FIGURE 50-1. Combined pancreas and kidney transplant. Digestive juices of the pancreas are drained into the intestine via an enteric anastomosis between the donor duodenum and the recipient ileum. Venous outflow can be either into the portal vein or to the peripheral circulation via the iliac vein as shown.
(Drawing courtesy Dr. David Sutherland.)
Venous Drainage
The majority of the venous drainage of the native pancreas is via the pancreaticoduodenal veins into the portal vein. Classically, venous drainage from a transplanted pancreas has been directed into the systemic circulation, but some centers are now employing the superior mesenteric vein to allow drainage into the portal vein, which is considered more physiologic but also more technically demanding.14,15 It has been suggested that presentation of pancreas alloantigens to the liver via portal drainage may provide an immunologic benefit,16 but this remains controversial and has not been confirmed in several prospective studies. An analysis of United Network for Organ Sharing (UNOS) registry data suggests that any immunologic advantage is dwarfed by current immunosuppression regimens.17 The portal vein route does result in lower peripheral circulating insulin levels.18,19 The continued use of portal drainage, despite being more technically demanding, highlights concerns that hyperinsulinemia may accelerate atherosclerosis. However, more atherogenic lipid profiles have not been identified with systemic drainage, and any potential benefit may be relatively small compared to the lipid changes associated with current immunosuppression regimens. Anecdotally, systemic drainage has also been linked to allograft-associated hypoglycemia, but these cases are rare and have not been well studied.20 Systemic venous drainage remains the more commonly performed procedure, and of enteric-drained grafts performed in the United States in the period 2002-2003, it was used in 80% of SPK, 79% of PAK, and 72% of PTA.4
ANTIREJECTION THERAPY
In 2006 in the United States, the most common maintenance immunosuppression for pancreas transplantation was tacrolimus and mycophenolate mofetil (MMF), followed by tacrolimus and sirolimus, although other agents such as cyclosporine and azathioprine are used in varying combinations at some centers. Although the majority of initial regimens still include corticosteroids, since 2000 there has been a movement towards “steroid-free” or early steroid withdrawal immunosuppression protocols in an attempt to reduce the adverse effects of corticosteroids. Induction immunosuppression is routinely employed, with the majority of recipients being given T-cell-depleting antibody treatment, particularly with polyclonal rabbit antithymocyte globulin (rATG—produced from rabbits immunized with human thymus thymoglobulin) or monoclonal alemtuzumab (Campath-1H). The nondepleting anti-CD25 antibodies basiliximab (Simulect) and daclizumab (Zenapax) are used less frequently. When compared to basiliximab induction, alemtuzumab has been associated with lower rates of acute rejection but higher risk of cytomegalovirus disease.21 Although rATG has been available for over 15 years, there has been a recent increase in interest after its use was recognized to expand antigen-specific CD4+CD25+Foxp3+ regulatory T cells, while the anti-CD25 antibodies or alemtuzumab do not.22
In pancreas transplant recipients, immunosuppression is often needed not only to control allograft rejection but also the autoimmunity of type 1 diabetes. In the setting of inadequate immunosuppression, autoimmunity may lead to rapid destruction of the insulin-producing cells over a matter of weeks, without rejection of the exocrine pancreas or other transplanted organs.23,24
Many adverse effects are seen with these immunosuppression agents, but only a few will be mentioned here. In general, higher doses of immunosuppressives are required for pancreas transplants than for kidney transplants alone, which is of concern, since there are increased risks of infection and malignancy with higher doses or duration of these medications.25 Post–renal transplant data indicate that the risk of skin cancer for a patient on immunosuppression varies by country and is cumulative; at 10 and 20 years, respectively, it goes from 10% to 40% in the Netherlands and 45% to 70% in Australia.26 As with any transplant requiring immunosuppression, posttransplant diabetes mellitus (PTDM) may develop after pancreas transplantation.27,28 In a series of 144 transplants followed for 39 months by the Mayo Clinic, 19% developed diabetes despite functioning grafts.29 Contributing variables included high pre-transplant BMI, high pre-transplant insulin requirements, and episodes of acute rejection.
Glucocorticoids may cause insulin resistance and also have direct inhibitory effects upon β-cell function.30,31 The impairment of glucose-dependent insulin secretion by tacrolimus is well documented,32 and worrisome negative effects of sirolimus, including decreased insulin release and deleterious effects on islet viability and proliferation, have also been identified.33–35 There are data suggesting that MMF can directly inhibit islet neogenesis as well.36 A final concern is the nephrotoxicity of calcineurin inhibitors.37 In spite of the proven beneficial effects of a pancreas transplant on a transplanted kidney,38,39 presumably due to euglycemia, drug-induced nephrotoxicity can be severe. Side effects seen with standard maintenance immunosuppression include nephrotoxicity, hypertension, hyperlipidemia, microvascular disease, glucose intolerance, gastrointestinal problems, weight gain, electrolyte abnormalities, skin changes, alopecia, and hirsutism (Table 50-1).
Judging efficacy of immunosuppression regimens is more difficult with pancreas transplants than for other solid organ transplants such as liver or kidney, where functional tests are readily available. For SKP transplants, rejection of the pancreas may be followed using kidney function as a surrogate marker. Detection of problems with the pancreas prior to permanent end-organ damage is more difficult for PAK and PTA procedures. For patients with bladder drainage, amylase output has been thought to be useful by some, although current approaches are more likely to employ serum amylase and lipase levels and pancreas biopsies.40,41
TRANSPLANT OUTCOMES AND IMPACT
The complex surgical procedures required for pancreas transplantation may be accompanied by significant mortality and morbidity, with patients potentially having long hospitalizations and readmissions for such problems as vascular thrombosis, intraabdominal infection, hemorrhage, graft pancreatitis, anastomotic leaks, and a variety of related problems.41,42 These risks, as well as those associated with lifelong immunosuppression, are weighed against the potential benefits of insulin independence and normoglycemia.
In an analysis of 937 transplants done at the University of Minnesota from 1994 to 2003, 313 grafts failed.43 Of these, 123 (39%) were technical failures, 119 (38%) were from rejection, 63 (20%) were from death with functioning grafts, and 4 (1%) were from primary nonfunction. Of the 123 technical failures, 52% were from thrombosis, 19% from infection, 20% from pancreatitis, 6.5% from leaks, and 2.4% from bladder drainage problems. There are many varied explanations for these outcomes, but an important variable appears to be the condition of the donated pancreas. For the follow-up period ending 2005, 1-year, 3-year, 5-year, and 10-year unadjusted pancreas graft survival by transplant type is shown in Fig. 50-2.7 Despite recent improvements in short-term graft function after solitary pancreas transplant, long-term graft function remains superior for SPK. Graft function at 5 years was 78% for SPK, 58% for PAK, and 51% for PTA.7 Graft function may be maintained for 10 or more years, greater than 20 years in some individuals, with fasting glucose and response to a meal or glucose challenge similar to nondiabetic individuals.44
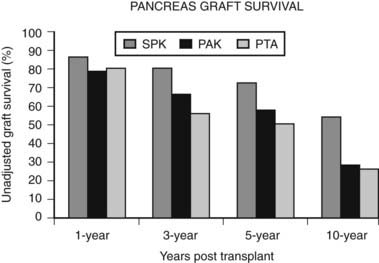
FIGURE 50-2. Pancreas graft survival among pancreas transplant recipients by type of transplant.
(Adapted from 2007 OPTN/SRTR Annual Report, Table 1.13.7)
Survival
For patients awaiting SPK, the survival rates are much lower than for patients with normal kidney function waiting for a solitary pancreas,10 no doubt owing to the dangerous combination of end-stage renal failure and hyperglycemia. It is clear that transplantation of a kidney alone improves survival in patients with diabetes and renal failure,45 and survival is better for living-donor kidney recipients than those receiving cadaver kidneys.46 However, even with the benefits of living-donor kidneys, SPK transplant recipients have better long-term survival as determined by analysis of UNOS data,46 but the difference is modest.
Retinopathy
Many studies have examined the impact of pancreas transplantation, often in combination with kidney transplantation, on preexisting retinopathy. Many of these studies have included mainly patients with longstanding, advanced retinopathy and showed little impact of transplantation on retinopathy.47–49 However, in patients with less advanced retinopathy, in particular nonproliferative retinopathy, stability50,51 or regression52,53 and improvement in macular edema51,54 has been noted after transplantation. In a recent study of individuals with GFR > 50 mL/min who underwent PTA, nonproliferative retinopathy improved (in 50%) or remained stable (in 50%) after transplant. In individuals with preexisting proliferative retinopathy, there was deterioration in some but to a lesser degree than in matched controls—14% and 57%, respectively.52
Nephropathy
The benefits of pancreas transplantation to the kidney are more apparent. Biopsy-proven diabetic nephropathy of transplanted kidneys is slowed by subsequent pancreas transplantation or even prevented by simultaneous transplant.38,55 Importantly, a longitudinal study found improvement of native kidneys after pancreas transplantation. Clinical improvement in urinary albumin was noted at 5 and 10 years posttransplant. On biopsy, no benefit was seen at 5 years, but biopsies at 10 years showed an impressive reversion of histology towards normal.39 This finding is complemented by recent findings of reduced albumin excretion after PTA.56
Neuropathy
The course of diabetes neuropathy benefits from pancreas transplantation, as demonstrated by modest improvements in nerve conduction velocity, parameters of autonomic neuropathy, and symptoms.57–60 Gastric emptying can also improve,61 but it is not clear how much can be attributed to glycemic control versus improved renal function.
Vascular Disease
The course of macrovascular disease after pancreas transplantation has not been extensively studied, but it is clear that risk factors are reduced.62–64 Moreover, reports suggest that carotid intima media thickness can diminish,64 and the progression of coronary atherosclerosis can be slowed and even regress.65 These latter findings still need to be confirmed with larger studies.
Quality of Life
The most obvious benefit of pancreas transplants is that patients feel their quality of life is improved, particularly with freedom from insulin injections, hypoglycemic episodes, and food restrictions. It must be remembered, however, that quality of life is a difficult parameter to evaluate.66,67 For example, it has been difficult to show that patients’ lives are improved using such standard criteria as whether they are more active or have better performance at work, and it has been difficult to show striking improvement using a variety of study methods.68 Nonetheless, patients seem happy to be free of their diabetes, which is of undeniable importance.
COSTS AND ORGAN AVAILABILITY
The costs of pancreas transplantation have been extensively analyzed by Stratta.69 For a typical SKP transplant in the United States, the cost is between $80,000 and $120,000, which includes hospital costs, professional fees, and organ acquisition charges. Complications requiring additional surgery and hospitalization can lead to much higher costs. In addition, the annual cost of related medications is about $15,000, and posttransplant monitoring is an additional $10,000 per year. It is important to consider this in the context of the pancreas transplant being added to the cost of a kidney transplant, which has a more pronounced impact upon health. The cost of dialysis is about $50,000 per year, while the cost of a kidney transplant from a cadaver donor is about $40,000 and from a living donor about $90,000.
Since SPK is the more commonly performed transplant type, pancreas transplants are closely linked to kidney availability. Unfortunately, there is a serious shortage of kidneys, which is particularly important for individuals with diabetes and end-stage renal disease, because once on dialysis, almost half are dead within 4 years. To further extend the donor pool by increasing living-donor renal transplants, there has been an effort to perform living-donor renal transplants at the same time as a cadaver pancreas becomes available (e.g., simultaneous cadaver-donor pancreas, living-donor kidney) or before a pancreas as a conventional PAK. Beyond encouraging living donation for kidneys, some centers have started to use extended donor criteria, including donation after cardiac death and wider acceptable age range for donation. In 2006, the number of pancreata recovered for transplantation compared to 1997 increased by 53%.7 Short-term outcomes with these extended donor criteria organs in SPK transplants have been comparable to those seen with conventional donors.70 Unfortunately, waiting-list time to transplantation has risen as well; for example, for SPK, median time to transplant went from 380 days in 1997 to 451 days in 2005.7 To further ease the shortage of organs, the U.S. Health Resources and Services Administration (HRSA) has recently launched a program to increase the numbers of available kidneys and pancreases.71
Islet Transplantation
The hypothesis that replacement of the endocrine pancreas by transplanting fragments of pancreas is not novel, with the first attempts to do so predating the discovery of insulin.72 The history of modern islet transplantation begins some 40 years ago when Paul Lacey proposed transplantation of the isolated islets of Langerhans.73 For a review of this fascinating history see Ref. 74. Progress has been slow and incremental. During the 1980s, reports suggested the feasibility of autologous islet transplantation in patients who underwent pancreatectomy for relief of chronic pain from pancreatitis.75 Despite improvements in islet isolation and purification, allotransplantation in recipients with preexisting type 1 diabetes proved significantly more difficult. Of 267 islet allografts performed between 1990 and 1998 in patients with type 1 diabetes, more than half completely failed within the first 2 months, with only 35% showing evidence of continuing function (C-peptide over 0.5 ng/mL), and 8% insulin independence beyond 1 year.76 More recently, insulin-independence rates of up to approximately 60% at 1 year posttransplant have been achieved, but only a minority (about 10%) maintain insulin independence at 5 years posttransplant.77
ISLET ISOLATION AND TRANSPLANTATION
Islets are isolated from donor pancreata using a mechanical and enzymatic digestion technique. A continuous digestion process disassembles the organ into fragments of decreasing sizes. At the end of the process, the small islet fraction is separated from the exocrine tissue using density gradient centrifugation.
Islets are either cultured for a short period of time (usually less than 48 hours) or immediately prepared for infusion. In allotransplantation, recipient-donor matching is made according to ABO compatibility, but strict human leukocyte antigen (HLA) matching is not currently done. Most commonly, the islets are infused into the hepatic portal vein via a percutaneous transhepatic ultrasound and angiography guided approach, but some centers perform small laparotomies with general anesthesia. The transplanted islets become wedged in the terminal branches of the portal vein and engraft (Fig. 50-3).
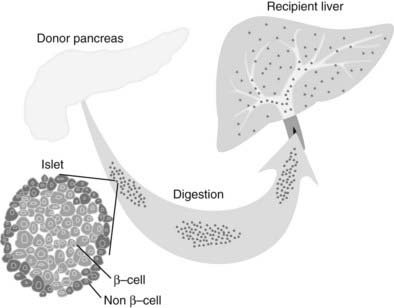
FIGURE 50-3. Islet transplantation in humans is usually done starting with a cadaver pancreas that is digested with a collagenase/protease mixture. The isolated islets are then introduced into the portal vein either by transhepatic angiography or via laparoscopy. The islets then are carried downstream and wedge in the hepatic sinusoids, whereupon they are vascularized by vessels from the recipient.
RECENT PROGRESS
In 2000, Shapiro and colleagues78 reported 100% insulin independence in seven individuals with type 1 diabetes after islet allotransplantation from multiple donors under what has become known as the Edmonton protocol. This result provided impetus to the islet transplant field worldwide. Existing centers accelerated their efforts, and other institutions embarked on establishing new islet programs. In 2001, the National Center for Research Resources of the National Institutes of Health established 10 islet resource centers in the United States with the mission of providing human islets for both clinical and research use. In addition, the Immune Tolerance Network of the National Institutes of Health, with help from the Juvenile Diabetes Research Foundation, began a trial at 9 centers to perform transplants in 40 subjects to try to reproduce the Edmonton results. As with the Edmonton trial, this multicenter trial recruited patients without kidney transplants who had life-threatening hypoglycemia episodes, severe glycemic instability, or advancing complications. The Immune Tolerance Network has confirmed that the Edmonton results can be reproduced in other centers, but better success rates occurred in more experienced centers than in the others.79 As reported by the Collaborative Islet Transplant Registry (CITR) in their 2008 Fifth Annual Report,80 between 1999 and 2007, 31 centers in North America performed 649 islet infusions in 325 recipients, while more than 200 patients received transplants in other parts of the world.
With increasing numbers of transplants, a number of advances have been reported. The Miami and Baylor groups reported successful transplants between cities, with pancreases flown from Houston to Miami and isolated islets flown back to Houston for implantation.81 This study and work from other groups shows that islets do not need to be transplanted immediately after isolation, as was done in the Edmonton trial, but can be placed in culture and transplanted 1 to 2 days later. Success in obtaining insulin independence from single donor transplants has also become more common.82,83 Building upon the Edmonton islets-alone approach, transplantation of islets after a kidney transplant has been explored and is now favored by a number of centers, as these patients will already require immunosuppression.84,85
In 2005, the Edmonton group reported 5-year follow-up data. Of the 44 Edmonton subjects achieving insulin independence, over half were back on insulin within 2 years, and at 5 years, only 10% were insulin independent.77 This general lack of durability has been confirmed in the 2008 CITR report. Of the 279 islet-alone recipients, as analyzed from the last infusion, 54% were insulin independent 6 months later, but only 22% were at year 3. The number on exogenous insulin with persistent graft function was about 25%, and the group with no detectable C-peptide was 34% at year 3 (the remaining 19% represents missing data). Over 60% of the recipients had hemoglobin A1c values below 7.0% at 6 months, and these values did not fall much over the 3 years, in part no doubt due to residual C-peptide secretion and treatment with exogenous insulin (Fig. 50-4).
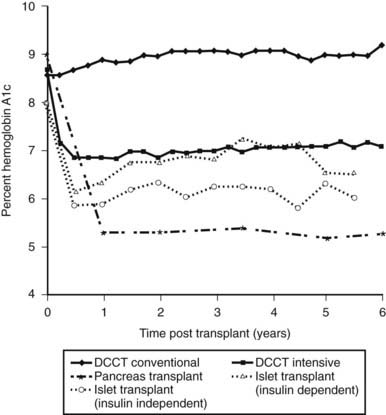
FIGURE 50-4. Comparison of hemoglobin A1c levels after successful pancreas or islet transplantation to levels obtained during the Diabetes Control and Complications Trial (DCCT).
(Data was obtained from the DCCT Research Group,1 Robertson et al,219 and Ryan et al.87)
The 2008 CITR report found a variety of variables that influenced insulin independence. Low recipient insulin requirements, low BMI, and better control led to better outcomes, as did shorter cold ischemia of the donated pancreas and the number of IEQs infused per body weight. There was also benefit for longer duration of diabetes and older age of the recipient. Islets from donors positive for CMV given to CMV-negative recipients led to poorer results. Donor O blood type also was correlated with success.80
RISKS AND BENEFITS
Several adverse side effects of islet transplants were highlighted by a report on six recipients from the National Institutes of Health islet transplantation program. This study employed the Edmonton approach of transplanting patients who were receiving immunosuppression for the first time.86 Several of the patients found the side effects of the immunosuppression to be so problematic that the drugs had to be discontinued, in one case due to sirolimus-induced pneumonitis.
Immunosuppression
The side effects of the immunosuppression under the Edmonton protocol are similar to those seen after other transplants and have been described in a number of clinical reports, as summarized in Table 50-2.77,87,88 The major side effects of sirolimus have been mouth ulcers, diarrhea, malaise, hypertension, and hyperlipidemia. Especially worrisome is the nephrotoxicity that can be found with calcineurin inhibitors—in particular, the tacrolimus that is part of the Edmonton protocol. As with other transplants, there is concern about immunosuppression leading to opportunistic infections and malignancy.25 The complications found in these early studies predicted similar events in other trials. Indeed, this is now well documented in the 2008 CITR report, in which out of 325 transplanted subjects, there were 440 severe adverse events, most related to immunosuppression.
Table 50-2. Adverse Events Associated With Clinical Islet Transplantation (Approximate Percent Incidence)
| Procedure-Related (%) | Immunosuppressive-Related (%) | |
|---|---|---|
| Elevated liver enzymes (50%) | Oral ulcers (89%) | Decline in GFR (50%) |
| Abdominal pain (50%) | Anemia (60%) | Proteinuria (50%) |
| Nausea/vomiting (50%) | Diarrhea (60%) | Peripheral edema (43%) |
| Fatty liver (long term) (20%) | Weight loss (50%) | Neutropenia (<10%) |
| Peritoneal hemorrhage (15%) | Fatigue (50%) | Tremor (<10%) |
| Portal vein thrombosis (4%) | LDL elevation (60%) | Acne (52%) |
| Gall-bladder puncture (3%) | Hypertension (50%) | Misc: Arthralgia, pneumonitis, hematuria, infection |
Islet Infusions
Although generally considered less invasive than surgery, percutaneous transhepatic angiography still poses significant risks. Most active centers have had some patients with bleeding after the procedure, which in some cases has required transfusions and even exploratory laparotomies. Thrombus in the segmental portal veins has been found with ultrasound examinations; these have been successfully treated with heparin, and fortunately, no cases of complete portal vein thrombosis have been reported. Gallbladder punctures have occurred. In one center, a patient experienced a hemothorax as a complication of the angiography. Some centers are finding that complications fall as the angiography team gains more experience.
Portal pressure is monitored during the islet infusion, which usually is a packed-cell volume of 2 to 6 mL; in the Edmonton series, pressures on average rose from 12 to 17 mm Hg, with there being a correlation with increasing packed-cell volume.89 Furthermore, when compared with the first transplant, pressure increases are higher with the second and third transplants. While this has not been associated with any complications, it does indicate that islet infusions lead to changes in the portal vasculature. Immediately following the islet infusions, transaminase levels often increase to levels two to five times normal, peaking at about 7 days and then returning to normal within 2 months.90 More modest increases in alkaline phosphatase are also found.
Hepatic Steatosis
Magnetic resonance imaging (MRI) evidence of hepatic steatosis has been reported by several groups.91,92 Of the 30 patients studied with MRI in Edmonton, 20% had patchy steatosis, which in one case disappeared following graft failure. Histologic findings concur with these imaging studies.92 This steatosis is likely due to high concentrations of insulin released locally by clumps of islet tissue, promoting localized triglyceride storage in hepatocytes. The possibility that this could lead to deleterious scarring has not been excluded, but liver function is being carefully followed in the patients, and no problems have been reported.
Glycemic Control and Hypoglycemia
The major benefit of islet transplantation has been improvement in glycemia. Protection from severe hypoglycemic events is striking; a prevalence of about 85% pre-transplant has been reduced to less than 10% throughout the first year after the last infusion. It was expected that there would be little hypoglycemia when subjects are insulin independent, but even when insulin treatment has been required, recipients enjoyed relative freedom from severe insulin reactions. Moreover, hypoglycemia awareness as assessed by Clarke hypoglycemic score was restored in both insulin-independent and insulin-requiring patients. In these patients, glycemic thresholds for activation of counterregulatory hormones were restored to normal even though the glucagon and epinephrine responses were not fully normalized.93 The glucagon secretion in response to hypoglycemia from islet grafts in the liver was very modest,94 which contrasts with the essentially normal responses found in recipients of whole pancreases.95
Unfortunately, even when recipients become insulin independent, they typically are not truly normoglycemic but instead can be classified as having impaired glucose tolerance, and some even meet the criteria for diabetes. This is in contrast with pancreas transplant recipients, who usually have normal glucose tolerance. As glucose levels deteriorate, some have reasoned that insulin sensitizers should be beneficial, yet anecdotal reports suggest that the use of metformin and thiazolidinediones have been of limited value. There has been some anxiety about using sulfonylureas because of their putative potential to cause β-cell apoptosis, but this risk in clinical situations is far from proven. There has been limited experience with exendin-4 treatment, with mixed results.96,97 Even after there is a return to insulin use, for patients accustomed to the marked glycemic fluctuations of brittle diabetes, this improved control with a far less demanding regimen is a welcome benefit, which often justifies continuation of immunosuppression until graft function is lost and unstable diabetes returns.
Relatively small studies from Milan, Miami, Edmonton, and a few other groups indicate that successful islet transplantation may have a protective effect on long-term diabetic complications,98,99 but larger well-controlled studies are still lacking. Several surrogate markers for vascular disease, including intima media thickness and endothelial function, have been shown to improve,84,100 and there are indications of some beneficial effects on other diabetic complications. Considering quality of life, most patients, even many of those still needing insulin, are very thankful to be free of hypoglycemia unawareness and severe insulin reactions.101
COSTS AND ORGAN AVAILABILITY
As with other transplants, the costs of islet transplantation are considerable. The startup costs include developing a pathogen-free space, purchasing equipment, and assembling an isolation team of five or more individuals, which typically costs over 2 million U.S. dollars. Costs for the actual transplants vary depending on healthcare costs.102 A recent example of costs for a patient in the United States was $188,000, which covered two isolations ($26,000), two transplants ($30,000), hospitalization ($14,000), medication for 1 year ($61,000), and monitoring for 1 year ($37,000), along with other costs (R.A. Dickey, oral communication, 2003).
There has been much discussion about the availability and allocation of pancreases that can provide islets for clinical trials and for research. In 2005, OPTN developed a pancreas allocation program to direct pancreases from donors older than 50 and/or a BMI of over 30 to islet transplantation.103 Pancreases from obese donors often provide high yields of excellent islets. While there has been the impression that islet yields from older donors are smaller and do less well, the pooled data in the 2008 CITR report failed to substantiate this concern. There continues to be a need to increase the number of high-quality pancreases available for islet and whole pancreas programs.103 Although the problem is not acute in 2008, the projected new clinical trails over the next few years may encounter shortages. There has been some preliminary success with the use of islets from pancreases of non-heart-beating donors,104 and there will be continued exploration of ways to expand the donor pool. The cost of pancreases for islet transplantation have remained problematic, since it is not uncommon for two or more isolations to be performed before a suitable islet preparation is obtained. This financial burden under current CMS accounting practices for the acquisition costs of organ procurement has been constraining, as laid out in a recent White Paper.105
Frontiers in Transplantation
IMMUNE MODULATION AND TOLERANCE
One of the dreams of transplantation is to induce tolerance, which means that treatment given only at the time of transplantation will somehow trick the recipient’s immune system into accepting transplanted foreign tissue as its own. In contrast to solid-organ transplants, the procedural risks of islet transplantation are considered modest, and graft failure is not acutely life threatening. The ability of islets to survive in culture also makes it possible to more readily manipulate the graft prior to transplant. As such, many investigators consider islet transplantation a good system in which to study tolerogenic strategies. Many different approaches currently being studied could lead to full or operational tolerance (Table 50-3).
Table 50-3. Approaches to Tolerance Induction
| Recipient Treatment Strategies |
| Graft Treatment Strategies |
Central tolerance refers to the process by which autoreactive T-cell clones are deleted as they migrate through the thymus. It has long been hoped that a similar process could be used to eliminate alloreactive T-cell clones. Several donor-specific tolerance strategies have been developed that mimic this central tolerance by leading to near-total and permanent elimination of donor-specific T-cell clones in the recipient. One such strategy is to directly introduce antigen into the thymus to aid in thymic selection. Posselt and colleagues demonstrated that injection of pancreatic islets directly into the thymus after treatment with anti-lymphocyte serum led to donor-specific unresponsiveness and long-term survival of islet grafts in a rodent model.106 To date, there has only been one report describing an attempt to apply this technique in a clinical setting with computed tomography (CT)-guided fine-needle intrathymic inoculation and simultaneous portal vein islet infusion and immunosuppression with an azathioprine, cyclosporine–based regimen. Although the patient never became insulin independent, there was evidence of continued graft function at 14 months.107 This approach has interesting immunologic implications, but the need for intrathymic injection, the temporal limitations of transplantation, and age-related atrophy of the thymus has made its clinical application difficult at best.
Another tactic is to use strategies that rely on the creation of mixed donor and recipient multilineage hematopoietic chimeras. This technique takes advantage of the ability of hematopoietic cells to home to areas of T-cell selection such as the thymus and to become dendritic cells capable of mediating selection. Thus, in chimeric hosts, donor-specific T cells are destroyed in peripheral immune tissues, and new thymic emigrants are depleted of donor-reactive T cells in the thymus.108 Using this approach, a small number of patients with refractory hematologic malignancies and renal failure have demonstrated tolerance after myeloablation followed by bone marrow transplantation and kidney transplant from the same donor.109,110 In islet transplantation, the induction of tolerance through mixed chimerism may have the added benefit of preventing autoimmune recurrence. Experiments by Megan Sykes’s group using nonmyeloablative conditioning in nonobese diabetic mice have demonstrated reversal of autoimmunity and acceptance of islet grafts.111 An ongoing trial at the University of Miami is using a steroid-free immunosuppressive regimen combined with infusion of CD34-enriched donor bone marrow cells.
Despite the rigorous nature of central selection, autoreactive T cells can escape thymic deletion into the periphery. As such, the normal immune system has mechanisms of peripheral tolerance to discourage autoimmunity. Regulatory T cells (Tregs) can suppress both antigen-specific and antigen-nonspecific cellular immune responses. A number of different strategies have been proposed to shift the balance between effector and regulatory T cells.
While some immunosuppressive drugs interfere with the survival, expansion, and/or function of Tregs, sirolimus treatment has been shown to expand Tregs in murine and human models. It is therefore often used as an immunosuppressive agent in combination with immune modulators. Most of these immune modulators have been developed in other clinical settings and then applied in islet transplant trials. For example, the serine protease α1-antitrypsin (AAT) reduces inflammation but is permissive of interleukin 2 (IL-2) activity. In a murine islet allotransplant model, treatment with AAT monotherapy for 14 days allowed graft function for up to 120 days without additional treatment and the development of antigen-specific T-regulatory cells.112 Since AAT is already available for the treatment of α1-antitrypsin deficiency and is considered relatively safe, it is expected to be rapidly translated to human clinical islet transplant trials. Investigation of regimens consisting of thymoglobulin and the anti-TNF agent etanercept or the anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) as induction agents in clinical islet transplants at the University of Minnesota are also underway.
The group of Judy Thomas at the University of Alabama has developed a primate anti-CD3-specific diphtheria-based immunotoxin. This compound is believed to deplete naive and memory T cells in blood and lymphatic compartments. 15-Deoxyspergualin is given concurrently to block proinflammatory cytokine production and maturation of dendritic cells by inhibiting nuclear translocation of nuclear factor κB (NF-κB).113 Using this technique, they have reported operational tolerance in approximately 85% of animals with normal β-cell function approximately 2 years after transplantation.114
In the two-signal model of T-cell activation, the costimulatory signal is essential for T-cell activation and effector function. Costimulatory blockade prevents clonal expansion and can achieve peripheral tolerance in several animal models. A number of costimulatory modifying agents, including anti-CD154 and CTLA4-Ig, are being actively investigated. Studies by Kenyon and colleagues have shown anti-CD154 treatment to be effective in a primate islet transplant model.115 Kirk and colleagues found that although administering anti-CD154 or CTLA4-Ig could significantly prolong renal allograft survival, treatment with both agents was much more efficacious. In a primate model, they saw improved graft survival and evidence of durable tolerance with subsequent nonresponsiveness to skin allografts from the original donors.116 Unfortunately, while anti-CD154/CTLA4-Ig combination therapy may produce some degree of transplantation tolerance in many animal models, this regimen does not enable long-term graft survival in mice with overt autoimmune diabetes.117 Given these findings, it is unlikely that simple deletion or costimulatory blockade alone will clinically prevent islet rejection in individuals already with underlying autoimmune diabetes. Despite these and other encouraging results in nonhuman primates, the future of anti-CD154 therapy is uncertain. CD154 is also expressed on platelets, and early clinical trials for rheumatoid arthritis with a humanized anti-CD154 blocking antibody (Hu5C8) were halted in the setting of thromboembolic complications.
On the other hand, CTLA4-Ig is progressing towards clinical trials. LEA29Y (belatacept), a mutant CTLA4-Ig molecule with increased binding activity, has been evaluated by Adams and colleagues in an allogeneic islet primate model. Their data suggest that this agent has promise as a component in a minimal immunosuppression regimen but not as a sole therapy.118 A clinical trial using sirolimus- and belatacept-based immunosuppression is underway.
Zheng et al have developed a strategy that avoids nonspecific inhibition, blockade of T-cell receptor signaling, or costimulation altogether and instead is designed to selectively eliminate activated cytopathic donor-reactive T cells while sparing immunoregulatory networks.119 Their approach takes advantage of activation-induced cell death (AICD), a method by which the body rapidly eliminates effector cells after antigen-dependent clonal expansion. AICD and passive cell death are both routine downstream consequences of T-cell activation and help maintain peripheral tolerance. By using sirolimus, which blocks the proliferation but not the apoptotic effects of IL-2, early clonal expansion can be limited without affecting subsequent apoptotic clearance via AICD. When sirolimus is given in combination with agonist IL-2 immunoglobulin fusion protein and antagonist IL-15 related cytolytic immunoglobulin fusion protein, this cocktail induces durable transplantation tolerance in mice with prior autoimmune diabetes.119 While the IL-2 promotes apoptosis of proliferating effector T cells, it is also a survival factor for immunoregulatory CD4+CD25+T cells. The mutant IL-15 operates by blocking normal IL-15-triggered antiapoptotic signals. Through these mechanisms, this treatment biases the immune response to one of Tregulatory cells over pathogenic T cells. Preliminary islet transplant experiments in nonhuman primates seem promising, with durable tolerance and no evidence of opportunistic infections (M. Koulmanda, X.X. Zheng, and T.B. Strom, unpublished observations).120
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









