FIGURE 17-1. The significant increase in reported cases of pituitary thyrotropin (TSH)-producing adenoma in the decade from 1989 to 1999, when ultrasensitive TSH assay and direct methods for free thyroid hormone measurement became available as first-line tests of thyroid function.
Pathology and Etiopathogenesis
The thyrotroph is the cell type of origin in TSH-omas. These tumors are nearly always benign; at present, transformation of a TSH-oma into a carcinoma with multiple metastases has been reported in only two patients.18,19 Most of them (72%) secrete TSH alone, although this often is accompanied by unbalanced hypersecretion of the α subunit. About one fourth of TSH-omas are mixed adenomas, characterized by concomitant hypersecretion of other anterior pituitary hormones, mainly growth hormone (GH), prolactin (PRL), or both, which are known to share with TSH the common transcription factor Pit-1. Indeed, hypersecretion of TSH and GH is the most frequent association (16%), followed by hypersecretion of TSH and PRL (10.4%) and occasionally TSH and gonadotropins (1.4%) (Fig. 17-2). No association with adrenocorticotropic hormone (ACTH) hypersecretion has been documented to date. Two ectopic TSH-producing adenomas have been documented in the pharyngeal hypophysis.20,21
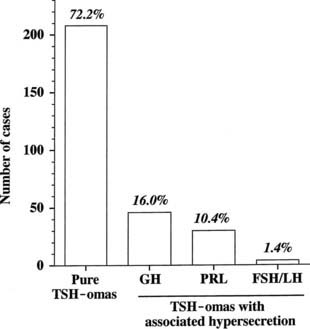
FIGURE 17-2. Classification of pituitary thyrotropin (TSH)-producing adenomas based on hormone secretion into circulation.
At morphologic and histopathologic analysis, most TSH-omas are macroadenomas (87%), frequently with fibrous consistency, even in the absence of prior surgery or radiotherapy, and high local invasiveness.22 However, previous thyroid ablation by surgery or radioiodine has deleterious effects on the size and invasiveness of the tumor (Fig. 17-3).4 In fact, invasive macroadenomas were found in 49% of patients who had undergone thyroid ablation versus 27% in those who were untreated, whereas the figure was reversed in patients with microadenomas (diameter <1 cm) or intrasellar macroadenomas. Therefore, previous thyroid ablation may induce an aggressive transformation of the tumor, as is observed in Nelson’s syndrome after adrenalectomy for Cushing’s disease.
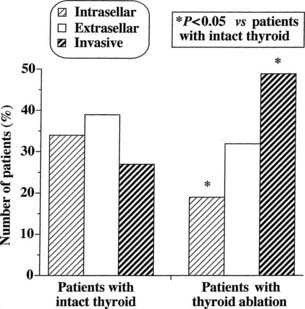
FIGURE 17-3. Effects of previous thyroid ablation on the size of pituitary thyrotropin (TSH)-producing adenomas. Intrasellar refers to both microadenomas and intrasellar macroadenomas, extrasellar to macroadenomas with suprasellar extension, and invasive to invasive macroadenomas. Data were calculated from 253 reported patients (163 with intact thyroid and 90 with thyroid ablation). Statistical analysis was carried out by Fisher’s exact test.
Light microscopy shows that adenoma cells are chromophobic, although they occasionally stain with basic or acid dyes. Ultrastructurally, adenomatous cells frequently appear monomorphous, even if they hypersecrete TSH, α subunit, and other pituitary tropins.23–26 Cells with abnormal morphologic features or mitoses,27 which may be misinterpreted as pituitary malignancy or metastases from distant carcinomas, are present in poorly differentiated adenomas that are characterized by the presence of fusiform cells with sparse and small secretory granules (80 to 200 nm). Indeed, there are no clear criteria of malignancy for TSH-omas except for the presence of metastases. It is worth noting that the first carcinoma reported in the literature exhibited a progressive malignant transformation accompanied by a decline in TSH and α subunit secretion.18
Immunostaining studies show the presence of TSH-β, either free or combined with the α subunit. In very few cases, a negative TSH-β immunostaining has been reported, possibly due to the extremely fast secretion rate of newly synthesized TSH molecules.4,34 With the use of double immunostaining, the existence of mixed TSH-α subunit adenomas composed of one cell type secreting α subunit alone and another cosecreting α subunit and TSH has been documented.28 In addition to α subunit, TSH frequently colocalizes with other pituitary hormones in the same tumoral cell29 or even in the same secretory granule.24,28,30,31 Nonetheless, positive immunohistochemistry panels for one or more pituitary hormones do not necessarily correlate with hypersecretion in vivo.32 Indeed, positive immunostaining for ACTH and gonadotropins without evidence of in vivo hypersecretion has been reported.33–37
TSH-omas have been shown to be monoclonal in origin,38 and several studies have screened a substantial number of adenomas for proto-oncogene activation32,33,39–41 or loss of antioncogenes,40,42 yielding negative results.4 A highly variable expression of thyrotropin-releasing hormone (TRH) and dopamine receptors was documented in several adenomas,43–45 whereas functional somatostatin receptors were detected constantly in TSH-omas,46–48 thus providing the rationale for their medical treatment with somatostatin analogues. Indeed, loss of heterozygosity at the locus of the somatostatin receptor type 5 gene appears to be associated with resistance to somatostatin analogues and a more aggressive phenotype.49
Recently, somatic mutations50 and aberrant alternative splicing51 of thyroid hormone receptor β have been reported, along with dysregulation of iodothyronine deiodinase enzyme expression and function.52,53 These findings at least in part may explain the defects in negative regulation of TSH secretion by thyroid hormones in some tumors.
Clinical Features
Patients with TSH-oma present with the signs and symptoms of either hyperthyroidism or the mass effect of an expanding intracranial tumor (Table 17-1). TSH-omas may occur at any age (range, 11 to 84 years), although most patients are in the third to sixth decade of life. Unlike the female predominance seen with other common thyroid disorders, TSH-omas occur with equal frequency in males and females. Goiter and clinical thyrotoxicosis are the most common presenting symptoms. Most patients presented with a long history of thyroid dysfunction, often mistakenly diagnosed as Graves’ disease, and one third had inappropriate thyroidectomy, radioiodine thyroid ablation, or both. Thus, patients with TSH-omas may present to the specialist with hyperthyroidism that has been refractory to previous therapeutic attempts. In general, clinical features of hyperthyroidism are milder than expected on the basis of circulating thyroid hormone levels. Moreover, individual patients with untreated TSH-oma were reported to be clinically euthyroid.10,54–56 This emphasizes the importance of systematic measurement of TSH and FT4 in all patients with pituitary tumor, to disclose those with central hyperthyroidism or central hypothyroidism. In some acromegalic patients, signs or symptoms of hyperthyroidism are missed, as they are overshadowed by those of acromegaly.24,57 Severe thyrotoxic features, such as atrial fibrillation, cardiac failure, and episodes of periodic paralysis,58–60 are observed in about one fourth of cases.
Table 17-1. Clinical Characteristics of Patients With Pituitary Thyrotropin (TSH-oma)
| Patients With TSH-oma % (n/total)* | |
|---|---|
| Age, years | 40.9 ± 14.5 (312)† |
| Sex, female | 55 (180/325) |
| Previous thyroid ablation | 33 (95/290) |
| Severe thyrotoxicosis | 29 (60/204) |
| Goiter | 93 (219/235) |
| Thyroid nodule(s) | 72 (46/64) |
| Macroadenomas | 87 (227/261) |
| Visual field defect | 40 (61/154) |
| Headache | 20 (23/117) |
| Menstrual disorders‡ | 33 (27/81) |
* n/total refers to the number of patients for whom the information was available.
‡ Data include women with or without associated prolactin (PRL) hypersecretion.
(Data from reports published until December 2007)
The presence of a goiter is the rule (93%), even in patients who have undergone previous partial thyroidectomy. Because the thyroid is intrinsically normal in this disorder, it may regrow even after near total resection as a consequence of TSH hyperstimulation. Occurrence of multinodular goiter has been reported in several patients,61 and differentiated thyroid carcinoma has been reported in other patients.62–65 Progression toward functional autonomy seems to be infrequent.66,67 In contrast to Graves’ disease, the occurrence of circulating antithyroid autoantibodies is similar to that found in the general population. Unilateral exophthalmos due to orbital invasion by pituitary tumor was reported in three patients with TSH-omas, whereas Graves’-associated bilateral ophthalmopathy was reported in five patients.4
Most patients bearing a TSH-producing macroadenoma seek medical attention with signs and symptoms of an expanding intracranial tumor. Indeed, as a consequence of tumor suprasellar extension or invasiveness, signs and symptoms of tumor mass prevail over those of thyroid hyperfunction in many patients. Visual field defects are present in 40% of patients and headache in one fifth. Moreover, partial hypopituitarism is common, and loss of gonadal function is present in about one third of patients.22,68 Galactorrhea was recorded in almost all patients with mixed TSH- and PRL-secreting tumors.69,70
Finally, TSH-omas may occur in families with multiple endocrine neoplasia type I71–73 and in McCune-Albright syndrome.74
Biochemical Findings
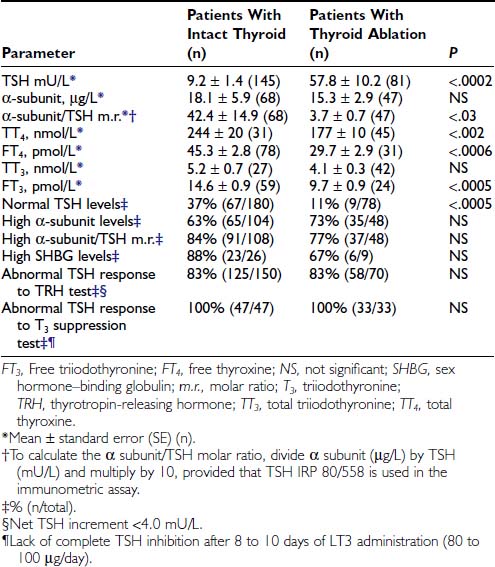
TSH AND THYROID HORMONE LEVELS
High concentrations of thyroid hormones in the presence of detectable TSH levels typically are present in patients with hyperthyroidism due to a TSH-oma or with resistance to thyroid hormone. In the case of replacement therapy for prior thyroidectomy or thyroid ablation, it is crucial to assess patients in steady state, as TSH levels need 4 to 6 weeks to adjust to a change in LT4 dose. Thus, the diagnosis of TSH-producing adenoma may be difficult to establish in any patient who has had a dramatic change in thyroid hormone replacement therapy resulting from physician instruction or poor compliance. Conversely, the finding of elevated TSH levels in patients who have undergone thyroid ablation and have been overtreated with LT4 should be regarded as a possible sign of previously undiagnosed TSH-oma.75
Various abnormalities in the pituitary-thyroid axis, as well as laboratory artifacts, may cause a biochemical profile similar to that of central hyperthyroidism. These different conditions are more common than are TSH-omas and resistance to thyroid hormone and should be excluded before an extensive clinical assessment of the possible presence of central hyperthyroidism is conducted. Familial or drug- or estrogen-induced increases in circulating thyroxine-binding globulin (TBG) or variants of albumin or transthyretin have led to increases in the levels of total serum thyroid hormone, particularly T4, thus producing a biochemical profile that may be confused with that of TSH-omas. Therefore, measurement of free thyroid hormones is mandatory in these conditions and should be performed by means of direct “two-step” methods (i.e., techniques by which contact between serum proteins and tracer can be avoided at the time of assay).76,77 Indeed, normal levels of total T4 were recorded in several patients with TSH-oma, and only the measurement of FT4 allowed the right diagnosis of central hyperthyroidism. Furthermore, inhibition of T4 to T3 conversion induced by iodine-containing drugs or nonthyroidal illness may cause hyperthyroxinemia and nonsuppressed TSH that are, however, associated with normal or low-normal T3. In clinically ambiguous situations, the differential diagnosis rests on recognition of the underlying disorder, as well as on documentation of normalization of thyroid function test results at a later stage or after recovery of drug withdrawal.
Several laboratory artifacts may cause falsely high serum levels of TSH or thyroid hormones (Table 17-2). The more common factors that interfere with TSH measurement are heterophilic antibodies directed against mouse gamma globulins78 or anti-TSH antibodies. However, preventing formation of the “sandwich” anti-TSH antibodies usually leads to an underestimation of the actual levels of TSH and rarely to an overestimation. The presence of anti-T4 or anti-T3 autoantibodies or both may cause FT4, FT3, or both to be overestimated, particularly when “one-step” analog methods are employed.77 Finally, because patients with a TSH-oma may have T3 toxicosis, as in other forms of hyperthyroidism, there is a need to measure T3, in particular, free T3, when T4 levels are normal.
Table 17-2. Circulating Factors That May Interfere With the Measurement of Pituitary Thyrotropin (TSH) or Total and Free Thyroid Hormones Giving Overestimation of the Actual Serum Levels of These Hormones and Thus Simulating the Presence of a TSH-Producing Adenoma
Heterophilic antibodies directed against mouse γ-globulins, leading to interference with monoclonal antibodies used in the immunometric assay* |
* This interference is commonly prevented by the addition of a few microliters of mouse serum to the assay buffer.
† Overestimation of TSH is very rare in the presence of such antibodies. This interference cannot be prevented, but it can be documented by performing dilution and recovery tests in the immunoassay.
‡ To prevent misdiagnosis, measure free T4 and free T3 by direct “two-step” methods.76,77
In TSH-omas, extremely variable levels of serum TSH and thyroid hormones have been reported (Table 17-3). It is interesting to note that in patients who were treated previously with thyroid ablation, TSH levels were dramatically higher than in untreated patients, although free thyroid hormone levels were still in the hyperthyroid range and the reduction of total thyroid hormone levels was minimal. The conserved sensitivity of tumoral thyrotroph cells to even small reductions in circulating free thyroid hormone levels is confirmed by the rapidly increased rate of TSH secretion during antithyroid drug administration.57
Although patients with TSH-oma have TSH-dependent hyperstimulation of the thyroid gland, any significant correlation between immunoreactive TSH and free thyroid hormone levels is lacking, even though only untreated patients are taken into account. Moreover, in one third of these patients, high levels of free thyroid hormones are associated with immunoreactive TSH levels within the normal range. Variations in the biologic activity of secreted TSH molecules most likely account for these findings.79 The first demonstration that circulating TSH in patients with TSH-oma may possess an enhanced bioactivity was made in one patient with a mixed GH-TSH–secreting pituitary adenoma in whom a ratio between biologic and immunologic activities of TSH significantly higher than that of controls was documented.24 Other studies indicate that the circulating TSH biologic/immunologic activity ratio may be normal, reduced, or increased in patients with TSH-oma,22,79,80 probably because of altered glycosylation of circulating TSH molecules. In fact, both intrapituitary and circulating TSH exist as multiple isoforms characterized by heterogeneity of oligosaccharide chains, which has a great impact on hormone biologic properties, such as biologic activity and metabolic clearance rate. Tumoral transformation may be accompanied by variable alterations in posttranslational processing within thyrotrophs, leading to the secretion of TSH molecules with peculiar glycosylation and biologic properties.79,81,82
GLYCOPROTEIN HORMONE α SUBUNIT
TSH-omas commonly secrete excessive quantities of the free α subunit, resulting in high levels of circulating free α subunit in two thirds of patients (see Table 17-3). This is another expression of the altered synthetic process within tumoral thyrotropes and represents a helpful diagnostic clue to the presence of a TSH-oma. Secretion of the α subunit in these tumors is in excess not only of the TSH-β subunit, but also of the intact TSH molecule. This generally results in a molar ratio of α subunit to TSH that is higher than 1. Although previous studies have suggested that a ratio greater than 1.0 is indicative of the presence of TSH-producing adenoma,83 similar values have been observed in normal controls, particularly in postmenopausal women, indicating the need for appropriate control groups matched for TSH and gonadotropin levels.4,61,84 It is interesting to note that microadenomas that frequently have α subunit levels within the normal range may show a high α subunit/TSH molar ratio. Furthermore, it has been suggested that extremely high levels of free α subunit might portend future malignant behavior, and that a spontaneous and marked decrease in both TSH and α subunit might indicate that the tumor is becoming less differentiated and might correlate with invasive and metastatic behavior.18
PARAMETERS EVALUATING PERIPHERAL THYROID HORMONE ACTION
Measurements of several parameters of peripheral thyroid hormone action both in vivo (basal metabolic rate, cardiac systolic time intervals, Achilles’ reflex time) and in vitro (sex hormone–binding globulin [SHBG], cholesterol, angiotensin-converting enzyme, osteocalcin, blood red cell sodium content, carboxyterminal cross-linked telopeptide of type I collagen [ICTP], and so on)85 may help in quantifying the degree of peripheral hyperthyroidism, particularly in patients with mild clinical signs and symptoms34,57,61,86–89 (see Table 17-3). In particular, evaluations of SHBG and ICTP may help to differentiate hyperthyroid patients with TSH-oma, in whom these parameters are elevated, from those with resistance to thyroid hormone, in whom they are in the range of those of euthyroid subjects.
Dynamic Testing
Several stimulatory and inhibitory tests have been employed to evaluate TSH secretory dynamics in patients with TSH-oma. None of these tests is of clear-cut diagnostic value, and the combination of some of them may enhance their accuracy in disclosing the pituitary adenoma. Among the stimulatory tests, TRH-induced TSH secretion is absent or blunted in 83% of patients (see Table 17-3). Although the α subunit response to the preceding stimulatory agents usually has paralleled that of TSH, discrepancy between α subunit and TSH response to TRH has been recorded in some cases. Such a discrepancy may be due to the presence of mixed adenomas, composed of distinct cell types that possess different receptor expression.24,28 Most TSH-omas are unable to increase TSH secretion after administration of dopamine antagonists such as domperidone or sulpiride. Long-term treatment with antithyroid drugs induces an increase in serum TSH levels in most patients because of both the high sensitivity of adenomatous cells to the reduction of circulating levels of FT4 and FT357 and recovered TSH secretion by normal thyrotrophs surrounding the adenoma in response to the activated feedback mechanism.4,90 In keeping with this are the observations of significantly higher TSH levels in patients who have undergone thyroid ablation, as well as the more active proliferation of tumoral cells in treated patients.
Among inhibitory tests, complete inhibition of both basal and TRH-stimulated TSH secretion after a T3 suppression test (Werner test: 80 to 100 µg/day of LT3 for 8 to 10 days) has never been recorded in a patient with a TSH-oma (see Table 17-3), although a slight TSH reduction may occur in a minority of patients. In patients who have undergone previous thyroid ablation, this test is the most sensitive and specific in documenting the possible presence of a TSH-oma. However, high doses of LT3 are contraindicated in elderly patients and in those with coronary heart disease. Dopamine (1 to 4 µg/kg body weight/min intravenously) or dopamine agonists, such as bromocriptine (2.5 mg orally), generally are ineffective in inhibiting TSH secretion, whereas native somatostatin or its analogues reduce TSH levels in most cases and may be predictive of the efficacy of long-term treatment in the majority of patients.61,91,92 We have demonstrated recently that long-term administration of long-acting somatostatin analogues in patients with central hyperthyroidism caused a marked decrease in both FT4 and FT3 circulating levels in all patients but one with TSH-omas, whereas patients with thyroid hormone resistance did not respond at all. Thus, administration of these analogues for at least 2 to 3 months can be useful in distinguishing the two forms of central hyperthyroidism.93
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree