the two transformations, a hypothesis that was reinforced by the description of patients with putative isolated 17,20-lyase deficiency. Consequently, reports59 that the 17α-hydroxylase and 17,20-lyase activities of neonatal pig testes copurified were initially received with great skepticism. This controversy of “one enzyme or two” persisted until the cDNA for bovine P450c17 was cloned and shown to confer both 17-hydroxylase and 17,20-lyase activities when expressed in nonsteroidogenic COS-1 cells.60 The human genome has one gene for P450c17,61 which is expressed in the adrenals and gonads,62 and not two tissue-specific isozymes as had been thought. A single 2.1-kb mRNA species yields a 57-kD protein in these tissues, and mutations in this gene produce a spectrum of deficiencies in 17-hydroxysteroids and C19 steroids.
Human P450c17 17-hydroxylates both pregnenolone and progesterone with approximately equal efficiency,63,64 but all other reactions show prominent differences between Δ4 and Δ5 substrates. The 17,20-lyase activity is about 50 times more efficient for the 17α-hydroxypregnenolone-to-DHEA reaction than for the 17α-hydroxyprogesterone-to-androstenedione reaction.63,64 Although the rate of the lyase reaction can be increased more than 10-fold by the addition of a molar excess of cytochrome b5,63–65 the Δ5 preference persists, and the lyase rate never quite reaches the rate of the hydroxylase reactions. In addition, human P450c17 16α-hydroxylates progesterone but not pregnenolone64; in the presence of cytochrome b5, it diverts about 10% of pregnenolone metabolism to a Δ16 andiene product63 that is also formed by this pathway in pigs and that acts as a pheromone in that species. Although experiments to study the chemistry of human P450c17 often require manipulations that could be considered nonphysiologic, the remarkable consistency for substrate preferences and kinetic constants observed for the modified, solubilized P450c17 expressed in Escherichia coli63,65 and native P450c17 expressed in yeast microsomes,64 or intact COS-1 cells,66 or that obtained from human tissues and cells,64,67 serve to verify these conclusions.
Given the diverse repertoire of reactions catalyzed by P450c17 in the classical pathways, it is not surprising that synthetic steroids such as dexamethasone68 and the enantiomer of progesterone,69 as well as planar drugs such as troglitazone,70 also bind to and inhibit P450c17. In addition, the 5α-reduced C21 steroids dihydroprogesterone (5α-pregnane-3,20-dione) and allopregnanolone (5α-pregnan-3α-ol-20-one) are excellent substrates for the 17α-hydroxylase activity of P450c1771 (Fig. 96-4A). Furthermore, 17α-hydroxylated allopregnanolone (5α-pregnane-3α,17α-diol-20-one; 17OH-Allo) is the most efficient substrate yet identified for the 17,20-lyase activity of human P450c17, and its cleavage to androsterone is minimally dependent on cytochrome b5,71 unlike 17α-hydroxypregnenolone metabolism to DHEA.63–65 The conversion of 17OH-Allo to androsterone by the 17,20-lyase activity of P450c17, first described in the testes of tammar wallaby pouch young,72 provides an alternative or “backdoor” pathway to DHT, by which DHT is produced without utilizing DHEA, androstenedione, and testosterone as intermediates73 (see Fig. 96-4B). Consequently, the presence of 5α-reductases in steroidogenic cells does not preclude the production of C19 steroids but rather paradoxically enhances the production of DHT by directing flux to 5α-reduced precursors of DHT.
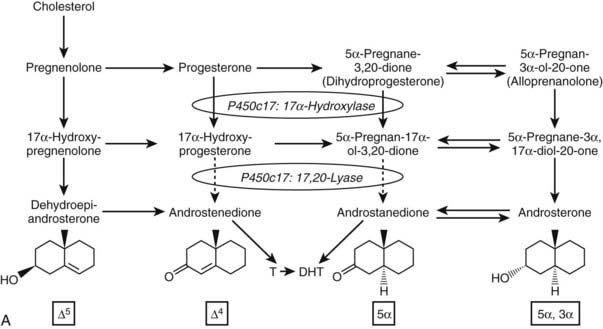
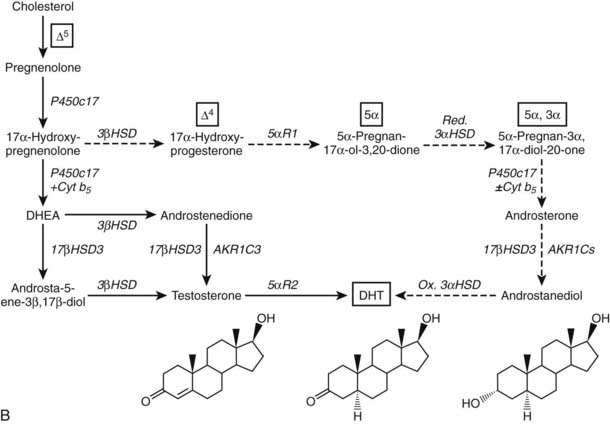
FIGURE 96-4. Reactions catalyzed by human P450c17 and pathways to C19 steroids. A, The four principal A/B-ring configurations of active endogenous steroids and their precursors: Δ5, Δ4, 5α, and 5α,3α (boxes and structures at bottom). Progesterone and 17α-hydroxyprogesterone can be 5α-reduced, and once the A-ring is saturated, these 5α-reduced steroids are substrates for reductive 3α-HSDs of the AKR1C family. Human P450c17 17α-hydroxylates all four classes of C21 steroids, but the 17,20-lyase activity is robust only with 17α-hydroxypregnenolone and 5α-pregnane-3α,17α-diol-20-one (Δ5– and 5α,3α-pathways, respectively). B, Two pathways to DHT using the different 17,20-lyase activities of human P450c17. In the conventional or Δ5-pathway (solid arrows), the 17,20-lyase activity of P450c17 requires cytochrome b5 to efficiently convert 17α-hydroxyprogesterone to DHEA, and testosterone is reduced in target tissues by 5α-reductase 2 (5αR2) to DHT. In the “backdoor” or 5α,3α-pathway (broken arrows), 5α-reduction by 5αR1 and 3α-reduction of C21 steroids occurs in the steroidogenic tissue prior to the 17,20-lyase reaction. In the best characterized pathway, 5α-pregnane-3α,17α-diol-20-one is cleaved to androsterone without requiring cytochrome b5 and reduced to androstanediol. Androstanediol is exported from the testis and metabolized to DHT by oxidative 3α-HSDs (Ox. 3α-HSD). Note that testosterone is not an intermediate in the backdoor pathway to DHT, that different isoforms of 5α-reductase appear to be involved in the two pathways, and that both reductive and oxidative 3αHSDs are required for the “backdoor” pathway. Structures of testosterone, DHT, and androstanediol are shown at bottom.
The backdoor pathway enables production of C19 steroids in the presence of abundant 3β-HSD activity, despite the poor 17,20-lyase activity of human P450c17 for 17α-hydroxyprogesterone, by using 17OH-Allo as the substrate for the 17,20-lyase reaction. The presence of 5α-reductase activity is a key requirement for the backdoor pathway. The best-studied example of 5α-reduction in a human steroidogenic tissue is the production of 5α-dihydroprogesterone in human corpus luteum by the type 1 enzyme.74 Human enzymes catalyze all of the other reactions required to complete this alternate route to DHT, and good evidence documents production of 5α-reduced androgens by the fetal adrenal, at least in some pathologic states. Consequently, it is possible that the backdoor pathway is the principal route to DHT in pathologic states in which 17α-hydroxyprogesterone accumulates, including 21-hydroxylase deficiency and P450 oxidoreductase deficiency (see the following). Androgen production by the backdoor pathway may explain why newborn girls with 21- and 11-hydroxlase deficiencies can be severely virilized, while those with 3β-HSD2 deficiency, whose adrenals cannot make 17α-hydroxyprogesterone, are minimally virilized.75 The fractional contributions of the conventional and backdoor pathways to DHT production during human sexual differentiation (at 8 to 12 weeks of gestation) and the expression of 5α-reductase in fetal adrenal and gonad tissues,76 however, are only beginning to be determined.
The chemistry of P450c17-mediated hydroxylations is believed to proceed via the common iron oxene species and “oxygen rebound” mechanism proposed for prototypical P450 hydroxylations.77 The mechanism of the 17,20-lyase reaction involving a carbon-carbon bond cleavage, however, is not known despite considerable study. The failure of hydrogen peroxide alone to support catalysis (as has been shown for some other P450-mediated deacylation reactions) and computer modeling studies suggest that the same heme-oxygen complex might participate in both hydroxylations and the 17,20-lyase reaction,78 but no conclusive evidence to exclude proposed mechanisms exist.
One consequence of the Δ5 preference of the human enzyme for the 17,20-lyase reaction is that most human C19 and C18 steroids derive from DHEA as an intermediate.67 This Δ5 preference allows for the phenomenon of adrenarche to occur in humans, an event that only takes place in large primates.79,80 However, Δ5-lyase activity is not sufficient for adrenarche to occur, because some monkeys (e.g., rhesus macaques) produce high amounts of DHEA throughout life, but most mammals (e.g., cattle, dogs, cats, etc.) never produce much DHEA.79 The biochemistry of P450c17, with its differential regulation of the 17α-hydroxylase and 17,20-lyase activities, provides clues to the genesis of this enigmatic process of adrenarche. P450c17 is a phosphoprotein, and phosphorylation selectively enhances the 17,20-lyase activity.81,82 It appears likely that the regulation of P450c17 phosphorylation, which is a dynamic balance between phosphorylation and dephosphorylation, plays an important role in adrenarche and pathologic hyperandrogenic states such as polycystic ovary syndrome.83 Whereas the kinase(s) responsible for P450c17 phosphorylation remain unknown, it is now apparent that the kinase activity is counterbalanced by protein phosphatase 2A, which in turn is regulated by cAMP via phosphoprotein SET.84 Cytochrome b5 also augments 17,20-lyase activity,64,82 and high expression of b5 in the zona reticularis of monkeys85 and humans86 suggests that the developmentally regulated expression of b5 might be a key event. The transcriptional regulation of cytochrome b5 in the adrenal is similar to that of P450c17,87 but mechanisms enabling zone-specific expression have not been elucidated. Finally, limiting steroid flux to the Δ5 pathway by lowering 3β-HSD activity in the zona reticularis (where most DHEA derives) potentiates the effect of increased 17,20-lyase activity.86,88
The initial description of 17α-hydroxylase deficiency was a case in which both 17α-hydroxylase and 17,20-lyase products were absent.89 When the gene for human P450c17 was cloned,61 patients with 17α-hydroxylase deficiency were found to harbor mutations in the CYP17A1 gene, and more than 40 mutations scattered throughout the CYP17A1 gene have been characterized,90 with mutations W406R and R362C being the most common and accounting for the high prevalence of 17α-hydroxylase deficiency in Brazil.91 The identification of CYP17A1 mutations causing apparent isolated 17,20-lyase deficiency is fraught with difficulty,92 but in the past decade, five cases of isolated 17,20-lyase deficiency caused by mutations in arginines 347 and 358 have been confirmed.93,94 Computer modeling studies demonstrate that R347H and R358Q neutralize positive charges in the redox-partner binding site.78,93 Biochemical studies confirm that mutations R347H and R358Q impair interactions of P450c17 with its electron donor POR and with cytochrome b5.95 Therefore, these cases of isolated 17,20-lyase deficiency are not caused by an inability of the mutant enzymes to bind the intermediate 17α-hydroxypregnenolone but rather are caused by subtle disturbances in interactions with redox partners.93,95 In contrast, mutation E305G has been shown to cause 17,20-lyase deficiency by selectively disrupting binding of 17α-hydroxypregnenolone and DHEA synthesis despite enhanced conversion of 17α-hydroxyprogesterone to androstenedione.96 This unusual variant of isolated 17,20-lyase deficiency provides further genetic evidence that the flux of androgens derived from conversion of 17α-hydroxyprogesterone to androstenedione in the minor Δ4 pathway is not sufficient to form normal male external genitalia. One of the first patients reported to have isolated 17,20 lyase deficiency was recently found to have a homozygous mutation in P450 oxidoreductase (G539R), further emphasizing the crucial role of efficient electron transfer in the 17,20 lyase reaction.97
P450C21
Microsomal P450c21 performs the 21-hydroxylation of the Δ4 steroids 17α-hydroxyprogesterone and progesterone, an essential step in the biosynthesis of both mineralocorticoids and glucocorticoids (see Fig. 96-2). The human P450c21 protein is found only in the adrenal glands; the extra-adrenal 21-hydroxylase activity found in other organs such as the liver and the aorta98 is not catalyzed by P450c2199 but appears to be catalyzed by CYP2C9, CYP3A4, and possibly CYP2C19 and other enzymes as well.100,101
The locus containing the CYP21 genes is among the most complex in the human genome and explains why 21-hydroxylase deficiency (affecting 1 of 14,000 live births) is one of the most common autosomal-recessive diseases. The CYP21A2 gene and the CYP21A1 pseudogene lie on chromosomal locus 6p21.1 in the midst of the human leukocyte antigen (HLA) locus. Because the HLA locus is highly recombinogenic, exchange between the CYP21A1 and CYP21A2 loci is common. Thus 85% of cases of 21-hydroxylase deficiency derive from micro- or macrogene conversion events where some or all of the CYP21A1 pseudogene replaces the corresponding area of the CYP21A2 gene, thus reducing the expression of the encoded P450c21 protein and/or impairing its activity.102 In addition, at least eight additional genes lie in this locus (Fig. 96-5), including the liver-specific C4A and C4B genes; the adrenal-specific “ZA” and “ZB” genes; and the ubiquitously expressed tenascin X or TNXB gene,103 the disruption of which is one cause of Ehlers-Danlos syndrome.104 Occasionally, a patient with 21-hydroxylase deficiency and Ehlers-Danlos syndrome will have a contiguous gene syndrome with tenascin X deficiency as well.105
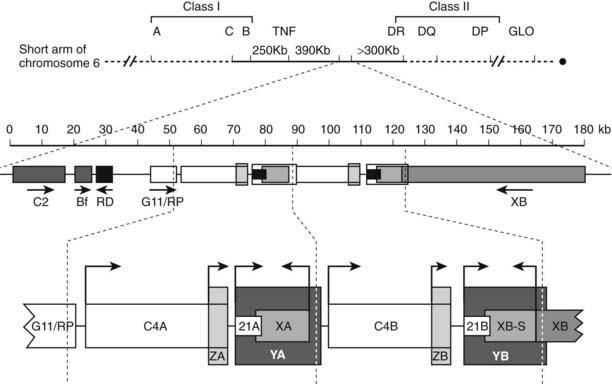
FIGURE 96-5. Genetic map of the human leukocyte antigen (HLA) locus containing the genes for P450c21. The top line shows the p21.1 region of chromosome 6, with the telomere to the left and the centromere to the right. Most HLA genes are found in the class I and class II regions; the class III region containing the CYP21 genes lies between these two. The second line shows the scale (in kb) for the diagram immediately below, showing (from left to right) the genes for complement factor C2, properdin factor Bf, and the RD and G11/RP genes of unknown function; arrows indicate transcriptional orientation. The bottom line shows the 21-hydroxylase locus on an expanded scale, including the C4A and C4B genes for the fourth component of complement, the “CYP21A” pseudogene (CYP21A1, 21A), and the active “CYP21B” gene (CYP21A2, 21B) that encodes P450c21. XA, YA, and YB are adrenal-specific transcripts that lack open reading frames. The XB gene encodes the extracellular matrix protein tenascin-X; XB-S encodes a truncated adrenal-specific form of the tenascin-X protein whose function is unknown. ZA and ZB are adrenal-specific transcripts that arise within the C4 genes and have open reading frames, but it is not known if they are translated into protein; however, the promoter elements of these transcripts are essential components of the CYP21A1 and CYP21A2 promoters. The arrows indicate transcriptional orientation. The vertical dotted lines designate the boundaries of the genetic duplication event that led to the presence of A and B regions.
Much less is known about the enzymology of P450c21 than of P450c17, but the available evidence suggests that unlike P450c17, P450c21 is not very sensitive to the abundance of POR or cytochrome b5. It is clear that genotype consistently predicts phenotype in very severe and very mild cases of 21-hydroxylase deficiency. In contrast, patients with P450c21 variants (e.g., the common Pro30Leu and Val281Leu mutations and less common mutations Arg339His and Pro453Ser), which have 20% to 50% of wild-type activity,102,106 can have various phenotypes, implying additional factors that can modify the clinical manifestations of 21-hydroxylase deficiency.
P450C11β AND P450C11AS
The classical descriptions of distinct deficiencies in 11β-hydroxylase, 18-hydroxylase (also called corticosterone methyl oxidase I, or CMOI), and 18-oxidase (CMOII) suggested that three enzymes executed these three respective transformations.107,108 Analogous to the scenario for P450c17, a single enzyme109 and corresponding gene110 were found in bovine adrenals that possessed all three activities. In contrast, humans have two genes named CYP11B1 and CYP11B2111 that encode the mitochondrial enzymes 11β-hydroxylase (P450c11β) and aldosterone synthase (P450c11AS), respectively, and rats but not mice have three functional CYP11B genes.112 Although P450c11β and P450c11AS both possess 11β-hydroxylase activities, P450c11AS also performs the two oxygenations at C18 required for aldosterone biosynthesis.113,114 Mutations in CYP11B1 cause 11β-hydroxylase deficiency,115 whereas defects in CYP11B2 cause either CMOI or CMOII deficiencies.116 Severe defects can impair all P450c11AS activities, leading to the clinical phenotype of CMOI deficiency,113 whereas P450c11β provides 11β-hydroxylase activity in the zona fasciculata. Fortuitous site-directed mutagenesis experiments of nature have found amino acid substitutions such as Arg181Trp plus Val386Ala, which mainly impair 18-oxidase activity and lead to CMOII deficiency.117
The coding regions of the CYP11B1 and CYP11B2 genes share 93% amino acid identity and the same exonic gene structure found in all mitochondrial P450 genes.118 Despite the sequence similarities of these tandem genes, located within 40 kb on chromosome 8q24.3, the expression of P450c11AS is restricted to the adrenal zona glomerulosa, whereas P450c11β is found in the zona fasciculata and zona reticularis. The regulation of P450c11β is driven mainly by cAMP in response to ACTH, whereas P450c11AS expression derives from potassium and angiotensin II activation of the protein kinase C pathway.119 Thus, under normal circumstances, 18-hydroxylase and 18-oxidase activities are restricted to the zona glomerulosa, where 17-hydroxylase activity is low, limiting the repertoire of steroids that can undergo 18-oxygenation.
Although the organization of two highly homologous, adjacent CYP11B1 and CYP11B2 genes on chromosome 8 is reminiscent of the genetics of the CYP21A1 and CYP21A2, gene conversion in the CYP11B locus occurs rarely.120 Instead, a clinical entity called glucocorticoid remediable aldosteronism (GRA) arises when an unequal crossing over of the CYP11B1 and CYP11B2 genes creates a third, hybrid gene in which the ACTH-regulated promoter of CYP11B1 drives expression of a chimeric protein with aldosterone synthase activity.121,122 As a result, 18-hydroxylase and 18-oxidase activities are ectopically expressed in the zona fasciculata, leading to elevated renin-independent production of aldosterone, as well as 18-oxygenated metabolites of cortisol. The expression of this gene is suppressed by blunting ACTH production with glucocorticoids such as dexamethasone, which is used for diagnosis and treatment.123 The prevalence of GRA varies from nil to as high 2% of referred patients with hypertension.124
The genetics of GRA has assisted in the precise identification of residues in P450c11AS that enable 18-oxygenase activities. Residues 288, 296, 301, 302, 325, and perhaps most importantly, 320 are critical for 18-oxygenase activities.125,126 Therefore, crossovers 3′ to codon 320 do not enable aldosterone synthase activity. These key residues lie in or near the I-helix, which contains the catalytically important threonine residue implicated in oxygen activation for almost all P450s; thus, these mutations would be expected to alter active site geometry.
P450ARO (AROMATASE)
The oxidative demethylation of C19 steroids, mainly androstenedione and testosterone, consumes three equivalents of molecular oxygen and NADPH, yielding formic acid and C18 steroids with an aromatic A-ring, hence the common name for this enzyme, aromatase. As is the case for P450scc, each subsequent oxygenation proceeds with greater efficiency, aiding in the completion of this transformation that is essential for estrogen biosynthesis in all animals.127 The mechanism of this aromatization must account for the incorporation of the final oxygen atom from molecular oxygen into the formic acid byproduct. The weight of evidence favors a hydroxylation at C2 of 19-oxo-androstenedione, followed by an enzyme-assisted rearrangement and tautomerization of the intermediate dienone to the phenolic A-ring.128
P450aro is expressed in steroidogenic tissues (ovarian granuloma cells, placenta), in brain, and in nonsteroidogenic tissues, especially fat and bone.127 The CYP19A1 gene for P450aro spans over 75 kb129 and contains five different transcriptional start sites130 with individual promoters that permit the tissue-specific regulation of expression in diverse tissues. P450aro is a glycoprotein, but glycosylation per se does not appear to affect activity.
Studies of patients with aromatase deficiency confirm that biologically significant estrogen synthesis derives entirely from this enzyme,131,132 although dietary phytoestrogens can provide some estrogen action in mice with targeted deletion of the aromatase gene.133 Although very few cases of aromatase deficiency have been described, they are highly informative “knockouts of nature” that illustrate principles of fetoplacental steroidogenesis. In fetuses homozygous for aromatase deficiency, the principal manifestation results from its deficiency in the placenta,131 because ovarian steroidogenesis is quiescent during fetal life.134 The fetal adrenal makes large amounts of C19 steroids, principally DHEA-S, much of which is 16α-hydroxylated in the fetal liver before undergoing metabolism via steroid sulfatases, 3β-HSD1, aromatase, and 17β-HSD1 in the placenta to produce estriol, the characteristic estrogen of pregnancy. Although huge amounts of estriol and estradiol are produced by the fetoplacental unit, estrogens are not needed for fetal development, the maintenance of pregnancy, or the onset of parturition; all of these processes proceed normally in fetuses lacking StAR, P450c17, or aromatase, or even in fetuses wholly lacking adrenal glands because of mutations in SF-1 or DAX-1.135 However, in the absence of placental aromatase activity, androgenic C19 steroids derived from the fetal adrenal are passed into the maternal circulation, causing marked virilization of the mother.131
Furthermore, in pregnancies in which the mother has poorly treated 21-hydroxylase deficiency, maternal testosterone values can exceed 300 ng/dL (a midpubertal value for males), yet the fetus is not virilized136 because the maternal testosterone is efficiently metabolized to estradiol by placental aromatase. Thus, placental aromatase is a key enzyme in protecting the fetus and mother from unwanted androgen exposure. After birth, individuals with aromatase deficiency grow normally and continue linear growth after completion of puberty, with males producing normal amounts of testosterone. However, when treated with estrogens, aromatase-deficient subjects fuse their epiphyses and cease linear growth.137 These observations provide powerful evidence that bony maturation and epiphyseal fusion in children is mediated by estrogens, not androgens, even in males. These observations have led to the experimental use of aromatase inhibitors in various disorders of accelerated bone maturation.
Redox Partner Proteins
The proteins collectively referred to as redox partners channel reducing equivalents from NADPH to the heme centers of P450 enzymes.3 Recent studies, however, suggest that these proteins act to promote catalysis by more than just their electron-transfer properties. Because of this, the precise nature of the interactions of the P450s with their redox partners is of considerable importance. Our understanding of these interactions has been greatly advanced by the x-ray crystal structures of these four proteins.
ADRENODOXIN (FERREDOXIN)
Adrenodoxin (Adx), also known as ferredoxin, is encoded by a gene on chromosome 11q22 that spans over 30 kb. Adx is a small (14 kD), soluble, Fe2S2 electron shuttle protein that resides either free in the mitochondrial matrix or is loosely bound to the inner mitochondrial membrane.138 Adx is expressed in many tissues, and its expression in steroidogenic tissues is induced by cAMP in parallel with P450scc.139
Bovine Adx consists of two domains,140 a core region and an interaction domain. The core region contains residues 1-55 and 91-end (bovine numbering), including the four cysteines whose sulfur atoms tether the Fe2S2 cluster to the protein. Residues 56 to 90 form the interaction domain, which is a hairpin containing a helix at its periphery that includes acidic residues critical for the interaction of Adx with P450scc141 (specifically, aspartates 72, 76, and 79, plus glutamate 73). The Fe2S2 cluster lies in a protuberance in the molecule at the junction of its two domains. The charged residues of Adx cluster in the interaction domain, giving the molecule a highly negatively charged surface above the Fe2S2 cluster (see Fig. 96-3A). This description of the Adx molecule concurs with earlier studies that showed that overlapping sets of negative charges on Adx drive Adx interactions with positive charges on both P450scc and adrenodoxin reductase (AdR).142 Because a preponderance of the evidence favors a model in which the same surface of Adx shuttles between AdR and the P450 to transport electrons,142,143 a model of how Adx interacts with AdR would approximate how mitochondrial P450s interact with Adx.
ADRENODOXIN (FERREDOXIN) REDUCTASE
Like Adx, adrenodoxin reductase (AdR) is widely expressed in human tissues, but its expression is two orders of magnitude higher in steroidogenic tissues.144 The primary RNA transcript from the 11-kb AdR gene145 on chromosome 17q24-q25146 is alternatively spliced, generating two mRNA species that differ by only 18 bp,147 but only the protein encoded by the shorter mRNA is active in steroidogenesis.148 Unlike most steroidogenic genes, the promoter for AdR contains six copies of GGGCGGG sequences,145 which is the canonical binding site for the transcription factor SP-1 typically found in “housekeeping genes.” Accordingly, cAMP does not regulate transcription of the AdR gene, as is the case for Adx and P450scc,144 implying that AdR plays additional roles in human physiology beyond steroidogenesis. Given their essential roles in the conversion of cholesterol to pregnenolone, no null mutations in AdR or Adx have been described in humans, and impairment of the Drosophila AdR homologue dare causes developmental arrest and degeneration of the adult nervous system owing to the loss of ecdysteroid production.149
Bovine AdR also consists of two domains, each comprising a β-sheet core surrounded by α-helices.150 The NADP(H)-binding domain is a compact region composed of residues 106 to 331 (bovine numbering), whereas the more open FAD domain, formed by the remaining amino- and carboxy-terminal residues, binds the dinucleotide portion of FAD across a Rossman fold, with the redox-active flavin isoalloxazine ring abutting the NADP(H) domain. By analogy to related structures, including glutathione and thioredoxin reductases, the nicotinamide ring of NADPH is modeled to lie adjacent to the flavin ring in a position to transfer its two electrons to the FAD. Intramolecular electron transfer occurs in the cleft formed by the angled apposition of these two domains. Within this cleft, basic residues abound, including arginines 240 and 244, which are important for interactions with Adx.142,151 Hypothetical docking of the two structures suggests that the negative surface of Adx fits elegantly into the positive surface of AdR, even with NADP(H) bound.150 Basic residues are also critical for the interaction of P450scc with the negative surface charges on Adx,143 so that AdR-Adx docking is expected to share some key features with the mitochondrial P450-Adx interaction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree