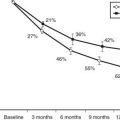
FIGURE 89-1. Histologic pattern of various benign tumors of the thyroid. A, Embryonal adenoma. B, Fetal adenoma. Note the sharp margin, capsule, and tiny follicles. C, Follicular adenoma. D, Hyperplastic variant of follicular adenoma. E, Colloid-filled variant.
(Courtesy Dr. Francis Straus, Department of Pathology, University of Chicago.)
The first distinction to be made among benign nodules is between functioning (“hot” on thyroid scan) and nonfunctioning (“cold”) nodules. Whereas a “hot” nodule is almost synonymous with a benign nodule, a “cold” nodule is not synonymous with a malignant nodule, because only a minority will turn out to be thyroid carcinomas. Cold nodules can be solid or cystic (around 10% to 20% of the total). However, mixed, solid-cystic forms are also common and should be considered solid in terms of frequency of malignancy. As a general rule, thyroid carcinoma is more common among solid, single, cold nodules.
Thyroid adenomas are usually monoclonal “new growths”17 that are formed in response to the same sort of stimuli that produce carcinomas. Heredity does not appear to play a major role in their appearance. One clue to their origin is that they are four times more common in women than in men, although no definitive relationship of estrogen to cell growth has been demonstrated. Thyroid radiation, chronic thyroid-stimulating hormone (TSH) stimulation, and oncogenes (see following) are believed to be related to the origin of these lesions; these are discussed in the following section on thyroid cancer.18 Of specific interest in relation to benign nodules is the observation by Parma and colleagues that activating mutations of the TSH receptor are the specific cause of most hyperfunctioning adenomas19 and the common involvement of ras gene mutations found in follicular adenomas.19 In view of the frequent discovery of ras gene mutation in follicular (and also papillary) thyroid carcinomas, the question is whether a ras mutated follicular adenoma should be considered as a pre-neoplastic lesion that should always be treated surgically.
Non-Neoplastic Nodules
These lesions are not true nodules but represent focal areas of glandular hyperplasia that arise spontaneously or, more commonly, as a consequence of previous partial thyroidectomies. Also, thyroid hemiagenesis may rarely be manifested as hyperplasia of the existing lobe and mimic a thyroid nodule. Nodule(s) associated with Hashimoto’s thyroiditis are the expression of lymphocytic infiltration and fibrosis. Nodules seen during the initial phase of subacute thyroiditis are a result of the inflammatory process that gives origin to typical granulomas.
Micronodules
Micronodules are nodules 1 cm or less in diameter. With the routine use of neck ultrasound, the discovery of micronodules is increasing, and nearly 50% of women older than 60 years have such nodularities. One view is that micronodules in general are not clinically relevant and, in the absence of other clinically suspicious findings, do not require investigation or treatment. The usual advice is to repeat thyroid ultrasound at regular intervals and reconsider therapy if growth is seen. However, in light of evidence that the frequency of malignancy is the same in micronodules or macronodules,12 an alternative view is that the former should be evaluated by FNAC under ultrasound guidance.
COURSE AND SYMPTOMS
Adenomas grow slowly, remain dormant for years, must reach a size of 0.5 to 1 cm before they can be palpated, and are typically asymptomatic. Thus, they often are discovered accidentally by the patient or the physician, and they rarely produce local symptoms.
About 70% of thyroid nodules or adenomas are hypofunctional in terms of accumulation of radioactive iodide and are “cold” on isotope scans. About 20% may be borderline in function and on isotope scan appear to have uptake similar to that of the remainder of the thyroid. One in 10 (or less) is hyperfunctional; these nodules concentrate iodide avidly, may suppress function of the normal gland, and may even produce thyrotoxicosis. This process, which typically occurs when the functioning nodule has grown large enough in diameter, is seen most often in older patients. Activating TSH receptor mutations have been found by Parma and coworkers to be the cause of most hyperfunctional adenomas19 and are common in “hot” nodules in patients with multinodular goiter.20,21 These mutations involve the extracellular loops of the transmembrane domain and the transmembrane segments, and in transfection studies, they have been proved to induce constitutive activation of the TSH receptor. Mutations of the stimulatory guanosine triphosphate–binding protein subunit are also present in some patients with hyperfunctioning thyroid adenomas.22 Hot nodules are almost invariably associated with low or suppressed serum TSH levels. The finding of subnormal serum TSH at the first patient evaluation is the hallmark of hyperfunctioning nodules and is the test of choice that should dictate the need for thyroid scanning. When serum TSH is normal, there is no need to perform routine thyroid scan.
Usually, a benign nodule, once formed, seems to be committed to this “lifestyle” indefinitely. However, pathologic evidence suggests that true follicular adenomas (or Hürthle cell adenomas) can transform into invasive carcinoma. Sequential change from hyperplasia to adenoma formation to invasive carcinoma has been found in patients with congenital goitrous hypothyroidism, and it can be produced experimentally in animals.
Interesting studies have described the metabolic function of nodules. Cold nodules are typically unable to transport iodide into the thyroid as a result of a specific deletion of some element of the transport mechanism.23 They are not able to maintain a concentration gradient for iodide between the thyroid cell and serum, although peroxidase function in the tissue may be intact.24,25 In such adenomas, thyrotropin is able to bind to the cell membrane and activate adenyl cyclase, but subsequent metabolic steps are lacking. Other “cold nodules” have been shown to lack peroxidase enzyme.26 These studies suggest that adenoma formation is associated with genetic mutational events that cause loss or dysfunction of specific enzymes in the iodide metabolic pathway. Recent cloning of the Na+/I− symporter (NIS) gene,27 the gene responsible for iodine uptake by the follicular cell, has confirmed this hypothesis, demonstrating that cold nodules in multinodular goiters and follicular adenomas have reduced NIS messenger RNA (mRNA) and protein expression, thus opening a new field of research for the development of novel therapeutic strategies based on gene manipulation.
CLINICAL EVALUATION AND MANAGEMENT OF NODULES
The aim of clinical evaluation is to detect nodules that should be referred to a surgeon. Among benign lesions, the aim is to differentiate between adenomas (functioning or nonfunctioning), cysts, and nodules in the context of an underlying benign thyroid disease. This differential diagnosis is extremely important decisions regarding the most appropriate therapy. Similarly important in benign lesions is the detection of clinical symptoms or signs (e.g., compression of the trachea or the esophagus, a recurrent nerve deficit) that per se could suggest a need for surgical therapy. The clinical and laboratory features associated with a high risk for cancer are listed in Table 89-3.
Table 89-3. Clinical Features Suggesting Malignancy of a Thyroid Nodule
Suspicious finding at thyroid ultrasounds (microcalcifications, hypoechogenicity, irregular margins or no halo, solid intranodule vascularity, more tall than wide) |
Factors that must be considered when a decision on management is reached include history of the lesion; age, sex, and family history of the patient; physical characteristics of the gland; the presence of local symptoms; laboratory evaluation results; and ultrasonographic features.
Personal History
The age of the patient is an important consideration because the ratio of malignant to benign nodules is higher in youth and lower in older age. In a study involving nonirradiated children with cold thyroid nodules, a twofold increased risk for thyroid cancer, regardless of sex,28 was found when compared with the rate for similar adults.2 In adult men, nodules are less common, and a greater proportion are malignant.
Rarely, the family history may be helpful in decision making regarding surgery. Patients with the heritable multiple endocrine neoplasia (MEN) syndrome, type 1, may have thyroid adenomas, parathyroid adenomas, islet cell tumors, and adrenal tumors, whereas patients with MEN type 2 may have pheochromocytomas, medullary thyroid carcinomas, hyperparathyroidism, and mucosal neuromas.29–31 Familial medullary cancer (without MEN) is also possible. Furthermore, we have noted that 6% to 12% of patients with differentiated thyroid carcinoma have one or (less frequently) more family members with a history of malignant (nonmedullary) thyroid neoplasm, most often papillary.32 Familial papillary thyroid tumors occur in Cowden’s disease, Gardner’s syndrome, and familial polyposis coli33 but most of the time are not associated with other manifestations (nonsyndromic). Recently, it has been reported that isolated familial papillary thyroid cancer displays the feature of “genetic anticipation,” that is, the tendency for a familial cancer to present at an earlier age and with a more aggressive phenotype in subsequent generations compared with the first generation.32 As is discussed in the following sections, a history of prior irradiation to the head or neck during infancy or childhood is strongly associated with the subsequent occurrence of carcinoma.34 A history of such radiation exposure and the presence of a palpable nodule or nodules must raise the possibility of thyroid cancer, which requires a cytologic diagnosis.
The epidemic of childhood papillary thyroid cancer observed in Belarus and Ukraine after the Chernobyl nuclear accident35 is believed to be the result of contamination from radioactive fallout, mainly iodine isotopes, which were released in huge amounts into the atmosphere. Several studies have reported no increase in the risk for thyroid cancer after diagnostic or therapeutic exposure to 131I. However, the possibility that many naturally occurring thyroid carcinomas may be caused by fallout radiation after nuclear tests, other radiation sources, or natural background radiation must be seriously considered.
The history of the neck lump itself is important. Recent onset, growth, hoarseness, pain, regional nodes, symptoms of brachial plexus irritation, and local tenderness all suggest malignancy but of course do not prove it. The usual cause of sudden swelling and tenderness in a nodule is hemorrhage into a benign lesion. Although the presence of a nodule for many years suggests a benign process, some cancers grow slowly. In our series, the average time from recognition of a nodule to the diagnosis of cancer was 3 years.
Coexisting benign thyroid disease is important in the evaluation of cancer risk associated with a thyroid nodule. A history of residence in an endemic goiter zone during the first decades of life is relevant and must raise the possibility of multinodular goiter as the true diagnosis. Hashimoto’s thyroiditis is often associated with discrete nodules, which are an expression of the autoimmune process. The frequency of thyroid carcinoma is not increased in patients with Hashimoto’s thyroiditis; however, Hashimoto’s thyroiditis is a common preexisting condition in patients in whom thyroid lymphoma develops.36 Higher risk for differentiated thyroid cancer has been noted in patients with Graves’ disease and cold thyroid nodules,36–39 and increased aggressiveness of such Graves’ disease–associated thyroid cancer has been proposed.40 In the experience of the authors and of other groups,41,42 the response to traditional therapy and the final outcome of patients with thyroid cancer and Graves’ disease are not different with respect to thyroid cancer patients without Graves’ disease.
Physical Examination Findings
In the era of thyroid ultrasound, evaluation of the thyroid gland has been enormously facilitated; however, accurate palpation of the thyroid gland and the cervical node chains is still of paramount importance in the evaluation of thyroid nodular pathology. It gives an idea of the number and size of the nodule(s), their consistency and motility, and the status of the rest of the thyroid gland, as well as the presence and the importance of lymph node involvement.
The adenoma typically is felt as a discrete lump in an otherwise normal gland, and it moves with the thyroid. Enlarged lymph nodes should be carefully sought, particularly in the area above the isthmus, in the cervical chains, and in the supraclavicular areas. Their presence suggests malignant disease unless a good alternative diagnosis (e.g., recent oropharyngeal sepsis, viral infection) is apparent. Fixation of the nodule to strap muscles or to the trachea is alarming. Characteristically, a benign thyroid adenoma is part of the thyroid and moves with deglutition, but it can be moved in relation to the strap muscles and within the gland substance to some extent. Pain, tenderness, or sudden swelling of the nodule usually indicates hemorrhage into the nodule but can also indicate invasive malignancy. Hoarseness may arise from pressure or from infiltration of a recurrent laryngeal nerve by a neoplasm. Obviously, the presence of a firm, fixed lesion associated with pain, hoarseness, or any one of these features should signal some degree of alarm. It is worth noting that these signs are not specific for the diagnosis of malignancy. In a study that correlated suspicious clinical features with the histologic diagnosis, the authors reported benign disease in 29% of patients with palpable cervical adenopathy, in 50% of patients with hard nodules, in 29% of patients with apparent nodule fixation, and in 17% of patients with true vocal cord paralysis.43 The converse situation, the absence of such characteristics, suggests but does not prove benignity. Fluctuance within the lesion suggests the presence of a cyst that is usually benign.
The presence of a diffusely multinodular gland, ascertained on the basis of palpation, ultrasound, or scanning, generally is interpreted as a sign of safety. Multinodular goiters coming to surgery have a significant prevalence of carcinoma (4% to 17%), but this finding is believed to be caused largely by selection of patients for surgery, and is not believed to be typical of multinodular goiter in the general population.44 If one area within a multinodular goiter seems different from the remainder of the gland on the basis of palpation or function, or has demonstrated rapid growth, or if two discrete nodules are found in a gland that is otherwise normal, one should consider malignant change rather than a benign multinodular goiter.
Occasionally, in addition to a nodule, the gland exhibits the diffuse enlargement and firm consistency of chronic thyroiditis, a palpable pyramidal lobe, and antibody test results that may be positive. These findings strongly suggest thyroiditis but do not disclose the nature of the nodule, which must be evaluated independently. It should be remembered that 14% to 20%45,46 of thyroid cancer specimens contain diffuse or focal thyroiditis.
Thyroid Function Tests
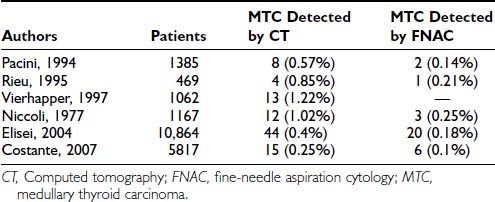
Unless a toxic adenoma is present, the patient is usually euthyroid, and this impression is supported by normal values for serum-free thyroxine (FT4), free triiodothyronine (FT3), and TSH. Low thyroid hormones or elevated TSH results should raise the question of thyroiditis. Several centers advocate measurement of serum antithyroid autoantibodies (antithyroglobulin [anti-Tg] and antithyroperoxidase [anti-TPO]) in every new patient in search of an underlying autoimmune thyroid disease. The serum Tg concentration may be elevated, as in all other goitrous conditions. Its increase is related mainly to the size of the nodule, rather than to its nature and to the size of the thyroid gland.47 Thus, serum Tg measurement is not a valuable tool in the differential diagnosis. On the contrary, elevation of circulating calcitonin levels in a patient with a thyroid nodule is almost always diagnostic of medullary thyroid cancer. Several prospective studies have shown that routine measurement of circulating calcitonin in thyroid nodules allows the preoperative diagnosis of medullary thyroid carcinoma with better accuracy than is seen with FNAC48–51 (Table 89-4). A large retrospective study involving more than 10,000 patients seen at a single institution has recently confirmed that on calcitonin screening, the incidence of medullary thyroid carcinoma (MTC) is 1 in 250 unselected thyroid nodules—higher than was previously believed. In addition, the study demonstrated that such screening offers the possibility of finding MTCs before they have metastasized, thus increasing the chance for definitive cure. Comparison with an historical group of patients with MTC diagnosed before the screening showed a significantly better long-term outcome for MTC detected by calcitonin screening.52 According to these authors, measurement of serum calcitonin should be included in the diagnostic evaluation of thyroid nodules at the first visit of the patient; this has been incorporated into the European Thyroid Association guidelines,53 but not the American Thyroid Association guidelines.54a Cost-effectiveness, one of the major issues in screening for thyroid nodules, has been calculated recently, and the result favored calcitonin screening.55 Calcitonin assay is, of course, mandatory in the presence of a suggestive family history or coincident features of the MEN2 syndromes.
Table 89-4. Medullary Thyroid Cancer Diagnosed by Routine Measurement of Serum Calcitonin and by Fine-Needle Aspiration Cytology in Nodular Thyroid Diseases
Routine measurement of serum calcium is advocated by several centers. The aim is not directly related to the diagnosis of thyroid nodules, but rather to detection of undiagnosed parathyroid adenomas.
Imaging
Thyroid Ultrasound
Thyroid ultrasound is becoming more and more popular in the first-line evaluation of thyroid nodules. Good technique demonstrates nodules, if they are larger than 3 mm; indicates cystic areas; and may reveal a capsule around the nodule and the size of the lobes. It often displays multiple nodules when only one is noted clinically, and it allows the discovery of suspicious lymph nodes in the neck. This technique is more sensitive than thyroid scan, is noninvasive, involves less time, allows serial examination, and usually is less expensive. From 3% to 20% of lesions are found to be totally or partially cystic. Purely cystic lesions are reported to have a lower incidence of malignancy than do solid tumors (3% vs. 10%), and diagnosis of a cyst raises the possibility of aspiration therapy.56 Some specific features (e.g., hypoechoic, solid, irregular halo, microcalcifications, shape) are indicative, although not diagnostic, of malignant nodules. The study of blood flow by Doppler ultrasonography may provide indicative information, and, very recently, a new technique called “elastographic ultrasound” was applied to the differential diagnosis with great specificity and sensitivity.57,58
Isotope Scans and Other Imaging Techniques
Isotope scintiscans provide only functional information regarding activity of the nodules; their use has been much reduced since neck ultrasound was introduced,59,60 because the same functional information is revealed by measurement of serum TSH levels. In cases of multinodular goiter, scintiscan is still useful in distinguishing the nodules to be submitted to FNAC (the cold one). Nodules that are hyperfunctional and that produce hyperthyroidism are rarely malignant, and those that accumulate iodide in concentrations equal to the surrounding normal thyroid tissue are usually but not always benign61,62 (Fig. 89-2). Cold nodules are typically benign, but when viewed the other way, most thyroid cancers are seen as inactive areas on thyroid scan. In practice, except for the specific case of a toxic nodule, scans are probably of little help in the differential diagnosis, and the tendency to omit scanning from diagnostic maneuvers is growing. Scintiscans can confirm the diagnosis of multinodular goiter and can show the presence of diffuse disease (e.g., Hashimoto’s thyroiditis) in some patients when nodularity is suspected.
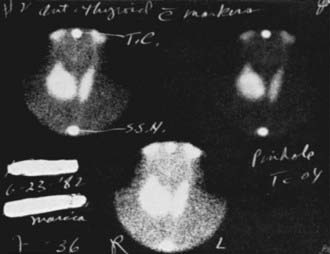
FIGURE 89-2. Scintillation scan view of a functioning nodule in a 36-year-old woman. At surgery, the nodule proved to be a mixed papillary-follicular neoplasm.
Other scanning techniques have not found a place in routine preoperative evaluation (Fig. 89-3). Computed tomography (CT) is expensive but occasionally useful, especially in unusually large substernal glands. Magnetic resonance imaging (MRI) is rarely necessary but is useful for identifying abnormal nodes. Fluorine-18-fluoro-deoxyglucose (FDG)–positron emission tomography (PET) is generating great interest in general oncology but has no particular role in the diagnosis of thyroid nodules, even if sporadic reports of positive uptake in malignant nodules have been published.63,64
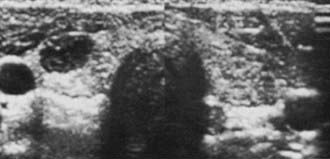
FIGURE 89-3. Sagittal echo scan of a palpable 2 cm single nodule showing a lesion in the R lobe. On aspiration, 2 mL of brownish colloid was obtained. The few cells present showed Hürthle cell changes.
Chest radiograph and soft tissue x-ray films of the neck are useful for ascertaining the presence of compression signs (e.g., indentation or deviation of the trachea), which are particularly common when the tumor is larger than 3 or 4 cm in diameter. Fine, stippled calcifications through the tumor (psammoma bodies) are virtually pathognomonic of papillary cancer. Patchy or “signet ring” calcification occurs in old cysts and degenerating adenomas and has no such connotation. The presence of such signs is very well detected by thyroid ultrasound.
Fine-Needle Aspiration Cytology
Although all the above mentioned procedures may provide some indication, only the results of FNAC can give a definitive answer regarding the nature of a thyroid nodule. FNAC has now been widely adopted after initial favorable reports by Walfish and colleagues65 and Gershengorn and colleagues.66 It has replaced the core needle biopsy previously used to provide a histologic diagnosis.67 In expert hands, adequate specimens can be obtained in more than 90% of patients, with a diagnostic sensitivity and specificity near or superior to 95%. Willems and Lowhagen, in reviewing a collected series of nearly 4000 surgically proven fine-needle aspiration (FNA) studies, found that 11.8% were considered malignant lesions.68 False-negative diagnoses of cancer were made in 6.6% to 27.5%, and false-positive diagnoses in only 0% to 2%. Currently, the results of FNAC are viewed as the “gold standard” for diagnosis in most cases, and they play a crucial role in the selection of patients for surgery,69–71 Gharib and coworkers analyzed data on 10,000 FNA procedures and found it to be the preferred first step in diagnosis.72 Diagnostic accuracy was nearly 98%, with fewer than 2% false positives and false negatives. Miller and colleagues compared FNA, large-needle aspiration, and cutting needle biopsy.73 They found that FNAC examination was able to detect almost all carcinomas, but they believe that cutting needle biopsy is a useful additional procedure, especially with larger (more than 2 to 3 cm) nodules.
FNAC should always be performed with ultrasound guidance, especially in smaller (or less discrete) or partially cystic nodules. It is demonstrated that, with respect to pre-FNAC years, various centers using the results of FNAC for therapeutic decisions have observed a 35% to 75% reduction in the number of patients sent to surgery; a twofold to threefold increase in the percentage of cancers found at surgery; and a variable, but constant, reduction in the cost of thyroid nodule management.
FNAC is performed with a 22 to 25 gauge needle. Specimen adequacy requires a minimum of two slides (from separate aspirates) showing at least six to eight cell clusters.74 The method is simple, inexpensive, and very well tolerated and, if necessary, may be repeated several times. Complications are very rare and consist mainly of hematomas. In several large series, it has been found that around 70% (range, 53% to 90%) of aspirates are classified as benign; 4.0% (1% to 10%) as malignant or suspicious for malignancy; 10% (5% to 23%) as indeterminate, mainly represented by “follicular neoplasia”; and 17% (15% to 20%) as inadequate for diagnosis.75–78 When the sample obtained is of good quality (i.e., high cellularity), the cytologic diagnosis of thyroid carcinoma, especially in the case of a papillary histotype, is highly reliable, and false-negative or false-positive results are very rare. Medullary thyroid carcinoma is diagnosed easily by cytology in classic cases, but sometimes the cellular pattern is atypical and can be interpreted as follicular and even papillary proliferation. Problems may arise in the case of thyroid lymphoma, because the smear may be composed of follicular cells mixed with lymphocytes, which can mimic chronic lymphocytic thyroiditis or may be confused with anaplastic carcinoma. Cytology of cystic nodules shows the presence of colloid, necrotic material, macrophages, and rare epithelial cells. In most cases, these lesions are benign, but the possibility of cystadenocarcinoma must be considered. The cytologic diagnosis of follicular or Hürthle cell neoplasia is particularly challanging. A variety of techniques have been applied to improve the accuracy of interpretation of FNA cytology in this setting, including staining with antibodies to thyroid peroxidase (TPO) and the search for MUC1 gene expression and telomerase activity, as well as galectin-3 expression.79–85 However, none of these potential markers has been entered into clinical practice because of conflicting results or low sensitivity.
The major limitation of FNAC is the inadequacy of specimens, even after repeat attempts. The rate of inadequacy is variable among different centers, with a realistic estimation of between 15% and 25%.75–76 Inadequacy raises the question of therapy for the nodule with nondiagnostic FNAC. Some authors recommend surgical treatment for all these nodules, whereas others select for surgery only those suspicious by other clinical or laboratory features. Even if only the most suspicious nodules with inadequate FNAC are selected for surgery, the yield of malignancy at histology is relatively low and ranges from 8% to 19%.74,86,87 In any case, patients should be carefully monitored by repeat FNAC and referred to a surgeon in the event that a nodule increases in size.
An additional indication for FNAC is the diagnostic evaluation of cervical nodes, both at initial evaluation and when a diagnosis of thyroid cancer has already been established. In the case of lymph nodes suspected of being of thyroid metastatic origin, FNAC may be integrated with the measurement of Tg in the liquid recovered when the needle used for the aspiration is washed. As is shown in Fig. 89-4, in the case of a metastatic lesion from differentiated thyroid carcinoma, this technique demonstrates the presence of high levels of Tg.88,89
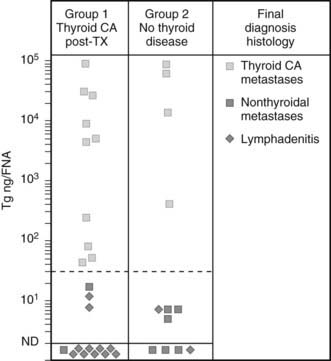
FIGURE 89-4. Concentration of thyroglobulin (Tg) in fine-needle aspirates of neck masses from patients with (group 1) or without (group 2) known thyroid cancer, according to the final diagnosis at histology.
(From Pacini F, Fugazzola L, Lippi F, et al: Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic thyroid cancer, J Clin Endocrinol Metab 74:1401,
In conclusion, FNAC should be performed on any thyroid nodule. In the case of multinodular goiter, FNAC should be performed on as many nodules as possible. The largest nodule is not necessarily the one associated with malignancy; thus, FNA should be performed under the guidance of sonographic features rather than on the basis of size.12 In dubious cases, FNAC may be repeated immediately or over the years, if the final decision is to not operate on the patient. It is worth mentioning that the preoperative diagnosis of thyroid carcinoma is useful not only for selecting patients to be operated on, but also for planning in advance the most appropriate surgical procedure.
Diagnostic Protocol
A possible practical diagnostic approach to patients with thyroid nodules is schematically represented in Fig. 89-5.
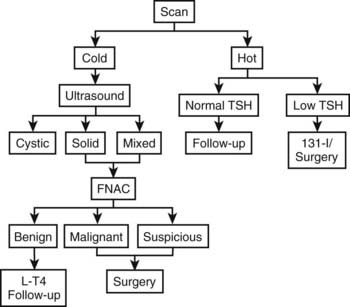
FIGURE 89-5. Flow chart for the diagnostic evaluation of thyroid nodules. FNAC, Fine-needle aspiration cytology; L-T4, levothyroxine; TSH, thyroid-stimulating hormone. Currently, isotope scanning is omitted unless there is evidence for excess thyroid hormone production.
Serum thyroid hormone and TSH measurement and thyroid ultrasound are performed as first-line exploration. Determination of antithyroid antibodies is calcitonin are also helpful.
If TSH is suppressed or low, a thyroid scan is performed to confirm the presence of an autonomously functioning nodule; the subsequent approach will depend on the presence of clinical or subclinical thyrotoxicosis and on the size of the nodule. If the nodule is cold and cystic, FNAC will be performed both as a therapeutic technique (evacuation) and as a diagnostic tool to detect the small percentage of cystic adenocarcinomas. If the nodule is cold and totally or partially solid, the therapeutic decision will depend on the results of FNAC.
THERAPY
A complete diagnostic evaluation, as previously outlined, is a prerequisite to determining the appropriate choice of treatment for thyroid nodules. The problem involves whether the nodule requires any therapy, and, if so, whether it is manageable by medical or surgical therapy.
Surgical Therapy
We favor selecting the malignant nodules for surgery, and we suggest medical therapy or follow-up for the others. However, surgical treatment should also be suggested for some benign lesions, either single or associated with multinodular goiter, when they are large enough to produce symptoms and signs of discomfort or aesthetic concern. Another surgical indication is for questionable nodules, including those characterized by follicular proliferation on FNAC.
If surgery is selected, we believe that it is crucial to work in conjunction with a surgeon who has frequent and continuous experience in thyroid surgery to obtain good results. This is not to say that resection of a thyroid lobe for a nodule is a difficult procedure; however, if more extensive surgery is required, and especially if total or near-total thyroidectomy and lymph node resection are indicated, it is imperative that the surgeon have the proper knowledge and experience to reduce the possibility of damage to recurrent laryngeal nerves and parathyroid glands.
Surgery for Nodules With FNAC Indicative of Malignancy
When the malignant nature of a nodule has been established by FNAC, the recommended surgical procedure should be total (or near-total) thyroidectomy regardless of the size of the nodule, without the need for frozen section examination, which has a rate of false-negative results in excess of false-positive results for FNAC (near zero). This procedure decreases the risk for local recurrence and is performed with almost no morbidity under expert hands. Moreover, it facilitates postsurgical radioiodine ablation and adequate follow-up.90–92 Surgery should be preceded by careful staging of the disease in the neck. This is accomplished by thyroid and neck ultrasound. Any suspicious lymph node must be submitted to confirmatory FNAC to alert the surgeon of the presence of metastatic disease. If positive nodes are seen, the surgeon should perform the most appropriate dissection of lymph node chains (central compartment, homolateral modified neck dissection, or bilateral). The need for routine dissection of the central node compartment in the absence of suspicious ultrasonographic findings is debated. Recent European and American guidelines53,54a suggest that the central neck should be explored and all nodes removed by the surgeon in cases of papillary and Hürthle cell thyroid cancer, but not follicular thyroid cancer. However, the benefits of prophylactic “en bloc” central node dissection in the absence of preoperative or intraoperative evidence of nodal disease are controversial. No evidence suggests that it improves recurrence or mortality rates, but it does permit accurate staging of the disease that may guide subsequent treatment and follow-up.
This approach, which emphasizes more extensive nodal surgery and less use of 131I, represents an interesting return to concepts in vogue and discarded five decades ago. Bonnet et al.54b reported a series of 115 patients with papillary tumors of 1 to 2 cm and no preoperatively recognized cervical nodes, whose surgery arbitrarily included en bloc dissection of midcervical (level VI) nodes and lateral (level III and IV) nodes, and sometimes level II and V nodes. Patients probably had more advanced tumors than are seen in average cases, in that 29% were invasive and 37% multifocal. This extensive nodal surgery changed therapy in 11%, in whom no nodes were found, and who therefore received no 131I. Complications included hypoparathyroidism and vocal cord paralysis (0.9% each). Certainly this “prophylactic” node dissection may preclude discovery of nodes at a later date, thus preventing a small number of patients from being exposed to 30 mCi of 131I. Whether this is a fair trade for the certain increase in side effects that would follow application of the approach in general practice remains very uncertain, and this approach cannot be recommended at present.
Surgery for Nodules With Indeterminate or Suspicious FNAC
Among solitary thyroid nodules with an indeterminate (“suspicious,” “follicular neoplasm,” or Hürthle cell neoplasm) biopsy, the risk for malignancy is approximately 20%.93–95 For solitary nodules that are repeatedly nondiagnostic on biopsy, the risk for malignancy is unknown but is probably closer to 5% to 10%.96 In these cases, the surgical procedure should be discussed with the patient. For those who prefer a more limited surgical procedure, thyroid lobectomy associated with frozen section examination is the recommended initial surgical approach.
Because of increased risk for malignancy, total thyroidectomy is indicated in patients with large tumors (>4 cm) when marked atypia is seen on biopsy, when the biopsy reading is “suspicious for papillary carcinoma,” in patients with a family history of thyroid carcinoma, in patients with a history of radiation exposure,97–99 in patients with bilateral nodular disease, and in those who prefer to undergo bilateral thyroidectomy to avoid the possibility of requiring a future surgery.
Surgery for Differentiated Thyroid Cancer Detected at Final Histology Without Total Thyroidectomy Performed
If a patient is referred after less than near-total thyroidectomy, completion thyroidectomy should be proposed in the case of a large tumor, multifocality, extrathyroidal extension, and/or vascular invasion or evidence of local or distant metastases, previous history of radiation exposure, or unfavorable histology.96,101 In cases of primary tumors between 10 and 20 mm in diameter that have been diagnosed at postoperative definitive histopathology, the indication for completion thyroidectomy should be discussed with the patient on the basis of the risks and benefits of reoperative surgery, including the potential risk for surgical morbidity. Depending on the size of the thyroid remnant, an effective alternative to completion thyroidectomy when the risk for persistent disease is low may be radioiodine ablation of residual thyroid tissue.102
Whenever surgery is performed for nodules with no suspicion of malignancy, the usual procedure is lobectomy, which is relatively harmless and has an incidence of complications approaching zero. Usually, patients are discharged within 2 to 3 days. Complications are more common when more extensive dissection is done, as will be discussed subsequently. The thyroid specimen itself, any abnormal areas in the gland, and any abnormal appearing lymph nodes should be examined immediately by frozen section. Differentiating benign from malignant thyroid lesions is admittedly difficult, especially with frozen sections, but experienced pathologists can make the distinction with a high degree of reliability. Occasionally, follicular lesions are believed by the pathologist to be benign at surgery, but permanent sections reveal changes that indicate malignancy. Reoperation with near-total thyroidectomy is probably desirable in these patients, because up to one third can be expected to have residual tumor in the contralateral lobe.103 To avoid these second operations, we recommend lobectomy and contralateral subtotal resection for very cellular follicular lesions as the initial procedure. Occasionally, a small papillary or follicular cancer is found in the pathologic specimen after the operation has concluded. If this cancer is less than 1 cm and has a well-demarcated single focus, and the patient is younger than 45 years, nothing further need be done therapeutically, but follow-up by periodic thyroid ultrasound is recommended. After surgery, all patients are maintained on replacement levothyroxine therapy in the hope of preventing recurrence of other nodules. Serum TSH should be maintained in the low-normal range.
Medical Therapy
Benign Solid Cold Nodule
Appropriate management of these nodules is strongly debated. A meta-analysis104 has indicated that about 25% respond to thyroxine treatment with a decrease in size, whereas the remainder remain unchanged, at least over several months. Some physicians believe that once malignancy has been ruled out, medical therapy is indicated for solid cold nodules with normal or subnormal thyroid function, especially when associated with thyroid enlargement. The drug of choice is levothyroxine. Some physicians advocate a dose sufficient to suppress pituitary TSH secretion as demonstrated by a serum TSH level less than 0.1 µU/mL. The rationale for this therapy is the unequivocal observation that TSH is, to some extent, a growth factor not only for the normal thyroid but also for thyroid nodules. Experimental and clinical evidence has shown that even mild iodine deficiency elicits subminimal increases in TSH levels, which leads first to glandular hyperplasia and later to multinodular goiter. On the other hand, the functional heterogeneity and the variable degree of mitogenicity of follicular cells upon stimulation by TSH offer an explanation for the appearance of a nodule without diffuse goiter. When the nodule and/or the goiter is of recent origin, suppression of TSH stimulation by levothyroxine is often sufficient to eliminate the nodule, or at least to reduce its size and that of the thyroid gland. In long-standing cases, both the nodules and the goiter are seldom cured, but a significant reduction in size and arrest of the progression are likely to occur.
Once instituted, levothyroxine therapy must go on for years to be effective.106 Age is very important in the selection of patients to be treated. Treatment is indicated in young patients and in adults up to about 45 to 50 years of age. In older patients, the opportunity to initiate suppressive therapy must be considered on an individual basis after other underlying diseases such as heart problems are excluded. However, if a patient is already receiving levothyroxine treatment and shows good compliance and no side effects, treatment can be continued after age 50 years and even after age 60 years with the daily dosage slightly decreased.
An alternative approach is to aim for a TSH of 0.3 to 1 µU/mL, because this level will have some suppressive effect, perhaps will inhibit the growth of nodules over subsequent years, and is free of the minimal risk for mild thyrotoxicosis.
At the other end of the spectrum of opinion are physicians who believe that thyroxine therapy is useless and who simply offer continued observation without treatment.
Another aspect to be considered is functional thyroid status. Before instituting levothyroxine therapy, to avoid iatrogenic thyrotoxicosis, one must be certain that the patient is perfectly euthyroid, and that serum TSH, measured with an ultrasensitive assay, is not already suppressed, as so often can happen in multinodular goiters with areas of functional autonomy.
The last important aspect is the dose to be given. The usual suppressive dose is between 1.5 and 2 µg/kg/day, administered in the morning. The dose is checked after 3 to 4 months by measuring FT3, which should stay in the normal range, and TSH, which should be in the range selected, with an FT4 value usually in the upper limit of the normal range. If results show that TSH is not suppressed, or that the patient has been overtreated, an appropriate dosage modification will be made, with another hormonal control determined 3 to 4 months later and then yearly.
Once the few precautions described above are observed, levothyroxine treatment is generally useful and safe. Our own experience and data from the literature indicate that significant shrinkage is obtained in 15% to 50% of the nodules, and that many others do not progress. Side effects on the heart and bone, described by some authors, are not observed when careful avoidance of subclinical thyrotoxicosis is maintained.107
When clinical signs of hyperthyroidism suddenly develop during levothyroxine therapy, one must suspect the occurrence of functional autonomy of the nodule(s), and levothyroxine treatment must be withdrawn immediately. An indication for referring the patient to a surgeon is an increase in size of the nodule during levothyroxine therapy. Such a situation is not unusual and, although regarded as suspicious, does not constitute definite evidence of malignancy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree