Figure 22-1. Anatomic relationship among the pineal gland, hypothalamus, and pituitary gland.
The pineal gland also receives parasympathetic and peptidergic innervation originating in the hypothalamus, mesolimbic system, and visual structures. These fibers contain a number of neurotransmitters such as somatostatin, serotonin, and histamine. The pineal gland also contains gonadotropin-releasing hormone (GnRH), thyrotropin-releasing hormone (TRH), vasotocin, and other peptides whose roles are yet to be defined.
Melatonin
The word melatonin is a derivative of the Greek words melas and tonein, coined because of the property of melatonin to lighten amphibian skin.2 Melatonin is N-acetyl-5-methoxytryptamine, an indolamine derivative of tryptophan. The molecule is extremely well conserved across the phyla and has been identified in all major taxa, including bacteria, unicellular eukaryotes, many plants species, and all animals.3 In the vertebrate, melatonin is primarily secreted by the pineal gland, although a variety of other tissues, including the retina, bone marrow, skin, lymphocytes, and gastrointestinal tract, also synthesize the hormone. Melatonin derived from the gastrointestinal tract can be released into the circulation after ingestion of high dietary tryptophan.4
SYNTHESIS AND SECRETION OF MELATONIN
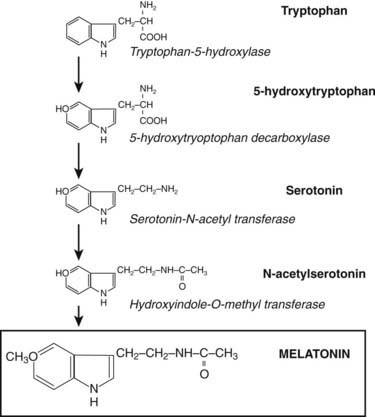
Through the neuroretinal pathways described above, the pineal gland becomes a neuroendocrine transducer, transmitting the SCN message into a hormonal code which signals that light or darkness has arrived. Indeed, the main function of the pineal gland is to translate SCN activity into the rhythmic release of melatonin, which in turn helps synchronize several daily and seasonal cycles. The synthesis of melatonin is presented in Fig. 22-2. The hormone is synthesized from the amino acid tryptophan, which is converted into serotonin prior to being processed into melatonin. Melatonin synthesis and secretion are greater during the dark phase compared to the light phase of the cycle. During the light phase of the photoperiod, SCN activity is high, resulting in low norepinephrine levels. Under reduced adrenergic activity, tryptophan is converted into serotonin in a two-step process via the intermediary, 5-hydroxytryptophan. At this point, serotonin does not come into contact with the enzyme responsible for converting it into melatonin. Therefore, plasma levels of melatonin are low during the light phase. However, with the arrival of the dark period, SCN activity becomes quiescent, and noradrenergic activity increases, resulting in activation of β-adrenergic receptors (and to a lesser extent α-adrenergic receptors) on the pinealocyte. The β-adrenergic receptors are coupled to cyclic adenosine monophosphate (cAMP)/protein kinase A signaling pathways that stimulate melatonin synthesis (Fig. 22-3). Stimulation of α-adrenergic receptors potentiates β-adrenergic function, resulting in a cascade that mobilizes calcium ions, phosphatidylinositol, diacylglycerol, and protein kinase C.5 This process requires serotonin to be first converted into N-acetyl serotonin by the enzyme serotonin-N-acetyltransferase, which in turn is converted into melatonin after coming into contact with the enzyme hydroxyindole-O-methyltransferase (HIOMT).6 The longer the dark phase, the longer the duration of melatonin secretion.
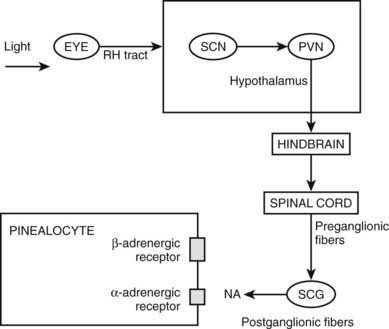
Figure 22-2. Pathways providing light and neural input to the pineal gland. The melatonin rhythm is synchronized to a 24-hour light/dark cycle primarily by the retina and retinohypothalamic projections to the SCN. RH, retinohypothalamic; SCN, suprachiasmatic nucleus; PVN, paraventricular nucleus; SCG, superior cervical ganglion; NA, norepinephrine.
Melatonin is not stored after synthesis but merely diffuses out of the pineal gland into the blood stream and cerebrospinal fluid. In the transition from light to dark, plasma melatonin concentrations increase from 2 to 10 pg/mL to 100 to 200 pg/mL.7 Melatonin levels start to rise during the evening, reach maximum levels in the middle of the night, and start decreasing in the early morning before sunrise. Although the melatonin rhythm is highly responsive to the light cycle, it does persist when people are placed for a few days in a dark room and does not immediately phase shift when the light schedule is altered.7 This indicates that the rhythm is not simply generated by the light/dark cycle but is free running and modulated by endogenous signals probably arising in the SCN. Indeed, the rhythm is abolished by lesioning of the SCN and persists, albeit in modified form, in the blind.8 The day-to-day pattern of melatonin secretion is extremely stable within an individual.9 However, the melatonin rhythm varies widely among individuals, in part owing to genetic determinants.9a In fact, a small number of healthy persons have no detectable melatonin in plasma at any time of day.10
Melatonin has a bi-exponential half-life, with a first distribution T½ of 2 minutes and a second of 20 minutes. The hormone is lipophilic and enters tissues rapidly. Up to 70% of melatonin is bound to albumin in plasma.11 In addition to blood, saliva, and urine, melatonin is also found in cerebrospinal fluid (CSF), the anterior chamber of the eye, and in many reproductive fluids, including semen, amniotic fluid, and breast milk.12 Melatonin levels in plasma, CSF, saliva, and urine become undetectable following removal of the pineal gland, demonstrating that the pineal gland is the main source of melatonin in these compartments.13
Melatonin secretion varies across the life cycle. Secretion of the hormone begins in the fourth month of postnatal life and then increases rapidly, peaking between the ages of 1 and 3. Nocturnal melatonin secretion then starts a marked decline over each decade of life14 (Fig. 22-4). Nocturnal melatonin concentrations can also be affected by drugs that interfere with the transmission of neurotransmitter signals to the pineal gland. These drugs include β-blockers, caffeine, and ethanol.15–17 Nocturnal melatonin secretion is also suppressed by exposure to environmental lighting. Indeed, there is a dose-dependent and spectral-sensitive acute suppressive effect of light on melatonin.18,19 Even low light levels found indoors can suppress nocturnal melatonin production. In addition to light, exercise and postural changes decrease plasma melatonin levels.20,21
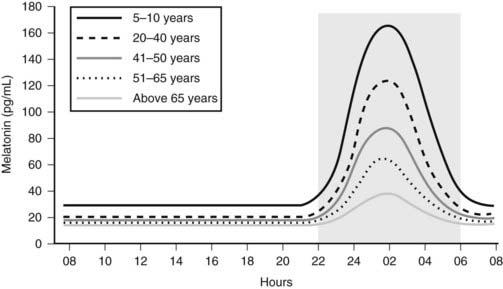
Figure 22-4. Circadian profile of serum melatonin across the life cycle; gray area indicates darkness.
Melatonin Receptors
In the mammal, there are at least two melatonin receptors, designated MT1 and MT2, which belong to the superfamily of G protein–coupled receptors containing the usual seven transmembrane domains. A third melatonin binding site, MT3, was found to be the enzyme quinone reductase 2.22 These receptors have distinct structures, chromosomal localization, and pharmacologic properties.23 The MT1 and MT2 receptors are differentially expressed across the nervous system. The highest density is found in the SCN, followed by the anterior pituitary and the retina.24 Studies have shown that the MT1 receptor in the SCN allows melatonin to inhibit firing of SCN neurons during the nighttime.24 The SCN MT2 receptor possibly mediates the effect of melatonin on SCN circadian rhythms.24 Both receptors are co-localized within the SCN and are coupled to multiple signaling pathways, with suppression of cyclic AMP production through a Gi-dependent process being the most common.23 The receptors can also stimulate phospholipase C, affecting various ion channels, as well as stimulating the mitogen-activated protein kinase cascade and the estrogen-dependent signaling cascade.25 Melatonin is a small, lipophilic compound which easily crosses membranes. In this regard, melatonin has been shown to bind to specific nuclear receptors of the retinoic acid receptor family26,27 and to calmodulin. Melatonin receptors have also been identified in the heart, kidney, liver, and many other peripheral tissues.28,29
The MT1 and MT2 receptors have different profiles for receptor desensitization. Exposure of the MT1 receptor to supraphysiologic concentrations of melatonin causes an increase in MT1 receptor density, with a concurrent decrease in affinity and functional sensitivity.24 In contrast, exposure of the MT2 receptor to physiologic concentrations of melatonin can induce a concentration and time-dependent receptor desensitization and internalization.30 The interplay between desensitization of the MT1 and MT2 receptors to daytime and nighttime physiologic concentrations of melatonin may promote changes in melatonin receptor function throughout the human circadian cycle. With the SCN ultimately controlling the production of melatonin, a feedback loop is created that may use melatonin as the regulatory control of SCN firing and phase-shifting functions.31 Desensitization of MT1 and MT2 receptors needs to be taken into account when administering melatonin to patients.
Melatonin Metabolism
Melatonin in the circulation is mainly inactivated by the liver’s cytochrome P450-specific mono-oxygenase, which first hydroxylates position C6 and then conjugates the product to a sulfate excreted into urine and feces as 6-sulfatooxymelatonin.32 The metabolism in nonhepatic tissue is different and is mainly through deacetylation. Melatonin can also be nonenzymatically metabolized in all cells and also extracellularly by free radicals.
Approximately 2% of circulating melatonin is excreted unchanged into urine and saliva. These body fluids can be assayed for melatonin.
Actions of Endogenous and Exogenous Melatonin
Seasonal and circadian rhythms serve an essential function in all living organisms. In conjunction with the SCN, melatonin signals the time of day and time of year to cells throughout the body and in so doing, modulates seasonal and circadian rhythms.
Seasonal variations in day length have reciprocal effects on melatonin secretion and can thus differentially affect plasma melatonin levels, depending on the location of the human population. For example, individuals living at high northern latitudes have a lengthening of melatonin secretion during winter nights.33 This may help explain how changes in the seasons affect reproductive vitality. In the laboratory setting, artificial shorting or lengthening of “night” will also adjust the duration of melatonin secretion.34
Circadian rhythms are biological processes that have a 24-hour periodicity even in the absence of external cues. Melatonin is involved in regulating several circadian cycles, including core body temperature and the sleep/wake cycle. The temporal organization of human circadian rhythms has been assigned the following terms: “biological day,” “biological night,” “biological dawn,” and “biological dusk.”35 During this 24-hour cycle, rising melatonin levels are associated with decreasing core body temperature, cortisol levels, and alertness. As melatonin levels wane, core body temperature, REM sleep propensity, and cortisol levels increase. It remains unclear whether there is a causal relationship between melatonin and these changes in the sleep/wake cycle. However, exogenously administered melatonin can phase shift body temperature, sleep timing, and the endogenous melatonin cycle.32 The ability of melatonin to phase shift circadian cycles has been exploited in the treatment of jet lag and shift-work disorders (see below).
SLEEP
The evening rise in melatonin precedes bedtime by about 2 hours and suggests a causal relationship. Indeed, the peak in plasma melatonin occurs when alertness is at its nadir. However, the sleep/wake cycle is regulated by both homeostatic and circadian processes and is only partially influenced by melatonin. The Smith-Magenis syndrome illustrates the association between melatonin and the sleep/wake cycle. The manifestations of this disorder include excessive daytime sleepiness and insomnia at night. Treatment of these patients with a β-adrenergic blocker decreases daytime melatonin levels and diminishes daytime sleepiness.36 Administration of physiologic and pharmacologic doses of melatonin to normal sleepers induces soporific symptoms. However, evidence of a direct relationship between endogenous melatonin and insomnia has been mixed.37,38 The number of reports that have measured plasma melatonin concentrations in sleep disorders is surprisingly low, considering its use as sleep-promoting agent for insomnia. Sleep disturbances have been described in some but not all pinealectomized patients, and administration of melatonin has sometimes been found to be efficacious in this setting.39
Although the exact role of endogenous melatonin in regulating human sleep remains to be fully elucidated, exogenous melatonin administration (0.1 to 0.3 mg) resulting in physiologic levels of melatonin will promote the onset and maintenance of sleep.32 Both a 1997 and a 2005 meta-analysis showed that melatonin administration decreases sleep latency, increases sleep efficiency, and increases total sleep duration in patients with primary insomnia.40,41 However, a meta-analysis published in 2006 which included patients with secondary causes of insomnia produced less clear-cut results.42
The sleep-promoting effect of melatonin has been linked to the inhibition of neuronal activity through activation of SCN MT1 receptors.43 A synthetic melatonin agonist, Ramelteon, which selectively activates MT1 and MT2 melatonin receptors, has been approved for the treatment of insomnia. Ramelteon shows 10-fold higher binding affinity for the MT1 compared to the MT2 receptor and 17 times higher affinity than melatonin for either receptor. The drug shows no affinity for a large number of other G protein–coupled receptors, enzymes, and neurotransmitter channels. In addition to Ramelteon, there are several other melatonin agonists under development.24
JET LAG
Jet lag is a syndrome associated with transmeridian travel resulting in disturbances to circadian rhythms. Manifestations of jet lag include problems sleeping, malaise, fatigue, and reduced performance. A similar syndrome may result from shift work and rarely from the transition to daylight savings time. Eastbound travel is generally associated with worse symptoms than westbound travel. The severity of jet lag is also proportional to the number of time zones crossed. Left untreated, the relationship between endogenous rhythms and environmental cues will come back into synchrony over several days to a week. The ability of melatonin to speed up the process of resynchronization accounts for its efficacy in the treatment of jet lag. Phase shifting of the neuronal firing rhythms has been linked to activation of the MT2 receptor at dusk, although MT1 may also be involved.44
For air travelers crossing five or more time zones going in the eastward direction, melatonin has been found to be effective in treating jet lag when administered close to the target bedtime at the travel destination.45 Doses from 0.5 to 5 mg are found to be effective. Treatment should begin the first evening upon arrival to the destination. Side effects of melatonin in this setting include daytime sleepiness, especially with the higher doses, dizziness, headaches, and loss of appetite.45 In addition to melatonin administration, proper nutrition, going to sleep according to the new time zone, exercising, and maximizing light exposure when awake can help alleviate jet lag symptoms.
Similar to individuals with jet lag, shift workers often have symptoms of fatigue, sleep disturbances, and gastrointestinal problems.46 Studies have observed marked variability in circadian melatonin profile in these workers.47 When administered at the desired bedtime during a night shift, melatonin seems to improve sleep and increase daytime alertness.46
Disorders in the sleep/wake cycle, core body temperature, and cortisol secretion are very common in blind people with no significant light perception.48 Many blind individuals have abnormal melatonin or 6-sulfatoxymelatonin circadian profiles.49,50 In these settings, melatonin has proven effective in phase-shifting the circadian clock and in so doing, stabilizing sleep onset and sometimes improving quality and duration of sleep.49,51
REPRODUCTION
In seasonal-breeding mammals, melatonin is involved in regulating the breeding cycle.52 The effects of melatonin on the reproductive system are generally antigonadotropic. Melatonin inhibits secretion of the gonadotropin hormones LH and FSH. This inhibitory affect is most likely due to inhibition of GnRH from the hypothalamus.53 For seasonal-breeding animals, seasonal changes in day length and thus melatonin secretion will up-regulate or down-regulate the gonadal axis. For example, during the shorter period of daylight that accompanies the nonbreeding season, melatonin levels are increased and down-regulate the gonadal axis. As the breeding season approaches and length of daylight increases, melatonin levels fall, allowing rejuvenation of the gonads.
Humans are not seasonal breeders, but melatonin may mediate the moderate seasonal fluctuations observed in human reproductive function.3 The increased conception rate seen in northern latitudes during the summer season has been reported to be caused by changes in gonadotropin induced by changes in melatonin secretion. Melatonin levels have been reported elevated in male infertility54 and in men with hypogonadotropic hypogonadism.55,56 Moreover, elevated melatonin levels have been reported in women with stress- and exercise-induced amenorrhea and in men with infertility.57 Exogenous melatonin administration has been shown to suppress LH levels in men and women and to reduce sperm motility in men.58
Melatonin has also been implicated in sexual maturation, where it may have inhibitory action on the onset of puberty. It has been proposed that a decline of melatonin below a threshold value may be a signal that activates GnRH pulsation in early puberty.3 Circumstantial evidence that melatonin is involved in sexual maturation includes the observation that children with precocious puberty have lower plasma melatonin levels, whereas children with delayed puberty exhibit higher nocturnal melatonin concentrations. However, a causal relationship between the pineal gland and human reproductive function is far from clear.
TEMPERATURE
Body temperature and plasma melatonin levels have a reciprocal profile; the 24-hour temperature nadir correlates with peak plasma melatonin levels.59 It has been estimated that approximately half of the nighttime decline in core temperature is induced by melatonin.60 Supporting a causal relationship between melatonin and body temperature are studies showing that exogenously administered melatonin will decrease body temperature in humans.61
BLOOD PRESSURE
There is circumstantial evidence that melatonin may be involved in the regulation of blood pressure. During the night, humans have lower blood pressure, heart rate, and cardiac output which are all associated with increases in plasma melatonin levels. There is an increased risk of myocardial infarction and stroke in the early morning when melatonin levels are low. Moreover, patients with coronary heart disease have lower melatonin levels and higher norepinephrine levels compared to persons without cardiac disease. It is known that the nocturnal surge in melatonin is blunted in patients with hypertension, especially nocturnal hypertension.62 To this end, studies have shown that melatonin (~2 mg) can lower nighttime blood pressure.63
IMMUNE SYSTEM AND CANCER
In animal models, melatonin has immunoenhancing properties and can reverse the immunosuppressive effects of acute stress, cancer drug therapy, and viral infections.64 Surgical and functional pinealectomy has been shown to reduce thymic and splenic weights in mice, rats, and hamsters corresponding to a reduction in B and T cells.65 Pinealectomy alters the activity of thymic enzymes and in the newborn rodent is associated with disorganization of thymic structures. In the rodent, neonatal pinealectomy also impairs immune function. Conversely, the administration of melatonin to rodents increases thymic and splenic weight, provides protection against the catabolic effects of dexamethasone on body weight and atrophy of the thymus and adrenal glands, and enhances immune responses to certain infectious agents.66 Melatonin administration increases the number of natural killer (NK) cells and monocytes in bone marrow and increases helper T-cell activity and interleukin-2 (IL-2) production in human lymphocytes.66 In general, these observations show that melatonin’s stimulatory effects on the immune system are best observed in states of immunosuppression. The role of pineal-derived and immune cell–derived melatonin on human immunity is not clear.
Melatonin is also reported to have oncostatic actions.3 Pinealectomized rats and hamsters have accelerated growth in a variety of transplanted cancers. Although chronic melatonin administration has oncostatic effects in breast and ovarian cancer in rodent models, high-dose melatonin did not alter survival in human melanoma patients.67 However, co-administration of melatonin and interferon gamma improved tumor regression in patients with metastatic renal cell carcinoma.68 Melatonin has also been shown to protect the host from negative effects of IL-2 while synergizing with the anticancer action of the cytokine.69 Much more information is needed to prove that melatonin will serve as a useful oncostatic medication in humans.
ANTIOXIDANT AND ANTI-AGING
At supraphysiologic concentrations, melatonin has antioxidant properties. Melatonin’s free-radical scavenging properties are due in part to its ability to modulate catalase, superoxide dismutase, and glutathione peroxidase. Compared to two well-known scavengers, glutathione and mannitol, melatonin is 4 times and 14 times more effective, respectively.70 The small molecule is both lipophilic and hydrophilic and easily enters the cytosol and nucleus, thus making it a potentially efficient antioxidant.71 However, the question still remains whether in physiologic concentrations, melatonin is an efficient free-radical scavenger. In a meta-analysis of three trials of melatonin treatment for the cognitive impairment associated with dementia, no evidence of benefit was seen.72
PSYCHIATRIC DISORDERS
Altered melatonin rhythms and levels have been observed in depression, mania, schizophrenia, anorexia nervosa, and other psychiatric disorders.73 However, there is no compelling evidence that melatonin contributes to the symptomatology associated with these psychiatric disorders.
Lesions of the Pineal Gland and Pineal Region
The pineal region can give rise to cysts and many types of neoplastic masses, including pineal cell tumors, germ cell tumors, gliomas, meningiomas, and metastases. Early detection and appropriate treatment of the neoplastic syndromes maximizes chances for better outcomes.
PINEAL GLAND CYSTS
A pineal cyst is a benign lesion lined by normal pineal parenchymal and glial cells. It is not uncommon for the endocrinologist to order a magnetic resonance imaging (MRI) scan of the brain during the evaluation of a pituitary disorder and also find an incidental pineal cyst. Indeed, the asymptomatic pineal cyst is a common neuroimaging finding seen on approximately 4% of MRI scans and observed in up to 40% of routine autopsies.74 The main importance in identifying a pineal cyst is to distinguish it from cystic tumors that also occur in this region and require treatment.
On computerized tomography (CT) images, pineal cysts are hypodense; occasionally there is evidence of hemorrhage. Cyst walls may or may not show contrast enhancement, and calcifications within the wall are found in about half of cases. On MRI images, the pineal cyst is round and smooth without intracystic trabeculations. It has low signal intensity on both T1WI and T2WI.75 Occasionally the imaging appearance of a benign pineal cyst is indistinguishable from small cystic neoplasms. For example, similar to pineal cysts, pineocytomas may be isointense with respect to CSF, but the latter usually have intratumoral trabeculations.
Pathologic analysis occasionally misdiagnoses benign pineal gland cysts as pineocytoma or pilocytic astrocytoma. This is an important error to avoid, since misdiagnosis could lead to inappropriate therapies. Errors are more likely to be made when small specimens were obtained at biopsy, making it difficult to determine whether the pineal tissue is normal or neoplastic.
Most pineal cysts are small, and patients are asymptomatic. Some cysts spontaneously resolve. One study observed that in 32 patients with pineal cysts, 75% remained stable over time,16% decreased in size or resolved, whereas only 8% increased in size.76 Small, asymptomatic cysts require no intervention.
Rarely, a pineal cyst becomes symptomatic.77 Symptomatic cysts tend to be larger than their asymptomatic counterparts. Although symptomatic pineal cysts have been reported in patients from 7 to 69 years of age, most occur be between 21 and 30 years of age. There is a 3:1 female predominance. Patients generally present with headaches as a result of acute hydrocephalus. A high degree of clinical suspicion is needed, because headache may be the only symptom. Headache may have either a prolonged intermittent or acute course.
The symptomatic patient is generally treated with either an open procedure or endoscopic stereotactic resection. Either procedure can cure the patient and eliminate the need for CSF shunting. Both procedures allow for both histologic sampling and relief of symptoms. Some surgeons prefer the stereotactic technique because morbidity is lower compared to an open procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree