THYROIDAL RADIOIODIDE UPTAKE
The iodide radioisotope usually is given orally in a capsule or in liquid form, and the quantity that is accumulated by the thyroid gland at various intervals is measured with a gamma scintillation counter. It is important to correct for the background activity of isotope circulating in the blood of the neck region (particularly during the early periods after administration). Background correction is achieved by subtracting counts obtained over the region of the thigh. A dose of the same radioisotope, usually 10%, placed in a neck “phantom” is also counted as a “standard.” The percentage of RAIU is calculated from the counts accumulated per constant time unit.
The percentage of RAIU 24 hours after the administration of radioiodide is most useful because in most instances, the thyroid gland has reached the plateau of isotope accumulation, and the best separation between high, normal, and low uptake is obtained at this time. Normal values for 24 hour RAIU in most parts of North America are 5% to 30%. In many other parts of the world, normal values range from 15% to 50%. Lower normal values are due to the increase in dietary iodine intake after the enrichment of foods, particularly mass-produced bread (150 µg of iodine per slice) containing this element. Over the past 3 decades, the mean ingestion of dietary iodine in the United States, although still within the recommended minimum for adults of 125 µg/day, has dramatically declined to approximately 240 to 300 µg/day for men and 190 to 210 µg/day for women.5 The inverse relationship between the daily dietary intake of iodine and the RAIU test is clearly illustrated in Fig. 77-1. Therefore, normal values of RAIU uptake will depend on the iodine content in a geographic region and also are related to age (with children having a higher iodine intake than adults). In Japan, the mean dietary iodine is six times higher than in the United States.
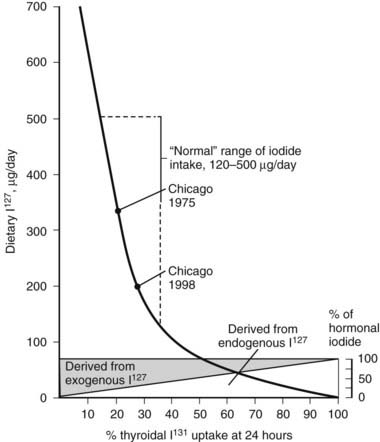
FIGURE 77-1. Relationship of 24-hour thyroidal radioiodide (131I) uptake (RAIU) to dietary content of stable iodine (127I). Uptake increases with decreasing dietary iodine. If iodine intake is below the amount provided from thyroid hormone degradation, the latter contributes a larger proportion of the total iodine taken up by the thyroid. With dietary habits in the United States, the average 24 hour thyroidal RAIU is below 20%.
(Data from DeGroot LJ, Reed Larsen P, Hennemann G, et al: The Thyroid and Its Diseases. New York, John Wiley & Sons, 1984.)
The intake of large amounts of iodide (>5 mg/day), mainly from the use of iodine-containing radiologic contrast media, antiseptics, vitamins, and drugs such as amiodarone, suppresses RAIU values to a level that is hardly detectable with the usual equipment and doses of isotope. Depending on the type of iodine preparation and the period of exposure, depression of RAIU can last for weeks, months, or even years. Even external application of iodide can suppress RAIU. It therefore is important to inquire about individual dietary habits and sources of excess iodide intake. Because dietary assessment of iodine ingestion can be somewhat inaccurate owing to the variable content of iodine added to various foods, measurement of iodine excretion is a more accurate assessment of the iodine balance. Spot urine iodine measurements were compiled from 1971 to 1974 and from 1988 to 1994 in the National Health and Nutrition Examination Surveys I and III, respectively, and have been found to be decreasing, in accordance with what was stated.6 Clinically, if one suspects that the patient had a large iodine load prior to an RAIU, a urine iodine measurement can be obtained. Urine iodine concentrations greater than 100 µg/day usually are associated with RAIU of 20% or less. Therefore, urine iodine can be useful in determining the feasibility of using RAIU.
RAIU is a measure of the avidity of the thyroid gland for iodide and its rate of clearance relative to the kidney, but results of this test do not equate with hormone production or release. Disease states resulting in excessive production of thyroid hormone most often are associated with increased thyroidal RAIU, and those causing hormone underproduction generally are associated with decreased thyroidal RAIU (Fig. 77-2). Some important exceptions to these rules include the high uptake values that are seen in certain hypothyroid patients and the low values noted in some hyperthyroid patients. Increased thyroidal RAIU with hormonal insufficiency can be caused by severe iodide deficiency and by most inborn errors of hormonogenesis (see Chapters 92 and 94). Lack of substrate in the former and specific enzymatic block of hormone synthesis in the latter cause hypothyroidism that is poorly compensated by TSH-induced thyroid gland overactivity. The increase in serum TSH, in response to the low circulating level of thyroid hormone, stimulates thyroidal iodine uptake by the NIS and hence increases RAIU. This can be a point of confusion for the clinician who is confronted with an increased RAIU in a patient who is suspected to have thyroiditis on the basis of blood tests. Alternatively, decreased thyroidal RAIU with hormonal excess typically is encountered in the syndrome of transient thyrotoxicosis (both deQuervain’s and painless thyroiditis) after the ingestion of exogenous hormone (thyrotoxicosis factitia), with iodide-induced thyrotoxicosis (Jod-Basedow disease), rarely in patients with metastatic functioning thyroid carcinoma or struma ovarii, and in patients with thyrotoxicosis who have a moderately high intake of iodide. High or low thyroidal RAIU as a result of low or high dietary iodine intake, respectively, might not be associated with significant changes in thyroid hormone secretion.
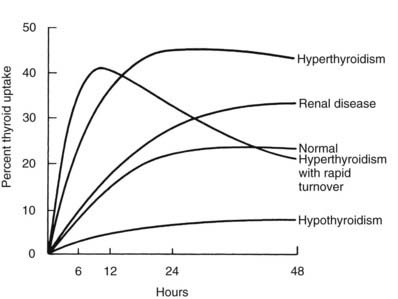
FIGURE 77-2. Examples of thyroidal radioiodide uptake curves under various pathologic conditions. Note the prolonged uptake in renal disease caused by decreased urinary excretion of the isotope and the early decline in thyroidal radioiodide content in some patients with thyrotoxicosis associated with a small but rapidly turning over intrathyroidal iodine pool.
(Data from DeGroot LJ, Reed Larsen P, Hennemann G, et al: The Thyroid and Its Diseases. New York, John Wiley & Sons, 1984.)
Various factors, including diseases that affect the value of the 24 hour thyroidal RAIU, are listed in Table 77-2. Several variations of the RAIU test have been devised that have particular value under special circumstances. Some of these variations are briefly described.
Table 77-2. Diseases and Other Factors That Affect 24 Hour Thyroidal RAIU
RAIU, Radioactive iodine uptake; TSH, thyroid-stimulating hormone.
EARLY THYROID RADIOIODIDE UPTAKE AND 99MTC UPTAKE MEASUREMENTS
The combination of severe thyrotoxicosis and a low intrathyroidal iodine concentration may result in an accelerated turnover rate of iodine in some patients. This produces a rapid initial uptake of radioiodide, which reaches a plateau before 6 hours, followed by a decline through release of the isotope in hormonal or other forms (see Fig. 77-2). Although this phenomenon is rare, some laboratories choose to routinely measure early RAIU, usually at 2, 4, or 6 hours. As was mentioned above, early measurements require accurate determination of the background activity contributed by circulating isotope. Radioisotopes with a shorter half-life, such as 123I and 132I, are more suitable in this context.
Because thyroidal uptake in the very early period after administration of radioiodide reflects mainly iodide trapping activity, 99mTc as the pertechnetate ion (99mTcO4−) may be used. In euthyroid patients, thyroid trapping is maximal at about 20 minutes and is approximately 1% of the administered dose. This test, when coupled with the administration of triiodothyronine (T3), has been used to evaluate thyroid gland suppressibility in thyrotoxic patients who are treated with antithyroid drugs.
PERCHLORATE DISCHARGE TEST
The perchlorate discharge test is used to detect defects in intrathyroidal iodide organification. It is based on the following physiologic principle. Iodide is “trapped” in the thyroid gland by an active transport mechanism that is mediated by NIS.7 Once in the gland, iodine is rapidly bound to thyroglobulin (Tg), and retention no longer requires active transport. Several ions, such as thiocyanate (SCN−) and perchlorate (ClO4−), inhibit NIS-mediated iodide transport and cause release of the intrathyroidal iodide that is not bound to thyroid protein. Thus, intrathyroidal radioiodine loss after the administration of an inhibitor of iodide trapping measures intrathyroidal iodide that is not protein bound and indicates the presence of an iodide-binding defect.
In the standard test, epithyroid counts are obtained every 10 or 15 minutes after the administration of radioiodide. Two hours later, 1 g of KClO4 is administered orally, and repeated epithyroid counts continue to be obtained for an additional 2 hours. We generally give this dose to patients 6 years or older and give 500 mg to children between the ages of 2 and 6 years. In normal individuals, radioiodide accumulation in the thyroid gland ceases after administration of the iodide transport inhibitor, and little or no loss of the accumulated thyroidal radioactivity occurs after induction of the “trapping” block. An organification defect is indicated if a loss of 5% or more is noted. The severity of the defect is proportional to the extent of radioiodide discharged from the gland and is complete when virtually all the activity accumulated by the gland is lost. The test is positive in the inborn defect of iodide organification caused by thyroid peroxidase (TPO) defects or by mutations in the chloride/iodide transport protein (pendrin) when associated with sensorineural deafness known as Pendred’s syndrome and in defects of the H2O2-generating enzyme, dual oxidase 2 (DUOX2), or its maturation factor, DUOXA2. The test may also be positive during the administration of iodide organification–blocking agents or after treatment with radioactive iodide.
IODINE SALIVA-TO-PLASMA RATIO TEST
An abnormal iodine (I−) saliva-to-plasma (S/P) ratio is pathognomonic of the iodine-trapping defect. The test can be carried out without interruption of thyroid hormone treatment. Furthermore, the measurement of I− S/P can distinguish between a trapping defect and thyroid agenesis, which cannot be determined by RAIU. The I− S/P ratio can be measured in a medical center without access to a gamma camera. The test is based on the observation that all tissues that normally concentrate iodide are affected by the trapping defect.8 The presence of an I− transport defect in the parietal cells of the stomach and the choroid plexus of these patients has been used diagnostically by measurement of the gastric fluid-to-plasma and cerebrospinal fluid (CSF)-to-plasma ratios of radioiodide, following the administration of isotope.
One hour after the oral administration of 5 µCi of Na125I, saliva is collected without stimulation over a period of 5 to 10 minutes. At the same time, a venous blood sample is obtained. After defrothing of the saliva and removal of cell debris by centrifugation (approximately 10 minutes at 500 × g) and separation of the serum, the S/P ratio of radioiodide is determined by counting equal volumes of these fluids in a gamma scintillation counter. A normal I− S/P is 25. Affected individuals who are not able to concentrate iodide and therefore cannot secrete it into their saliva have a very low S/P. An I− S/P of approximately 1 is diagnostic of a complete trapping defect, and a value between 1 and 20 is consistent with a partial defect.
Measurement of Hormone Concentration and Other Iodinated Compounds and Their Transport in Blood
The tests most commonly used for evaluating thyroid hormone–dependent metabolic status consist of measurements of free thyroid hormone concentrations. This approach is used because of the development of simple, sensitive, and specific methods for measuring these iodothyronines, and because of the lack of specific tests for direct measurement of the metabolic effects of these hormones on target tissues. Other advantages are the requirement of only a small blood sample and the large number of determinations that can be completed by a laboratory during a regular workday. In fact, although a clinician may entertain a diagnosis of thyroid dysfunction, the certainty of the diagnosis is confirmed only after measurement of the thyroid hormone concentrations and thyrotropin (TSH, see later).
The principal source of all hormonal iodine-containing compounds or their precursors is the thyroid gland, whereas peripheral tissues are the source of the products of their degradation. Their chemical structures and normal concentrations in serum are given in Fig. 77-3. It is important to note that the concentration of each substance is dependent not only on the amount synthesized and secreted by the thyroid gland, but also on its affinity for carrier serum proteins, distribution in tissues, rate of degradation, and, finally, clearance.
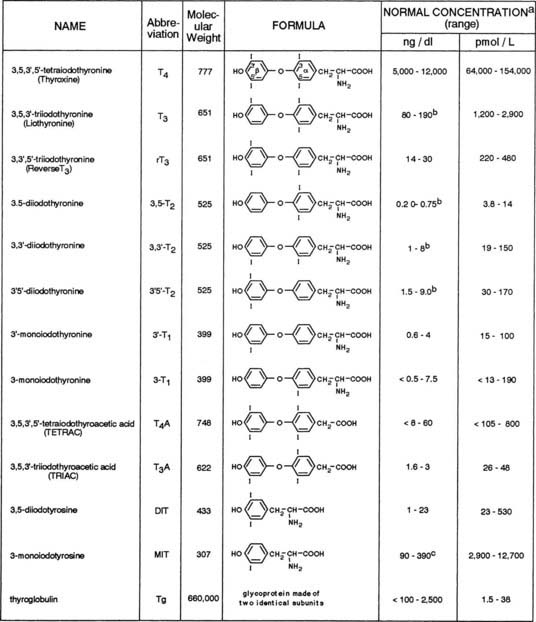
FIGURE 77-3. Iodine-containing compounds in the serum of healthy adults. A, Iodothyronine concentrations in the euthyroid population are not normally distributed. Therefore, calculation of the normal range on the basis of 95% confidence limits for a Gaussian distribution is accurate. B, Significant decline with old age. C, Probably an overestimation because of cross-reactivity by related substances.
Quantitatively, the major secretory product of the thyroid gland is thyroxine (T4), with T3 being next in relative abundance. They are synthesized and stored in the thyroid gland as part of a larger molecule, Tg, which is degraded to release the two iodothyronines in a ratio favoring T4 by 10- to 20-fold. Under normal circumstances, only minute amounts of Tg escape into the circulation. On a molar basis, it is the least abundant iodine-containing compound in blood. With the exception of T4, Tg, and small amounts of diiodotyrosine (DIT) and monoiodotyrosine (MIT), all other iodine-containing compounds that are found in normal human serum are produced mainly in extrathyroidal tissues by a stepwise process of deiodination of T4. An alternative pathway of T4 metabolism that involves deamination and decarboxylation but retention of the iodine residues gives rise to tetraiodothyroacetic acid (TETRAC) and triiodothyroacetic acid (TRIAC).9,10 Conjugation to form sulfated iodoproteins also occurs. Sulfoconjugates of T4, T3, and reverse T3 (rT3) have been identified in human biological fluids. Additionally, maternal serum levels of 3,3′-diiothyronine sulfate (T2S) may reflect on the status of fetal thyroid function. Circulating iodalbumin is generated by intrathyroidal iodination of serum albumin. Small amounts of iodoproteins may be formed in peripheral tissues or in serum by covalent linkage of T4 and T3 to soluble proteins. The physiologic function of circulating iodine compounds other than T4 and T3 remains unknown, with the exception of rT3. rT3 levels are elevated during fasting and during significant nonthyroidal illness. In such instances, measurement of rT3 can help the clinician to distinguish between these conditions and central hypothyroidism.
MEASUREMENT OF TOTAL THYROID HORMONE CONCENTRATION IN SERUM
Iodometry
Because iodine is an integral part of the thyroid hormone molecule, it is not surprising that determination of the iodine content in serum was the first method used over 6 decades ago for the identification and quantitation of thyroid hormone.11 Measurement of protein-bound iodine was the earliest method used routinely for the estimation of thyroid hormone concentration in serum. This test measured the total quantity of iodine precipitable with serum proteins, 90% of which is T4. The normal range was 4 to 8 mg of iodine per deciliter of serum.
Efforts to measure serum thyroid hormone levels with greater specificity and with lesser interference from nonhormonal iodinated compounds led to the development of measurement of butanol-extractable iodine and T4 iodine by column techniques. All such chemical methods for the measurement of thyroid hormone in serum have been replaced by ligand assays, which are devoid of interference by even large quantities of nonhormonal iodine-containing substances.
Radioimmunoassays
Concentrations of thyroid hormones in serum can be measured by radioimmunoassays (RIAs). The principle of these assays relies on competition between the hormone being measured with the same isotopically labeled compound for binding to a specific class of immunoglobulin G (IgG) molecule present in the antiserum. In assays for thyroid hormones, the hormone needs to be liberated from serum hormone–binding proteins, mainly thyroxine-binding globulin (TBG). Methods used to achieve such liberation include extraction, competitive displacement of the hormone being measured, and inactivation of TBG.12–14 Rarely, circulating antibodies against thyronines develop in some patients and interfere with RIAs carried out on unextracted serum samples. Depending on the method used for the separation of bound from free ligand, the values that are obtained may be spuriously low or spuriously high in the presence of such antibodies.
The wide choice of commercial kits available for most RIA procedures makes these assays accessible to all medical centers. RIAs have been adapted for the measurement of T4 in small samples of dried blood spots on filter paper and are used in screening for neonatal hypothyroidism.
Despite the ready availability of these kits, the specificity of the various antibodies can result in a twofold difference in hormone measurement when assessed by the College of American Pathologists Proficiency Testing Program.15
Nonradioactive Methods
Serum concentrations of T4 and T3 have been measured by radioimmunoassay since the early 1970s and more recently have been measured by nonisotopic methods. Assays for total T4 and T3 in unextracted serum include a reagent such as 8-anilinonaphthalene sulfonic acid that blocks T4 and T3 binding to serum proteins, so that total hormone is available for competition with the assay antibody. Assays were then developed that were based on the principle of the radioligand assay but do not use radioactive material. These assays, which use ligand conjugated to an enzyme, have largely replaced RIAs. The enzyme-linked ligand competes with the ligand being measured for the same binding sites on the antibody. Quantitation is carried out by spectrophotometry of the color reaction developed after addition of the enzyme substrate. Both homogeneous (enzyme-multiplied immunoassay technique) and heterogeneous (enzyme-linked immunosorbent) assays for T4 have been developed. In the homogeneous assays, no separation step is required, thus providing easy automation. In one such assay, T4 is linked to malate dehydrogenase to inhibit enzyme activity. The enzyme is activated when the T4-enzyme conjugate is bound to T4-specific antibody. Active T4 conjugates to other enzymes such as peroxidase and alkaline phosphatase have also been developed. This assay has also been adapted for the measurement of T4 in dried blood samples used in mass screening programs for neonatal hypothyroidism. Other nonradioisotopic immunoassays use fluorescence excitation for detection of the labeled ligand, a technique that is finding increasing application. Such assay methods use a variety of chemiluminescent molecules, such as 1,2-dioxetanes, luminol and derivatives, acridinium esters, oxalate esters, and firefly luciferins, as well as many sensitizers and fluorescent enhancers. One such assay that uses T4 conjugated to β-galactosidase and fluorescence measurements of the hydrolytic product of 4-methylumbelliferyl-β-d-galactopyranoside has been adapted for use in a microanalytic system requiring only 10 µL of serum. One commercial electrochemiluminescence immunoassay by Roche Elecsys Systems (Mannheim, Germany) uses a competitive test principle with antibodies specifically derived against T4 or T3. Endogenous T4 or T3 released by the action of 8-anilino-1-naphthalene sulfonic acid competes with the added biotinylated T4 derivative for the binding sites on the antibodies labeled with the ruthenium complex. Only 15 µL of sample is required. Application of the voltage to the reaction mixture induces chemiluminescent emission, which is measured by a photomultiplier.
Additionally, quantitative measurement of T4 and T3 can be done by high-performance liquid chromatography,16 gas chromatography, and mass spectrometry.17–20
Serum Total T4
The usual concentration of total T4 (TT4) in adults ranges from 5 to 12 µg/dL (64 to 154 nmol/L). When concentrations are below or above this range in the absence of thyroid dysfunction, they are usually the result of an abnormal level of serum TBG. Such abnormalities are commonly seen during the hyperestrogenic state of pregnancy and during the administration of estrogen-containing compounds, which results in a significant elevation of serum TT4 levels in euthyroid individuals. Similar elevations can be seen in subjects with different forms of hepatitis, and if not appreciated the patient can be misdiagnosed as having hyperthyroidism. Far less commonly, TBG excess is inherited.21
Serum TT4 is virtually undetectable in the fetus until midgestation. Thereafter, it rapidly increases and reaches high normal adult levels during the last trimester. A further acute but transient rise occurs within hours after delivery. Values remain above the adult range until 6 years of age, but subsequent age-related changes are minimal, so in clinical practice, the same normal range of TT4 applies to both sexes and all ages.
Small seasonal variations and changes related to high altitude, cold, and heat have been described. Rhythmic variations in serum TT4 concentration are of two types: variations related to postural changes in serum protein concentration22 and those resulting from true circadian variation. Postural changes in protein concentration do not alter the free T4 (FT4) concentration, however.
Although levels of serum TT4 below the normal range are usually associated with hypothyroidism and above this range are associated with thyrotoxicosis, it must be stressed that the TT4 level does not always correspond to the FT4 concentration, which represents the metabolically active fraction (see below). The TT4 concentration in serum may be altered by independent mechanisms: (1) an increase or decrease in the supply of T4, as is seen in most cases of thyrotoxicosis and hypothyroidism, respectively; (2) changes caused solely by alterations in T4 binding to serum proteins; and (3) compensatory changes in the serum TT4 concentration caused by high or low serum levels of T3. Conditions associated with changes in serum TT4 and their relationship to the metabolic status of the patient are listed in Table 77-3.
Table 77-3. Conditions Associated With Changes in Serum TT4 Concentration and Relationship to Clinical Status
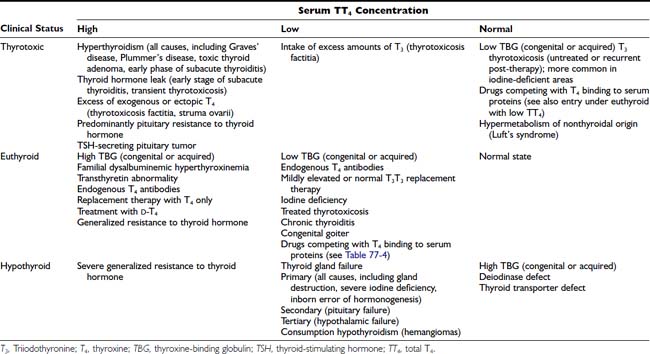
Serum TT4 levels are low in conditions that are associated with decreased TBG concentrations, in the presence of abnormal TBGs with reduced binding affinity (see Chapter 93), and when the available T4-binding sites on TBG are partially saturated by competing drugs present in blood in high concentration (Table 77-4). Conversely, TT4 levels are high when the serum TBG concentration is high. In this situation, the person remains euthyroid provided that feedback regulation of the thyroid gland is intact.
Table 77-4. Compounds That Affect Thyroid Hormone Serum Transport Proteins
| Substance | Common Use |
|---|---|
| Increase TBG Concentration | |
| Estrogens22,396,397 | Ovulation suppressants and anticancer |
| Heroin and methadone398 | Opiates (in addicts) |
| Clofibrate399 | Hypolipidemic |
| 5-Fluorouracil400 | Anticancer |
| Perphenazine401 | Tranquilizer |
| Tamoxifen402,403 | Chemotherapy |
| Raloxifene403–405 | Osteoporosis |
| Decrease TBG Concentration | |
| Androgens and anabolic steroids406,407 | Virilizing, anticancer, and anabolic |
| Glucocorticoids408 intracranial pressure | Antiinflammatory and anti-immunosuppressive; decrease |
| l-Asparaginase409 | Antileukemic |
| Nicotinic acid410,411 | Hypolipidemic |
| Interfere With Thyroid Hormone Binding to TBG and/or TTR | |
| Salicylates and salsalate387,412 | Antiinflammatory, analgesic, and antipyretic |
| Carbamazepine387,412 | |
| Diphenylhydantoin and analogues413,414 | Anticonvulsive and antiarrhythmic |
| Diazepam415 | Antianxiety |
| Furosemide416 | Diuretic |
| Sulfonylureas417 | Hypoglycemic |
| Dinitrophenol411 | Uncouples oxidative phosphorylation |
| Free fatty acids417 | |
| o,p′-DDD418 | Antiadrenal |
| Phenylbutazone419 | Antiinflammatory |
| Halofenate420 | Hypolipidemic |
| Fenclofenac392,421 | NSAID |
| Mefenamic | NSAID |
| Diclofenac | NSAID |
| Heparin (IV)422 | Anticoagulant |
| Enoxaparin422 | |
| Orphenadrine423 | Spasmolytic |
| Monovalent anions (SCN−, C104−)424 | Antithyroid |
| Thyroid hormone analogues, including dextroisomers425 | Cholesterol reducing |
NSAID, Nonsteroidal antiinflammatory drug; o,p′-DDD, 2,4′-dichlorodiphenyldichloroethane (mitotane); TBG, thyroxine-binding globulin; TTR, transthyretin.
Although changes in transthyretin (TTR) concentration rarely give rise to significant alterations in TT4 concentration, the presence of a variant serum albumin with high affinity for T4 or antibodies against T4 produce apparent elevations in the measured TT4 concentration, whereas the metabolic status remains normal. The variant albumin is inherited as an autosomal dominant trait termed familial dysalbuminemic hyperthyroxinemia (FDH; see Chapter 93).
Another possible cause of discrepancy between the observed serum TT4 concentration and the metabolic status of the patient is divergent changes in serum total T3 (TT3) and TT4 concentrations with alterations in the serum T3/T4 ratio. The most common situation is that of an elevated TT3 concentration. The source of T3 may be endogenous, as in T3 thyrotoxicosis, or exogenous, as during ingestion of T3. In the former situation, contrary to the common variety of thyrotoxicosis, elevation in the serum TT3 concentration is not accompanied by an increase in the TT4 level. In fact, the serum TT4 level is normal and occasionally low. This finding indicates that the pathogenesis of T3 thyrotoxicosis is the direct secretion of T3 from the thyroid gland rather than the peripheral conversion of T4 to T3. Ingestion of pharmacologic doses of T3 results in thyrotoxicosis associated with severe depression of the serum TT4 concentration. Moderate hypersecretion of T3 can be associated with euthyroidism and a low serum TT4 concentration. This situation, occasionally referred to as T3 euthyroidism, may be more prevalent than T3 thyrotoxicosis. It is believed to constitute a state of compensatory T3 secretion as a physiologic adaptation of the failing thyroid gland, such as after treatment of thyrotoxicosis, in some cases of chronic thyroiditis, or during iodine deprivation. The serum TT4 concentration is also low in normal persons receiving replacement doses of T3. Conversely, serum TT4 levels are above the upper limit of normal in 15% to 50% patients treated with exogenous T4 and having normal serum TSH. Because of the relatively slow rate of metabolism and the large extrathyroidal T4 pool, the serum concentration of the hormone varies little with the time of sampling in relation to ingestion of the daily dose.
Serum Total T3
Triiodothyronine is principally responsible for the effects of thyroid hormones on the target organs. T3 is formed extrathyroidally via 5′ deiodination of T4. Thus, serum T3 concentration reflects the functional state of the peripheral tissue rather than the secretory performance of the thyroid gland. Like T4, 99% of T3 is present in protein-bound form. However, the affinity of T3 to TBG is 10-fold lower than that of T4.
Normal serum TT3 concentrations in the adult range from 80 to 190 ng/dL (1.2 to 2.9 nmol/L). Sex differences are small, but age differences are more dramatic. In contrast to serum TT4, the TT3 concentration at birth is low, about half the normal adult level. It rises rapidly within 24 hours to about double the normal adult value, followed by a decrease over the subsequent 24 hours to a level in the upper adult range, which persists for the first year of life. A decline in the mean TT3 level has been observed in old age, although not in healthy subjects,23,24 which suggests that a fall in TT3 might reflect the prevalence of nonthyroidal illness rather than an effect of age alone. Although a positive correlation between serum TT3 level and body weight has been observed, this might be related to overeating.25 Rapid and profound reductions in serum TT3 can be produced within 24 to 48 hours of total calorie or carbohydrate-only deprivation.
Most conditions that cause serum TT4 levels to increase are associated with high TT3 concentrations. Thus, serum TT3 levels are usually elevated in thyrotoxicosis and reduced in hypothyroidism. However, in both conditions, the TT3/TT4 ratio is elevated relative to normal euthyroid persons. This elevation is due to the disproportionate increase in serum TT3 concentration in thyrotoxicosis and a lesser diminution in hypothyroidism relative to the TT4 concentration. Accordingly, measurement of the serum TT3 level is a more sensitive test for the diagnosis of hyperthyroidism, and measurement of TT4 is more useful in the diagnosis of hypothyroidism.
Under certain conditions, changes in the serum TT3 and TT4 concentrations are disproportionate or occur in the opposite direction (Table 77-5). Such conditions include the syndrome of thyrotoxicosis with normal TT4 and FT4 levels (T3 thyrotoxicosis). In some patients, treatment of thyrotoxicosis with antithyroid drugs normalizes the serum TT4 but not the TT3 level and produces a high TT3/TT4 ratio. In areas of limited iodine supply and in patients with limited thyroidal ability to process iodide, euthyroidism can be maintained at low serum TT4 and FT4 levels by increased direct thyroidal secretion of T3. Although these changes have a rational physiologic explanation, the significance of discordant serum TT4 and TT3 levels under other circumstances is less well understood.
Table 77-5. Conditions That May Be Associated With Discrepancies Between the Concentration of Serum TT3 and TT4
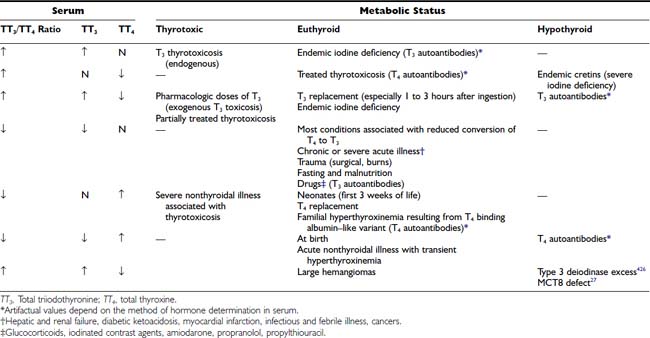
The most common cause of discordant serum concentrations of TT3 and TT4 is a selective decrease in serum TT3 caused by decreased conversion of T4 to T3 in peripheral tissues. This reduction is an integral part of the pathophysiology of a number of nonthyroidal acute and chronic illnesses and calorie deprivation (see Chapter 86). In these conditions, the serum TT3 level is often lower than that commonly found in patients with frank primary hypothyroidism. However, no clear clinical evidence of hypometabolism is found in this situation. In some individuals, decreased T4-to-T3 conversion is an inherited condition.26 A combination of high TT3 and low TT4 is typical in subjects with loss-of-function mutations in the iodothyronine cell membrane transporter, MCT8.27
A variety of drugs are responsible for producing changes in the serum TT3 concentration without apparent metabolic consequences. Drugs that compete with hormone binding to serum proteins decrease serum TT3 levels, generally without affecting the free T3 (FT3) concentration (see Table 77-4). Some drugs such as glucocorticoids28 depress the serum TT3 concentration by interfering with the peripheral conversion of T4 to T3. Others, such as phenobarbital,29 depress the serum TT3 concentration by stimulating the rate of intracellular hormone degradation and clearance. Most have multiple effects. These effects are combinations of those described above, as well as inhibition of the hypothalamic-pituitary axis or thyroidal hormonogenesis.
Changes in serum TBG concentration have an effect on the serum TT3 concentration similar to that on TT4. The presence of endogenous antibodies to T3 can also result in apparent elevation of serum TT3, but as in the case of high TBG, it does not cause hypermetabolism.
Administration of commonly used replacement doses of T3, usually on the order of 75 µg/day or 1 µg/kg body weight per day,30 results in serum TT3 levels in the thyrotoxic range. Furthermore, because of rapid gastrointestinal absorption and a relatively fast degradation rate, the serum level varies considerably according to the time of sampling in relation to hormone ingestion.
MEASUREMENT OF TOTAL AND UNSATURATED THYROID HORMONE–BINDING CAPACITY IN SERUM
The concentration of thyroid hormone in serum is dependent on its supply, as well as on the abundance of hormone-binding sites on serum proteins; therefore, estimation of the latter has proved useful in the correct interpretation of values obtained from measurement of the total hormone concentration. These results have been used to provide an estimate of the free hormone concentration, which is important in differentiating changes in serum total hormone concentrations caused by alterations in binding proteins in euthyroid patients from those caused by abnormalities in thyroid gland activity that give rise to hypermetabolism or hypometabolism.
In Vitro Uptake Tests
In vitro uptake tests measure the unoccupied thyroid hormone–binding sites on TBG. They use labeled T3 or T4 and some form of synthetic absorbent to measure the proportion of radiolabeled hormone that is not tightly bound to serum proteins. Because ion exchange resins often are used as absorbents, the test became known as the resin T3 or T4 uptake test, which describes the technique rather than the entity measured.
The test is usually carried out by incubating a sample of the patient’s serum with a trace amount of labeled T3 or T4. The labeled hormone, not bound to available binding sites on TBG present in the serum sample, is absorbed onto an anion exchange resin and measured as resin-bound radioactivity. Values correlate inversely with the concentration of unsaturated TBG. Various methods use different absorbing materials to remove the hormone that is not tightly bound to TBG. Labeled T3 generally is used because of its less firm, yet preferential, binding to TBG. Depending on the method, typical normal results for T3 uptake are 25% to 35% or 45% to 55%. Thus, it is more valuable to express results of the uptake tests as a ratio of the result obtained in a normal control serum run in the same assay as the test samples. Normal values will then range on either side of 1.0, usually from 0.85 to 1.15.
Uptake of tracer by the absorbent is inversely proportional to the number of unsaturated binding sites (unoccupied by endogenous thyroid hormone) in serum TBG. Thus, uptake is increased when the amount of unsaturated TBG is reduced as a result of excess endogenous thyroid hormone or a decrease in the concentration of TBG. In contrast, uptake is decreased when the amount of unsaturated TBG is increased as a result of a low serum thyroid hormone concentration or an increase in the concentration of TBG. Because the test can be affected by either or both independent variables—serum total thyroid hormone and TBG concentrations—the results cannot be interpreted without knowledge of the hormone concentration. As a rule, parallel increases or decreases in serum TT4 concentration and the T3 uptake test indicate hyperthyroidism and hypothyroidism, respectively, whereas discrepant changes in serum TT4 and T3 uptake suggest abnormalities in TBG binding. However, abnormalities in hormone and TBG concentrations can coexist in the same patient. For example, a hypothyroid patient with a low TBG level will typically show a low TT4 level and normal T3 uptake results (Fig. 77-4). Several nonhormonal compounds, because of structural similarities, compete with thyroid hormone for its binding site on TBG. Some are used as pharmacologic agents and thus may alter the in vitro uptake test, as well as the total thyroid hormone concentration in serum. A list is provided in Table 77-4.
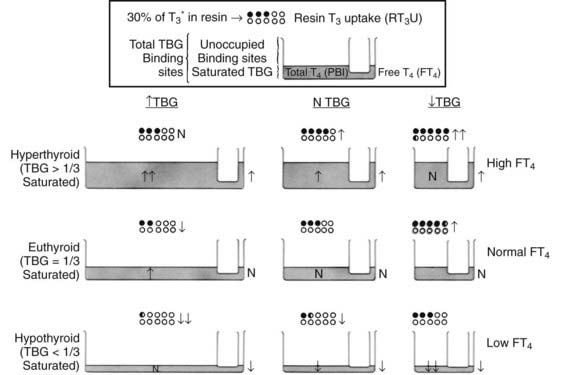
FIGURE 77-4. Graphic representation of the relationship between the serum total thyroxine (T4) concentration, the resin triiodothyronine uptake (rT3U) test, and the free T4 (FT4) concentration in various metabolic states and in association with changes in thyroxine-binding globulin (TBG). The principle of communicating vessels is used as an illustration. The height of fluid in the small vessel represents the level of FT4; the total amount of fluid in the large vessel, the total T4 concentration; and the total volume of the large vessel, the TBG capacity. Dots represent resin beads; black dots represent those carrying the radioactive T3 tracer (T3*). The rT3U test result (black dots) is inversely proportional to the unoccupied TBG-binding sites represented by the unfilled capacity of the large vessel.
TBG and TTR Measurements
The concentrations of TBG and TTR in serum can be estimated by measurement of their total T4-binding capacity at saturation or measured directly by immunologic techniques.31,32
The TBG concentration in serum can be determined by RIA,32 and both TBG and TTR can be measured by Laurell’s rocket immunoelectrophoresis, by radial immunodiffusion, or by enzyme immunoassay; commercial methods are available. The true mean value for TBG is 1.6 mg/dL (260 nmol/L), with a range of 1.1 to 2.2 mg/dL (180 to 350 nmol/L) in serum. In adults, the normal range for TTR is 16 to 30 mg/dL (2.7 to 5.0 mmol/L). Concentrations of TBG and TTR in serum vary with age, gender, pregnancy, and posture. Determination of the concentration of these proteins in serum is particularly helpful for evaluation of extreme deviations from normal, as in congenital abnormalities of TBG. In most instances, however, the in vitro uptake test, in conjunction with the serum TT4 level, gives an approximate estimation of the TBG concentration.
ESTIMATION OF FREE THYROID HORMONE CONCENTRATION
Most thyroid hormones in the blood are bound to serum protein carriers, thus leaving only a minute fraction of free hormone in the circulation that is capable of mediating biological activities. A reversible equilibrium exists between bound and unbound hormone, and it is the latter that represents the fraction of the hormone capable of traversing cellular membranes to exert its effects on body tissues. Although changes in serum hormone-binding proteins affect both the total hormone concentration and the corresponding circulating free fraction, in a euthyroid person, the absolute concentration of free hormone remains constant and correlates with the tissue hormone level and its biological effect. Information concerning this value is probably the most important parameter in the evaluation of thyroid function because it relates to the patient’s metabolic status, although other mechanisms exist for the cell to control the active amount of thyroid hormone via autoregulation of receptors33 and regulation of deiodinase activity.34,35 Rarely, a defect in thyroid hormone transport into cells would abolish the free hormone and the metabolic effect correlation.27
With few exceptions, the free hormone concentration is high in thyrotoxicosis, low in hypothyroidism, and normal in euthyroidism, even in the presence of profound changes in TBG concentration, provided that the patient is in a steady state. Notably, the FT4 concentration may be normal or even low in patients with T3 thyrotoxicosis and in those ingesting pharmacologic doses of T3. The concentration of FT4 may be outside the normal range in the absence of an apparent abnormality in thyroid hormone–dependent metabolic status. This situation is frequently observed in severe nonthyroidal illness, during which both high and low values have been reported. As expected, when a euthyroid state is maintained by the administration of T3 or by predominant thyroidal secretion of T3, the FT4 level is also depressed. More consistently, patients with a variety of nonthyroidal illnesses have low FT3 levels. This decrease is characteristic of all conditions associated with depressed serum TT3 concentrations caused by diminished conversion of T4 to T3 in peripheral tissues by deiodinase enzymes (see Chapter 75). Both FT4 and FT3 values may be out of line in patients receiving a variety of drugs (see below). Marked elevations in both FT4 and FT3 concentrations in the absence of hypermetabolism are typical of patients with the inherited condition of resistance to thyroid hormone (see Chapter 94). The FT3 concentration is usually normal or even high in hypothyroid individuals living in areas of severe endemic iodine deficiency. Their FT4 levels are, however, normal or low. Free hormone concentrations also do not reflect the metabolic status of the patient with inherited defects in hormone transport into cells of hormone metabolism.36
Direct Measurement of Free T4 and Free T3
Direct measurement of absolute FT4 and FT3 concentrations is technically difficult and until recently has been limited to research assays. To minimize perturbations of the relationship between free and bound hormone, these hormones must be separated by ultrafiltration or by dialysis involving minimal dilution and little alteration in pH or electrolyte composition. The separated free hormone is then measured directly by RIA or chromatography.37 These assays are probably the most accurate available, but small, weakly bound, dialyzable substances or drugs may be removed from the binding proteins, and the free hormone concentration measured in their presence might not fully reflect the free concentration in vivo. Direct immunometric assays adapted to automation, although not reliable under specific conditions, have replaced more labor intensive methods (see below).
Isotopic Equilibrium Dialysis
This method has been the gold standard for the estimation of FT4 or FT3 for more than 40 years. It is based on a determination of the proportion of T4 or T3 that is unbound, or free, and thus is able to diffuse through a dialysis membrane (i.e., the dialyzable fraction). To carry out the test, a sample of serum is incubated with a trace amount of labeled T4 or T3. The labeled tracer rapidly equilibrates with the respective bound and free endogenous hormones. The sample is then dialyzed against buffer at a constant temperature until the concentration of free hormone on either side of the dialysis membrane has reached equilibrium. The dialyzable fraction is calculated from the proportion of labeled hormone in the dialysate. The contribution from radioiodide present as contaminant in the labeled tracer hormone should be eliminated by purification38 and by various techniques of precipitation of the dialyzed hormone.39 FT4 and FT3 levels can be measured simultaneously by addition to the sample of T4 and T3 labeled with two different radioiodine isotopes. Ultrafiltration is a modification of the dialysis technique. Results are expressed as the fraction (dialyzable fraction of T4 or T3) or percentage (%FT4 or %FT3) of the respective hormones that dialyzed, and the absolute concentrations of FT4 and FT3 are calculated from the product of the total concentration of the hormone in serum and its respective dialyzable fraction. Typical normal values for FT4 in adults range from 1.0 to 3.0 ng/dL (13 to 39 pmol/L), and those for FT3 range from 0.25 to 0.65 ng/dL (3.8 to 10 nmol/L).
Results achieved by these techniques generally are comparable to those determined by direct one-step methods (see below) but are more likely to differ with extremely low or extremely high TBG concentrations or in the presence of circulating inhibitors of protein binding, especially in situations of nonthyroidal illness. The measured dialyzable fraction may be altered by the temperature at which the assay is run, the degree of dilution, the time allowed for equilibrium to be reached, and the composition of the diluting fluid. The calculated value is dependent on an accurate measurement of TT4 or TT3 and may be incorrect in patients with T4 or T3 autoantibodies. Some of these problems, particularly those arising from dilution, can be surmounted by using commercially available dialysis methods or ultrafiltration methods of free from bound hormone that do not necessitate serum dilution. In addition, the antibody that is used on the ultrafiltrate must be of high affinity to achieve acceptable precision.
Index Methods
Because determination of free hormone by equilibrium dialysis is cumbersome and technically demanding, many clinical laboratories have used a method by which an FT4 index (FT4I) or an FT3 index (FT3I) is derived from the product of the TT4 or TT3 (determined by immunoassay) and the value of an in vitro uptake test (see above). Although the results are not always in agreement with the values obtained by dialysis, these techniques are rapid and simple. They are more likely to fail at extremely low or extremely high TBG concentrations, in the presence of abnormal binding proteins, in patients with nonthyroidal illness, or in the presence of circulating inhibitors of protein binding.
The theoretical contention that the FT4I is an accurate estimate of the absolute FT4 concentration can be confirmed by the linear correlation between these two parameters. This statement is true provided that results of the in vitro uptake test (T3 or T4 uptake) are expressed as the thyroid hormone–binding ratio, which is determined by dividing the tracer counts bound to the solid matrix by counts bound to serum proteins. Values are corrected for assay variations by using appropriate serum standards and are expressed as the ratio of a normal reference pool. The normal range is slightly narrower than the corresponding TT4 in healthy euthyroid patients with a normal TBG concentration. It is 6.0 to 10.5 mg/dL (77 to 135 nmol/L) when calculated from TT4 values. The FT4I is high in thyrotoxicosis and low in hypothyroidism, irrespective of the TBG concentration. Euthyroid patients with TT4 values outside the normal range as a result of TBG abnormalities have a normal FT4I. Lack of correlation between the FT4I and the metabolic status of the patient has been observed in the same circumstances as those described for similar discrepancies when the FT4 concentration was measured by dialysis.
Methods for estimation of the FT3I are also available but are rarely used in routine clinical evaluation of thyroid function. Like the FT4I, it correlates well with the absolute FT3 concentration. The test corrects for changes in TT3 concentration resulting from variations in TBG concentration.
Estimation of Free T4 and Free T3 Based on TBG Measurements
Because most T4 and T3 in serum are bound to TBG, their free concentrations can be calculated from their binding affinity constants to TBG and molar concentrations of hormones and TBG. A simpler calculation of the T4/TBG and T3/TBG ratios yields values that are similar to but less accurate than the FT4I and FT3I, respectively.
Two-Step Immunoassays
In these assays, the free hormone is first immunoextracted by a specific bound antibody (first step), frequently fixed to the tube (coated tube).40 After washing, labeled tracer is added and is allowed to equilibrate between the unoccupied sites on the antibody and those of serum thyroid hormone–binding proteins. The free hormone concentration will be inversely related to the antibody-bound tracer, and values are determined by comparison to a standard curve. Values that are obtained with this technique are generally comparable to those determined by direct methods. They are more likely to differ in the presence of circulating inhibitors of protein binding and in sera from patients with nonthyroidal illness.
Analogue (One-Step) Immunoassays
In these assays, a labeled analogue of T4 or T3 directly competes with the endogenous free hormone for binding to antibodies.41 In theory, these analogues are not bound by the thyroid hormone–binding proteins in serum. However, various studies have found significant protein binding to the variant albumin-like protein, to TTR, and to iodothyronine autoantibodies. Such binding results in discrepant values in other assays in a number of conditions, including nonthyroidal illness, pregnancy, and familial dysalbuminemic hyperthyroxinemia (FDH).42 A growing number of commercial kits are available, some of which have been modified to minimize these problems.43,44 Nonetheless, their accuracy remains controversial, although such commercial methods are increasingly being adopted in the routine clinical chemistry laboratory. Commercially available kits for measurement of free T4 values are compared in Table 77-6.
Table 77-6. Commercial Free T4 Methods
| Name | Methodology | Manufacturer |
|---|---|---|
| Amberlite MAB | Serum free T4 inhibits binding of peroxidase anti-T4 monoclonal antibody T3-coated solid phase. | Amersham, UK |
| Chiron ACS:180 | Serum free T4 competes with acridinium ester labeled T4. Anti-T4 antibody linked to magnetic particles. | Chiron Diagnostics, MA, USA |
| AxSYM | Anti-T4 coated microparticles. T3-alkaline phosphatase binds to the unoccupied sites. | Abbott Labs, IL, USA |
| Elecsys | Anti-T4 antibody labeled with ruthenium. Unoccupied antibody binds to biotinylated T4, which is linked to streptavidin-coated microparticles. Magnetic separation. | Boehringer Manheim, IN, USA |
| Diagnostic Product Immulite | T4 analogue tracer (does not bind TBG or TTR) competes with serum free T4 for a limited number of T4 antibody binding sites. Alkaline phosphatase–labeled antianalogue binds to solid phase, and generated signal is inversely proportional to free T4. | Diag Prod, CA, USA |
| Corning Nichols Dialysis | Dialysis against 12-fold buffer volume, followed by radioimmunoassay of T4 in the dialysate. | Nichols Institute, CA, USA |
Automated Measurement of Free T4 and Free T3
During the 1990s, through the introduction of random access immunoassay analyzers that operate with chemiluminescent or fluorescent labels, measurements of free thyroid hormones became automated and therefore allowed rapid processing of multiple samples. Although the initial financial burden of such equipment is considerable, they reduce labor costs, demand few handling skills on behalf of the operator, and provide random access so that samples can be tested on demand. Precision studies have shown highly reproducible data with this approach.45,46 Comparison of results between different automated analyzers and with manual free thyroid hormone assays, including the gold standard of equilibrium dialysis, has revealed good correlation over a broad range of free thyroid hormone concentrations.47,48
Considerations in Selection of Methods for the Estimation of Free Thyroid Hormone Concentration
No single method for the estimation of free hormone concentration in serum is infallible in the evaluation of thyroid hormone–dependent metabolic status. Each test has inherent advantages and disadvantages depending on specific physiologic and pathologic circumstances. For example, methods based on measurement of total thyroid hormone and TBG cannot be used in patients with absent TBG secondary to inherited TBG deficiency. Under such circumstances, the concentration of free thyroid hormone is dependent on interaction of the hormone with serum proteins that normally play a negligible role (TTR and albumin). When alterations in thyroid hormone binding do not affect T4 and T3 equally, discrepant results of FT4I are obtained when labeled T4 or T3 is used in the in vitro uptake test. For example, euthyroid patients with FDH and those who have endogenous antibodies with greater affinity for T4 will have high TT4 but a normal T3 uptake test, which will result in an overestimation of the calculated FT4I. In such instances, calculation of the FT4I from a T4 uptake test may provide more accurate results. Conversely, reduced overall binding affinity for T4, which affects T3 to a lesser extent, will underestimate the FT4I derived from a T3 uptake test. Similarly, use of T4 and T3 uptake for estimation of the free hormone concentration is satisfactory in the presence of alterations in TBG concentration but not with alterations of the affinity of TBG for the hormone.
Methods based on equilibrium dialysis are most appropriate for estimation of the free thyroid hormone level in patients with all varieties of abnormal binding to serum proteins, provided that the true concentration of total hormone has been accurately determined. All methods for the estimation of FT4 may give high or low values in patients with severe nonthyroidal illness who are believed to be euthyroid. This finding has been attributed, at least in part, to the presence of inhibitors of thyroid hormone binding to serum proteins, as well as to the various adsorbents that are used in the test procedures. Some of these inhibitors have been postulated to leak from tissues of the diseased patient. Such discrepancies are even more pronounced during transient states of hyperthyroxinemia or hypothyroxinemia associated with acute illness, after withdrawal of treatment with thyroid hormone, and in patients with acute changes in TBG concentration (see Chapter 93).
The contribution of various drugs that interfere with binding of thyroid hormone to serum proteins or with the in vitro tests should also be taken into account in the choice and interpretation of tests (see Table 77-4). Although the free thyroid hormone concentration in serum would appear to determine the amount of hormone that is available to body tissues, factors that govern their cell membrane uptake, transport to the nucleus, and functional interactions with nuclear receptors and cofactors ultimately determine their biological effects.
MEASUREMENTS OF IODINE-CONTAINING HORMONE PRECURSORS AND PRODUCTS OF DEGRADATION
The last 4 decades have witnessed the development of RIAs for the measurement of a number of naturally occurring, iodine-containing substances that have little, if any, thyromimetic activity. Some of these substances are products of T4 and T3 degradation in peripheral tissues. Others are predominantly, if not exclusively, of thyroidal origin. Because they are devoid of significant metabolic activity, with the exception of rT3, measurement of their concentration is of value only in the research setting for detecting abnormalities in the metabolism of thyroid hormone in peripheral tissues, as well as defects of hormone synthesis and secretion. The application of tandem mass spectrometry in the measurement of these substances undoubtedly will result in a better understanding of their changes and their pathophysiologic implications.
3,3′,5′-Triiodothyronine or Reverse T3
rT3 is principally a product of T4 degradation in peripheral tissues, namely, liver and kidney (see Chapter 75). It is also secreted by the thyroid gland, but the amounts are practically insignificant.49 rT3 is an inactive product of T4 degradation. Thus, measurement of the rT3 concentration in serum reflects both tissue supply and metabolism of T4 and identifies conditions that favor this particular pathway of T4 degradation.
When total rT3 (TrT3) is measured in unextracted serum, a competitor of rT3 binding to serum proteins must be added. Several chemically related compounds may cross-react with the antibodies. The strongest cross-reactivity is observed with 3,3′-diiodothyronine (3,3′-T2), but such cross-reactivity does not present a serious methodologic problem because of its relatively low level in human serum. Although cross-reactivity with T3 and T4 is less likely, these compounds are more often the cause of rT3 overestimation because of their relative abundance, particularly in thyrotoxicosis. Free fatty acids interfere with the measurement of rT3 by RIA.50 The normal range in adult serum for TrT3 is 14 to 30 ng/dL (0.22 to 0.46 nmol/L), although varying values have been reported. It is elevated in subjects with high TBG and in some individuals with FDH.51 Serum TrT3 levels are normal in hypothyroid patients who are treated with T4, which indicates that peripheral T4 metabolism is an important source of circulating rT3. Values are high in thyrotoxicosis and low in untreated hypothyroidism. High values are normally found in cord blood and in newborns.
With only a few exceptions, notably uremia and human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), serum TrT3 concentrations are elevated in all circumstances that cause low serum T3 levels in the absence of obvious clinical signs of hypothyroidism. These conditions include, in addition to the newborn period, a variety of acute and chronic nonthyroidal illnesses, calorie deprivation, and the influence of a growing list of clinical agents and drugs (Table 77-7).
Table 77-7. Agents That Alter the Extrathyroidal Metabolism of Thyroid Hormone
| Substance | Common Use |
|---|---|
| Inhibit Conversion of T4 to T3 | |
| PTU427–429 | Antithyroid |
| Glucocorticoids (hydrocortisone, prednisone dexamethasone)214 | Antiinflammatory and immunosuppressive; decrease intracranial pressure |
| Propranolol430,431 | Adrenergic blocker (antiarrhythmic, antihypertensive) |
| Interleukin-6432 | Cancer therapy |
| Iodinated contrast agents: ipodate (Oragrafin), iopanoic acid (Telepaque)433,434 | Radiologic contrast media |
| Amiodarone435–437 | Antianginal and antiarrhythmic |
| Clomipramine438 | Tricyclic antidepressant |
| Stimulate Hormone Degradation | |
| Diphenylhydantoin243,439 | Anticonvulsive and antiarrhythmic |
| Carbamazepine440 | Anticonvulsant |
| Phenobarbital440 | Hypnotic, tranquilizing, and anticonvulsive |
| Rifampin441 | Antituberculosis drug |
| Ritonavir | Antiviral |
| Sertraline442 | Depression |
| Decrease Absorption/Increase Fecal Excretion of Thyroid Hormone | |
| Cholestyramine443 Colestipol | Hypolipidemic resins |
| Soybeans444 | Diet |
| Calcium carbonate445 | |
| Ferrous sulfate355 | Anemia |
| Sucralfate446 | Antiulcer |
| Aluminum hydroxide353,447 | Antacid |
PTU, Propylthiouracil; T3, triiodothyronine; T4, thyroxine.
The current clinical application of TrT3 measurement in serum is in the differential diagnosis of conditions associated with alterations in serum T3 and T4 concentrations when thyroid gland and metabolic abnormalities are not readily apparent.
The dialyzable fraction of rT3 in normal adult serum is 0.2% to 0.32%, or approximately the same as that of T3. The corresponding serum free rT3 (FrT3) concentration is 50 to 100 pg/dL (0.77 to 1.5 pmol/L). In the absence of gross TBG abnormalities, variations in serum FrT3 concentration closely follow those of TrT3. Reverse T3 is measured by RIA in serum, plasma, or amniotic fluid samples. Antigen from patient samples competes with radioactive tracer (125I-rT3) for binding sites on an antibody. After incubation, the amount of tracer that is bound to antibody will be inversely proportional to the amount of antigen in the sample. The radioactivity of the tracer bound to antibody is counted, and the amount of antigen or rT3 from the patient’s serum is determined from this. Typically, 0.1 mL of undiluted sample is required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








