FIGURE 44-1. The metabolic syndrome, current view. A combination of overnutrition, inactivity, and genetic factors interact to produce a state of metabolic dysregulation that leads to insulin resistance, hyperinsulinemia, and a proinflammatory state. In individuals who are genetically predisposed, this in turn leads to one or more of the indicated disorders and often others. Nearly all of these disorders are associated with an increased risk of coronary heart disease. A considerable body of evidence suggests that the metabolic dysregulation involves cellular lipid metabolism and may be mediated by abnormalities in the regulation of the fuel-sensing enzyme AMPK. NAFLD/NASH, Non-alcohol fatty liver disease/non-alcoholic steatohepatitis; PCOS, polycystic ovary syndrome.
From a clinical perspective, the importance of the metabolic syndrome is attributable principally to three factors: (1) It antedates the multiple disorders with which it is associated, (2) it may be a target for the prevention and therapy of these disorders, and (3) it is extremely common. Based on recent adult treatment panel (ATP III) diagnostic guidelines15 (see following discussion), it has been estimated in the population evaluated in the National Health and Nutrition Survey (NHANES) study that upwards of 50 million people in the United States above the age of 20 have the metabolic syndrome, including 40% of all individuals above the age of 60 (Fig. 44-2).16 Likewise, a high prevalence of the metabolic syndrome has been found in many other countries,1,17 and its prevalence is increasing in adolescents and children in parallel with the pandemic of obesity in this population.18–20 This chapter will update the reader on the present status of the metabolic syndrome (formally referred to as syndrome X or the insulin resistance syndrome).14,21 Emphasis will be placed on describing novel mechanisms and factors that are transforming our previous conceptions about its pathogenesis and treatment. In addition, proposed guidelines for the diagnosis of the metabolic syndrome will be discussed, as will the special problems that need to be considered when attempting to treat and/or prevent it. For other perspectives, the reader is referred to several excellent recent reviews.15,22–24
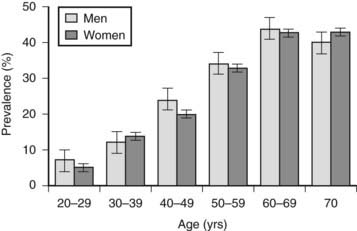
FIGURE 44-2. Prevalence of the metabolic syndrome in the United States according to age. Based on National Health and Nutrition Examination Survey (NHANES) data and ATP III criteria (see Table 44-3).
(Data from Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among U.S. adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287:356–359, 2002.)
History
The clustering of the major components of the metabolic syndrome, such as obesity, type 2 diabetes, hypertension, and dyslipidemia, has long been recognized25; however; its delineation as a distinct entity took place only after its linkage to insulin resistance, hyperinsulinemia, and cardiovascular disease became more apparent.21,26,27 Insulin resistance has been defined as a state (of a cell, tissue, system, or body) in which greater than normal amounts of insulin are required to elicit a normal biological response.28 In humans, it is currently diagnosed on the basis of high levels of plasma insulin, either fasting or during a glucose tolerance test, or by a decreased rate of glucose infusion during a euglycemic-hyperinsulinemic clamp.8 The much greater prevalence of insulin resistance in patients with type 2 rather than type 1 diabetes was appreciated over 60 years ago, based on their substantially higher insulin requirement29,30 and diminished response to exogenous insulin.31 Shortly after the development of the insulin immunoassay by Yalow and Berson,32 this suspicion was confirmed,33,34 and other disorders associated with insulin resistance and hyperinsulinemia were identified, including coronary heart disease and several of its risk factors,21,35–40 as well as obesity itself (see Fig. 44-1). In general, most adults with insulin resistance and hyperinsulinemia are obese (BMI > 29) or overweight (BMI 25 to 29). However, a significant percentage are normal weight by BMI but show increases in abdominal fat (central obesity)4,8 and in intrahepatic and intramyocellular triglyceride41 and/or enlarged fat cells.5,42 The presence of central obesity has been shown to correlate strongly with an individual’s predisposition to most of the diseases indicated in Fig. 44-1, including coronary heart disease,43,44 and this is the reason waist circumference is one of the diagnostic criteria for the metabolic syndrome, as will be discussed later.
Hyperinsulinemia and insulin resistance are also present in normal-weight offspring of people with type 2 diabetes,7,45–47 hypertension,48 and hypertriglyceridemia8,49,50 and in individuals at increased risk for coronary heart disease,51–54 suggesting that they are early markers or pathogenetic factors for these disorders. Studies such as these, plus the presence of a high rate of ischemic heart disease in patients with type 2 diabetes at the time of diagnosis (20% to 50%)55–57 and to a somewhat lesser extent individuals with impaired glucose tolerance,58 have led to the suggestion that treatment of the metabolic syndrome at an early stage may be needed for preventing coronary heart disease.8,22,39,59,60
Pathophysiology
INSULIN RESISTANCE AND COMPENSATORY HYPERINSULINEMIA
The presence of insulin resistance in otherwise normal offspring of people with type 2 diabetes, hypertriglyceridemia, and hypertension has led to the notion that it is a causal factor for the metabolic syndrome or an early pathogenetic event.21 According to this widely held view, insulin resistance affects a number of organs (e.g., muscle, liver, adipose tissue), and hyperinsulinemia due to increased insulin secretion by the pancreatic β cell and decreased insulin degradation by the liver is a compensatory phenomenon.14,21,35 The observation that therapies that increase insulin sensitivity and lower plasma insulin levels, such as lifestyle modification (diet and exercise)61–63 and treatment with metformin61 or thiazolidinediones,64–65 prevent or delay the onset of diabetes in individuals with glucose intolerance is compatible with this notion, as is the efficacy of these therapies in some people with other disorders associated with the metabolic syndrome, such as nonalcoholic fatty liver disease66,67 and the polycystic ovary syndrome.69,70 Left unexplained by this hypothesis, however, is the molecular mechanism by which insulin resistance develops initially and how it leads to hyperinsulinemia. Also, the possibility that hyperinsulinemia is the more primary of the two events or occurs simultaneously with the insulin resistance has not been ruled out.70
THE INSULIN SIGNALING CASCADE
Hypothetically, the metabolic syndrome could be related to genetic abnormalities in the insulin signaling cascade. In keeping with this possibility, mutations of IRS1 and IRS2, the initial targets of the insulin receptor tyrosine kinase, have been shown to lead to insulin resistance and diabetes in transgenic mice.71,72 Evidence that these or other genetic defects in the insulin signaling cascade are common in humans with the metabolic syndrome or type 2 diabetes and account for observed signaling defects73 is still lacking, however.
THE LIPID THEORY
Insulin resistance and hyperinsulinemia in humans and experimental animals have been linked to obesity and dysregulation of cellular lipid metabolism in a wide variety of circumstances.73–76 Early studies focused on free fatty acids (FFA) released from adipose tissue and assumed that insulin resistance in skeletal muscle was in some way the result of elevated plasma FFA levels. More recently, it has become apparent that insulin resistance is associated with alterations in lipid metabolism in tissues other than skeletal muscle, and that its appearance is affected by a number of newly discovered hormones and intracellular regulatory mechanisms. In addition, it has been demonstrated that the metabolic syndrome occurs in people who lack adipose tissue as well as in those with excess adiposity, and that in both groups, it is associated with triglyceride deposition in ectopic sites such as muscle, liver, and visceral fat. In this section, we will attempt to review the current status of this increasingly complex but intriguing area. Three distinct but often interrelated mechanisms that have been put forth to explain the link between altered lipid metabolism and components of the metabolic syndrome will be discussed.
Excess Free Fatty Acids
Over 40 years ago, Randle and his co-workers77 first demonstrated that elevated circulating free fatty acid levels diminish insulin-stimulated glucose utilization by a perfused rat heart preparation. They presented evidence that this effect occurred within minutes, and that it was associated with enhanced mitochondrial fat oxidation that led to both inhibition of glucose oxidation at the pyruvate dehydrogenase step and an increase in the cytosolic concentration of citrate. They also demonstrated that the increase in citrate inhibited glycolysis at phosphofructokinase, and that the secondary increase in glucose 6-phosphate inhibited hexokinase and secondarily diminished insulin-stimulated glucose uptake78 (Fig. 44-3). It was suggested that a similar mechanism might account for the insulin resistance observed in humans with obesity or type 2 diabetes, in both of whom plasma FFA levels were known to be elevated.79 Over the next 25 years, most investigators were unable to reproduce these findings in skeletal muscle, however,80,81 and for this reason the contribution of elevated plasma FFA levels to the insulin resistance observed in most humans with obesity, type 2 diabetes, and other metabolic syndrome–associated disorders remained unclear.
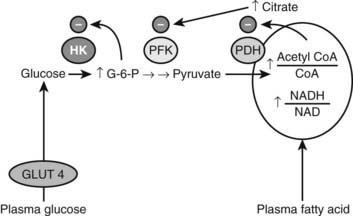
FIGURE 44-3. Inhibition of glucose uptake and oxidation by fatty acids as described in heart muscle by Randle, Garland, Hales, and Newsholme (1964, 1965). See text for details.
(Adapted from Shulman GI: Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176, 2000.)
In 1991, Boden et al.82 demonstrated that raising plasma FFA (by infusing a lipid emulsion with heparin to activate lipoprotein lipase activity) in humans during a euglycemic-hyperinsulinemic clamp inhibits insulin-stimulated glucose uptake and its incorporation into glycogen by leg muscle. Importantly, they found that this effect required 4 to 6 hours and not minutes to become evident, and that it was not accompanied by an increase in citrate. Subsequent investigations by Shulman’s laboratory in which 31P magnetic resonance spectroscopy (MRS) was used to measure intracellular glucose-6-phosphate noninvasively found that its concentration was reduced, suggesting that free fatty acids principally inhibit insulin-stimulated glucose transport or phosphorylation activity83 and not the phosphofructokinase reaction, as suggested by Randle. More recently, studies by the same group, in which 13C MRS was used to assess intracellular free glucose concentrations, revealed that insulin-stimulated glucose transport and not phosphorylation was the step inhibited by high fatty acids.84
Other studies have demonstrated that insulin resistance in human muscle, caused by infusing lipids to increase plasma FA, is associated with impaired insulin signaling,73 increases in the concentrations of muscle triglyceride, long-chain fatty acyl CoA85 and diacylglycerol, and increases in protein kinase C (PKC) activity and the translocation of various PKCs from the cytosol to a membrane fraction.76,86–88 Another finding was a decrease in IKBα abundance, suggesting activation of NF-κB and proinflammatory events.88 As will be discussed later, similar abnormalities have been found in rodent liver following a sustained exposure to fatty acids,89–92 in rodent muscle in a wide variety of states associated with insulin resistance,76,86,87 and in liver and muscle of massively obese, insulin-resistant humans with type 2 diabetes.93,94 Thus the intracellular changes produced by an excess of free fatty acids are associated with insulin resistance in many tissues.
A still unanswered question is whether an increase in plasma FFA is an early pathogenetic event in the metabolic syndrome. Elevated concentrations of plasma FFA, attributable to increased adipose tissue mass, and the relative insensitivity of large fat cells and visceral fat to insulin35,85 are present in people with obesity and type 2 diabetes, and they appear to contribute to insulin resistance when these disorders are established.74,85,95,96 On the other hand, even some massively obese individuals remain insulin sensitive, suggesting that other factors are involved97 (see discussion in Adipose Tissue). Also unclear is whether plasma FFA are elevated in individuals with the metabolic syndrome in its very early stages. Only modest increases in plasma FFA, if any, have been observed in normal-weight, insulin-resistant individuals who are at risk for developing diabetes because of family history.45,98
Altered Fatty Acid Metabolism: Malonyl Coenzyme A, Mitochondria, and Adenosine Monophosphate–Activated Protein Kinase
A second abnormality in lipid metabolism that could lead to insulin resistance is a disturbance in fatty acid metabolism in which the oxidation of cytosolic long-chain fatty acyl CoA (FACoA) by mitochondria is impaired and its esterification and metabolism by other non-mitochondrial processes is enhanced.76,99 This could occur if either the intrinsic ability of mitochondria to oxidize fatty acid is decreased or the activity of carnitine palmitoyl transferase 1, the enzyme that regulates the transfer of cytosolic long-chain fatty acyl CoA into mitochondria, is diminished.
Malonyl CoA
Altered fatty acid partitioning between the mitochondria and cytoplasm as a cause of insulin resistance was first suggested by studies in denervated rat muscle, in which enhanced diacylglycerol (DAG) synthesis and PKC activation were observed,100 and later by similar findings in muscle of obese, insulin-resistant KKAy mice.101 In these and other instances, insulin resistance in rodent muscle correlated with an increase in the concentration of malonyl CoA,76 an allosteric inhibitor of carnitine palmitoyltransferase. As shown in Figure 44-4, an increase in malonyl CoA by decreasing the oxidation of cytosolic FACoA would increase its availability for the formation of DAG, triglycerides, ceramide, and possibly other factors linked to insulin resistance. In keeping with such a mechanism, it has been demonstrated by McGarry99,102 that treatment with etomoxir, a pharmacologic CPT1 inhibitor, concurrently increases triglyceride accumulation and causes insulin resistance in rat skeletal muscle. Also supporting this notion are a number of other findings, including the following: mice lacking functional acetyl CoA carboxylase 2 (ACC2, the isoform that generates the malonyl CoA that regulates CPT1) are more insulin sensitive than control rats103,104; the administration of an ACC inhibitor diminishes obesity and insulin resistance in fat-fed rats105; and antisense oligonucleotides directed at hepatic ACC1 and ACC2 diminish both hepatic steatosis and insulin resistance in mice fed a high-fat diet.106 Finally, low rates of fatty acid oxidation have been reported in pre-obese humans,107,108 Zucker diabetic rats,109 and interleukin-6 (IL-6) knockout mice110 prior to the onset of diabetes and obesity. Malonyl CoA was not assayed in any of these studies; however, in the two rodent models, a decrease in the activity of AMP-activated protein kinase (AMPK) was found, suggesting that its concentration was elevated111,112 (see section on AMPK).
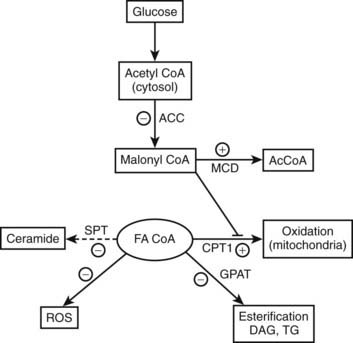
FIGURE 44-4. AMPK concurrently regulates multiple aspects of fatty acid metabolism. By phosphorylating and inhibiting ACC and activating MCD, AMPK diminishes the concentration of malonyl CoA. This relieves the inhibition of CPT1 by malonyl CoA and results in both increased fatty acid oxidation and a decrease in the availability of cytosolic FACoA for TG, DAG, and ceramide synthesis, and possibly lipid peroxidation and protein acylation. Conversely, AMPK activation independently decreases the expression of GPAT, fatty acid synthase (not shown), and SPT, decreasing respectively the synthesis of glycerolipids, fatty acids (de novo), and ceramide (de novo). A decrease in AMPK produces the opposite effects. The basis for the ability of AMPK to inhibit oxidant stress (ROS generation) in some settings is not known. Whether AMPK activation enhances or inhibits a process or an enzyme in this scheme is denoted by plus and minus signs, respectively. Not shown in the diagram is that AMPK achieves these changes in some instances by phosphorylating the indicated enzyme and/or in other instances by regulating its expression. In addition, AMPK can enhance mitochondrial biogenesis and function by enhancing the expression of the transcriptional coactivator PGC1α. ACC, Acetyl CoA carboxylase; CPT1, carnitine palmitoyltransferase 1; DAG, diacylglycerol; FA CoA, cytosolic long chain fatty acyl CoA; GPAT, glycerophosphate acyltransferase; MCD, malonyl CoA decarboxylase; ROS, reactive O2 species.
(Adapted from Ruderman N, Prentki M: AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3:340–351, 2004.)
Mitochondrial Dysfunction
Altered fatty acid partitioning in muscle and other tissues could also occur if fatty acid oxidation is depressed as a consequence of mitochondrial dysfunction. Decreases in mitochondrial function, and in some instances number and size, have been found in muscle of individuals with type 2 diabetes associated with obesity,113 in lean insulin-resistant elderly individuals,114 and in lean insulin-resistant offspring of diabetic parents.7 In the latter case, the reduction in mitochondrial function could be attributed to a similar reduction in mitochondrial content.115 Likewise, decreases in the mRNA for PGC1α, a transcriptional coactivator that enhances genes for mitochondrial biogenesis and function, have been observed in some116 but not all115 studies of nondiabetic first-degree relatives of patients with type 2 diabetes, as well as in individuals with type 2 diabetes and impaired glucose tolerance.117 Whether these mitochondrial changes are hereditary or secondary to metabolic events (e.g., lipotoxic changes due to abnormalities in intracellular lipid metabolism) or abnormalities in AMPK regulation (see next section) remains to be determined. However, given the key role for increased intracellular fatty acid metabolites in mediating insulin resistance, decreased mitochondrial function leading to decreased fatty acid oxidation will certainly exacerbate the problem. Also to be determined is whether the changes observed in the offspring of diabetic parents reflect a difference in muscle fiber type, since the ratio of mitochondrial-rich type 1 fibers to glycolytic type 2 fibers may be decreased in these individuals.7,118
AMP-Activated Protein Kinase
AMPK (Box 44-1) is a fuel-sensing enzyme that appears to play a key role in regulating both cellular metabolism and mitochondrial function. In addition, an increasing body of evidence has suggested that its dysregulation could be a cause of the metabolic syndrome (animal studies) as well as a target for its prevention and therapy (human and animals studies).3,24,75
Box 44-1. AMP-ACTIVATED PROTEIN KINASE (AMPK)
AMPK is a heterotrimer containing α, β, and γ subunits, each of which has at least two isoforms. The α subunit contains the catalytic site; the β-subunit, a glycogen binding domain; and the γ subunit, two AMP-binding sites. All three subunits are necessary for full activity.270,271 In general, AMPK is found in the cytosol of a cell; however, the α2 isoform of the enzyme is also present in the nucleus.271 Decreases in the energy state of a cell, as reflected by increases in the ratio of AMP/ATP, activate AMPK by a number of mechanisms, including allosteric activation and especially covalent modification due to phosphorylation of its catalytic subunit on Thr-172 as a result of the actions of the AMPK kinase LKB1 and the inhibition of the ability of protein phosphatase 2C to dephosphorylate this site.272,273 When activated, AMPK activates a number of processes that increase ATP generation including fatty acid oxidation and glucose transport (in skeletal muscle) and it decreases others that consume ATP, but are not acutely necessary for survival, such as fatty acid and triglyceride synthesis. In addition, AMPK can alter the expression of a wide variety of genes including several that alter mitochondrial function (e.g., PGC1α, UCP3) and lipid synthesis (SREBPIC). The effects of AMPK activation that could account for its ability to diminish lipid accumulation, cell dysfunction and insulin resistance are depicted in Figs. 44-3 and 44-4. Some of the factors that activate it in vivo are listed in Table 44-2.
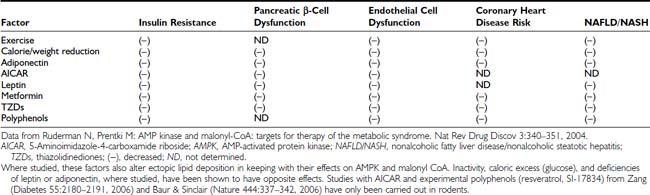
As shown in Fig. 44-4, when AMPK is activated (e.g., during exercise or in some tissues by caloric deprivation), it phosphorylates and inhibits acetyl CoA carboxylase (ACC), the enzyme that catalyzes the synthesis of malonyl CoA. By a still undetermined mechanism, it activates malonyl CoA decarboxylase, an enzyme that catalyzes malonyl CoA degradation.119 In addition, AMPK concurrently inhibits the use of cytosolic FACoA for diacylglycerol, triglyceride, and ceramide synthesis, and at least in endothelial cells120 and adipocytes (see Adipose Tissue), it diminishes the generation of lipid peroxides and the activation of NF-κB caused by elevated concentrations of fatty acids.75,120,121(and unpublished data) Thus AMPK appears to protect cells against lipotoxicity and to do so by multiple actions. In addition to modulating these events acutely, AMPK chronically regulates the effects of transcriptional regulators (e.g., SREBP1C) that govern the synthesis of acetyl CoA carboxylase and other key enzymes. Of specific relevance to this chapter, decreases in AMPK activity and increases in malonyl CoA are both associated with insulin resistance in muscle (Table 44-1) and liver in many situations, and failure to activate AMPK in the fat cell during lipolysis is associated with increases in oxidative stress and inflammation.121(and unpublished observations) In addition, hormones and other factors that decrease AMPK in peripheral tissues, such as glucocorticoids,122 TNF-α,123 and resistin,124 cause insulin resistance. Conversely, numerous endogenous hormones (e.g., adiponectin and leptin) and pharmacologic agents (e.g., metformin, thiazolidinediones, α-lipoic acid) that diminish insulin resistance have been demonstrated to activate AMPK and diminish the concentration of malonyl CoA in experimental animals, as do exercise and treatment with the AMPK activator AICAR (5-aminoimidazole-4-carboxamide riboside)75 (Table 44-2).
Table 44-2. Effect of Therapies That Activate AMPK in Humans and/or Rodents on Various Manifestations of the Metabolic Syndrome
Closely linked to mitochondrial theories of insulin resistance is PGC1α (PPARγ-coactivator 1α), a transcriptional coactivator whose expression is increased when AMPK is activated (e.g., by exercise or AICAR).125–128 In skeletal muscle, and likely in other tissues, PGC1α regulates mitochondrial biogenesis and the expression of multiple genes governing oxidative phosphorylation, including those for citric acid cycle enzymes and electron transport proteins.116,117,129 PGC1α polymorphisms have been reported in populations with type 2 diabetes in Denmark130 and Japan.131 Also, modest but coordinated decreases in the expression of many PGC1α-mediated genes have been reported in muscle of overweight/obese middle-aged individuals with type 2 diabetes or impaired glucose tolerance.116,117 A decreased capacity for exercise (VO2 max) was also observed in these subjects, in keeping with the findings of earlier studies in similar patient groups.118,132–134
In keeping with its effect on PGC1α, it has been shown that AMPK is necessary for mitochondrial biogenesis.128 Whether the various hormones (e.g., adiponectin, leptin, catecholamines) and pharmacologic agents (thiazolidinediones, metformin) reported to activate AMPK in peripheral tissues also increase PGC1α expression and stimulate mitochondrial biogenesis and enzyme activity in skeletal muscle and other tissues, or whether agents that decrease AMPK (e.g., endocannabinoids and glucocorticoids) have the opposite effect, are active areas for study. Also requiring study is the relative role of Ca2+ calmodulin–dependent protein kinase, which like AMPK is activated during exercise and increases PGC1α expression.135
Adipose Tissue
Overview
A number of lines of investigation have linked abnormalities in adipose tissue to the pathogenesis of the metabolic syndrome. First, as already noted, elevated plasma FFA levels, attributable to an increase in their release from adipocytes in obese or centrally obese individuals, correlate with the presence of insulin resistance in most patients.85,136 Second, when the function of the adipocyte as a store for lipid is impaired,137,138 as it is in many insulin-resistant obese individuals and people with various lipodystrophies (see later discussion), fatty acids are deposited as triglyceride in ectopic sites such as muscle, liver, and visceral fat, and this is associated with insulin resistance, inflammation, and other manifestations of cellular dysfunction (lipotoxicity).139 Recent studies suggest that markedly obese individuals with diminished levels of the lipid droplet proteins CIDE A and perilipin in adipose tissue have an impaired ability to deposit lipid in their adipocytes, a higher rate of lipolysis, and they are insulin resistant.97 In contrast, in comparably obese individuals with normal levels of these lipid droplet proteins, increased lipolysis and insulin resistance were not observed. Thus differences in the capacity of the adipocyte to store lipid may be a key determinant of whether obesity leads to the metabolic syndrome. Finally, the adipocyte can release hormones such as leptin and adiponectin that in multiple tissues activate AMPK, diminish ectopic lipid, and enhance insulin action. In addition, it can release proinflammatory cytokines such as TNF-α,140 resistin,124 and plasminogen activator inhibitor (PAI)12 that could cause insulin resistance.141 As already noted, diminished AMPK activity, which results in large increases in oxidative stress and possibly inflammatory changes in the adipocyte when lipolysis is stimulated, might contribute to the latter.121(and unpublished data)
Adiponectin
Of the various adipokines, adiponectin, also referred to as ACRP30, has been most closely linked to insulin resistance in humans. It is produced exclusively or at least predominantly in adipose tissue and is present in the circulation in trimeric, hexameric, and high-molecular-weight (HMW) forms. The biological relevance of the three oligomers142,143 and still other forms of adiponectin is incompletely understood. These considerations aside, there is abundant evidence that low immunoassayable adiponectin levels in plasma (accounted for mainly by the HMW form) are present in obese individuals and in individuals at risk for type 2 diabetes144,145 and coronary heart disease,146 even in the absence of overt obesity. In addition, polymorphisms of the adiponectin gene have been associated with the metabolic syndrome in some populations and a predisposition to type 2 diabetes in others.144
In keeping with these findings in humans, in mice fed a high-fat diet, genetically knocking out adiponectin causes glucose intolerance and insulin resistance147; and conversely, overexpression of full-length148 or globular149 adiponectin attenuates the severity of atherosclerosis in apoE knockout mice. In addition, the administration of full-length (HMW) adiponectin and the globular subunit have been shown respectively to diminish hepatic lipid accumulation in ob/ob mice with non-alcoholic fatty liver disease (NAFLD)150 and insulin resistance in fat-fed rats.151
Adiponectin, like exercise, activates AMPK and stimulates AMPK-mediated events such as glucose transport and fatty acid oxidation in muscle152,153 and inhibition of glucose production by liver.154 In addition, adiponectin has antiinflammatory actions.155 Whether the insulin-sensitizing effect of adiponectin is AMPK-mediated has not been proven definitively; however, treatment with thiazolidinediones increases plasma adiponectin, and the insulin-sensitizing effect of these agents and their ability to activate AMPK are both markedly attenuated in adiponectin knockout mice.156,157
Leptin
Since its discovery by Friedman and his co-workers,158 interest in leptin has for the most part focused on its role as an appetite suppressant. However, it has also been recognized for some time that leptin increases oxidative metabolism and fatty acid oxidation in peripheral tissues, owing both to a direct action159 and to an effect on the hypothalamus that appears to be mediated by the sympathetic nervous system.160 As first reported by Minokoshi and Kahn and their co-workers, both the direct and centrally-mediated effects of leptin on peripheral tissues are associated with AMPK activation,161 whereas its action on hypothalamic nuclei is associated with a decrease in AMPK activity.162
When leptin is lacking or its receptor is not functioning, lipid accumulates in many tissues, and cellular damage may result. Unger et al.163,164 have reported that in the Zucker diabetic fatty rat (ZDF), such ectopic lipid accumulation occurs in liver, muscle, and the pancreatic β cell, and that it antedates the presence of diabetes and pancreatic β-cell apoptosis. They coined the term lipotoxicity to describe this phenomenon and have proposed that a major action of leptin is to prevent the accumulation of ectopic lipid and related events (e.g., increased ceramide and oxidant stress) that cause lipotoxicity (apoptosis, mitochondrial dysfunction, inflammation, insulin resistance). Of particular note for this review, decreases in AMPK activity and an increased concentration of malonyl CoA have been found in tissues of the fa/fa and the ZDF rat, rodents with a functionally deficient leptin receptor, and the ob/ob mouse, which does not synthesize leptin.112 Furthermore, treatment with the AMPK activator, AICAR,112 as well as the TZD troglitazone165 and other means of AMPK activation including exercise,166 prevent the ectopic lipid accumulation, pancreatic β-cell damage, and the development of diabetes in the ZDF rat.
Vascular Endothelial Cells
An impressive case has been made that atherogenesis is essentially an inflammatory response to a variety of risk factors, and the consequences of this response include acute coronary and cerebrovascular syndromes.167 An early site at which this inflammatory response appears to occur is the endothelial cell168; indeed, increases in NF-κB expression have been observed in endothelium at sites predisposed to atherosclerotic plaque formation169 and in endothelial cells exposed to elevated concentrations of glucose170 and free fatty acids.171 Likewise, impaired endothelium-dependent relaxation and increases in circulating adhesion molecules (VCAM1, ICAM, selectins), markers of cellular dysfunction and incipient atherosclerotic vascular disease, have been observed in humans with type 2 diabetes and the metabolic syndrome172,173 and in normal individuals in whom plasma FFA levels are increased by a lipid infusion.174 Conversely, endothelial cell dysfunction is diminished in humans by factors that diminish the proinflammatory state, including exercise and caloric restriction175,176 and by treatment with thiazolidinediones.172,177 As already noted, all of these interventions have been reported to activate AMPK in rodents. Studies with endothelial cells in culture also support such a protective role for AMPK. Thus increases in oxidative stress and NF-κB-mediated gene expression observed in cultured endothelium (HUVEC) incubated with palmitate are inhibited by AICAR and other AMPK activators.120,178,179 Also, AICAR and (where studied) expression of a constitutively active AMPK have been shown to inhibit apoptosis, mitochondrial dysfunction, DAG synthesis, and the development of insulin resistance (diminished Akt activation) in HUVEC incubated in a high-glucose medium.180 Finally, it has recently been demonstrated that the administration of atorvastatin by gavage activates AMPK and eNOS in the rat aorta,181 and it has a similar effect when incubated with cultured endothelial cells (HUVEC). Whether this accounts for the anti-atherogenic effect of statins attributed to its antiinflammatory action is an intriguing possibility.182
Liver
Changes similar to those in muscle and the endothelial cell occur in the liver in insulin-resistant states. Thus, as in muscle, an association between hepatic lipid deposition and insulin resistance has been clearly demonstrated in humans.183,184 Also, in rats infused with a lipid emulsion to increase plasma FFA levels during a euglycemic-hyperinsulinemic clamp89,185 or with short-term fat feeding,91 the development of hepatic insulin resistance is associated with increases in DAG content, PKC activation, and a decrease in IKBα abundance—changes almost identical to those observed in human muscle.88 Similar alterations in PKC have been noted in the livers of massively obese, insulin-resistant humans93 and fat-fed rats with hepatic steatosis.91 Also, knockdown of PKCe expression by antisense oligonucleotides protects rats from fat-induced hepatic insulin resistance.186 Finally, as will be discussed later, the metabolic and inflammatory changes observed in the liver of patients with NAFLD/NASH closely resemble those attributable to lipotoxicity in other organs.75
Pancreatic β Cell
Insulin resistance in muscle and liver does not initially result in hyperglycemia because it is accompanied by hyperinsulinemia. As noted previously, it is unclear whether the hyperinsulinemia is compensatory or results from the same factors that cause insulin resistance, in which event it might occur at the same time or even precede it.70,187 In this context, it is noteworthy that increases in plasma FFA have been shown acutely to increase insulin secretion in certain settings, whereas chronic increases in the concentration of saturated fatty acids and glucose cause dysfunction and damage to the β cell and ultimately result in apoptosis.75,99,180,188 Work from a number of laboratories163,164,189,190 has both delineated the events that lead to these phenomena in the β cell and revealed their similarity to the events observed in endothelium and other cells when exposed to high concentrations of FFA or glucose. As already noted, in the ZDF rat, the leptin-receptor deficient rodent characterized by Unger,163 the activity of AMPK in multiple tissues is depressed, and treatment with troglitazone, AICAR, or caloric deprivation prevent or at least markedly attenuate the development of β-cell damage and dysfunction and hyperglycemia.112,165 Likewise, it has been demonstrated that AMPK activation prevents the apoptosis and mitochondrial dysfunction observed in pancreatic β cells when incubated with saturated fatty acids at a high glucose concentration.188 Finally, although theories linking triglyceride accumulation to β-cell dysfunction are attractive and have led to interesting hypotheses,22,177,191,192 to our knowledge there have been no definitive studies showing that triglyceride accumulates in human islets in patients with type 2 diabetes.
Molecular Mechanisms of Insulin Resistance and Cellular Dysfunction According to the Lipid Theory
Based on studies reviewed in the preceding sections, a model can be proposed in which insulin resistance and cellular dysfunction are due to an increase in intracellular fatty acid metabolites such as diacylglycerol87,88,193 and cytosolic LCCoA, possibly secondary to dysregulation of AMPK (Figs. 44-5 and 44-6). According to this scheme, such changes activate a serine–threonine kinase cascade that includes conventional and/or novel protein kinase C isoforms,87,88,194,195 IKKB,196,197 and Jun-activated kinase (JNK-1), one or more of which phosphorylate serine residues on IRS-1 (in muscle).85 Similar changes in IRS1 in response to hyperglycemia may also result from activation of the mTOR/p70s6k signaling mechanism.198 Serine phosphorylation of IRS-1 in turn impairs its ability to associate with P13-kinase, leading to a diminished activation by insulin of Akt and PKC zeta, glucose transport, glycogen synthesis, and other insulin-stimulated downstream events. Recent studies demonstrating that transgenic mice with a muscle-specific alteration in IRS-1 Ser→Ala are protected from fat-induced insulin resistance in skeletal muscle strongly support this hypothesis.199 Similar changes appear to occur in liver, except that the inhibitory actions of insulin on gluconeogenesis and glycogenolysis are impaired,85,91,186 and IRS1 and IRS2 may be differentially affected.200 Also possibly involved in this chain of events are increases in oxidative and ER stress, ceramide synthesis, NF-κB activation, and NF-κB-mediated gene expression that could explain, at least in part, the proinflammatory state associated with the metabolic syndrome.73,75,201 Interestingly, the hallmark of this insulin-resistant state is an increase in intracellular triglyceride in liver and muscle that can be quantified noninvasively with magnetic resonance imaging.202,203 Triglyceride accumulation in muscle and liver is generally regarded as a marker of lipid-induced insulin resistance and cellular dysfunction rather than a cause.139 On the other hand, by providing an additional source of intracellular free fatty acids, it could play a more pathogenetic role.
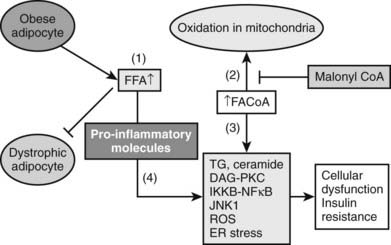
FIGURE 44-5. The pathogenesis of the metabolic syndrome—the lipid hypothesis. According to the proposed scheme, cellular dysfunction and insulin resistance in the setting of the metabolic syndrome can result from multiple events that alter lipid metabolism, including (1) increased plasma FFA and inflammatory cytokines (4) secondary to impaired triglyceride storage and enhanced lipolysis in the fat cell; (2) increased FACoA and inappropriately normal or decreased mitochondrial fatty acid oxidation in liver, muscle, and possibly other tissues due to increases in the concentration of malonyl CoA and other factors (see Fig. 44-4); and (3) increased esterification of FACoA to form triglyceride (TG), diacylglycerol (DAG), and in some tissues ceramide, in increased amounts. By mechanisms only partially understood, these abnormalities can in turn lead to activation of various protein kinase C isoforms, oxidant stress (ROS) and endoplasmic reticulum (ER) stress, and activation of the IKKB-NF-κB system. As described in the text, a decrease in AMPK activity could predispose to all of these abnormalities and AMPK activation could prevent them (see also Figs. 44-4 and 44-6).
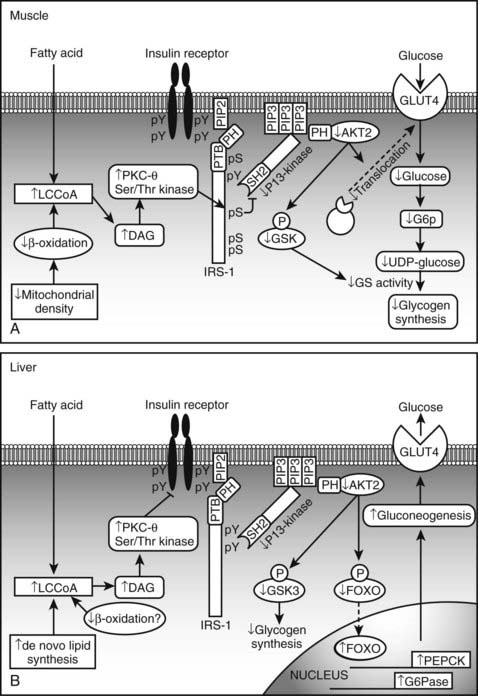
FIGURE 44-6. Proposed mechanism of fatty acid induced insulin resistance in skeletal muscle (A) and liver (B). A, Muscle. Increases is intramyocellular LCCoA and DAG, due to increased fatty acid delivery and/or decreased mitochondrial fatty acid oxidation, trigger a serine/threonine kinase (Ser/Thr) cascade initiated by nPKCs and possibly involving IKKB and/or JNK-1. This ultimately induces serine/threonine phosphorylation of critical IRS-1 sites in muscle, that inhibit IRS-1 tyrosine phosphorylation and activation of PI 3-kinase. This in turn results in reduced insulin-stimulated glucose transport and glycogen synthesis. B, Liver. Increases in intracellular DAG, due to enhanced lipogenesis and/or decreased mitochondrial fatty acid oxidation, activate PKCs (ε and/or possibly δ), which bind to and inactivate the insulin receptor kinase. This results in reduced insulin-stimulated IRS-1 and IRS-2 tyrosine phosphorylation, and decreased insulin activation of PI 3-kinase and AKT2. This in turn leads to diminished GSK3 and FOXO phosphorylation, which respectively decrease insulin-stimulated liver glycogen synthesis and hepatic gluconeogenesis. DAG, Diacylglycerol; FOXO, forkhead box protein O; GLUT, glucose transporter; G6P, glucose-6-phosphate; GSK3, glycogen synthase kinase 3; IRS, insulin receptor substrate; IKKB, IκB kinase β; JNK-1, Jun-activated kinase 1; LCCoA, long-chain acylcoenzyme A; PEPCK, phosphoenolpyruvate carboxykinase; PI 3-kinase, phosphatidylinositol-3-kinase; PTB, phosphotyrosine-binding domain; PH, pleckstrin homology domain; SH2, src homology domain.
(Adapted from Savage DB, Petersen KF, Shulman GI: Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87:507–520, 2007.)
The Hypothalamus, Food Intake, and Insulin Resistance
Leptin, which activates AMPK in peripheral tissues by a direct action, diminishes AMPK activity in the hypothalamus. This in turn leads to decreased food intake and activation of the sympathetic nervous system and secondary to this, further activation of AMPK in peripheral tissues.198 Conversely, glucocorticoids increase AMPK in the hypothalamus, leading to an increase in food intake, and they decrease AMPK and cause insulin resistance in peripheral tissues [see discussion of Cushing’s Syndrome in reference 122, Christ-Crain et al., 2008]. Various antipsychotic drugs have also been found to activate AMPK in the hypothalamus, and like glucocorticoids, they increase food intake and cause insulin resistance.204 Why some agents that decrease or increase AMPK activity in the hypothalamus have opposite effects on peripheral tissues is not known. On the other hand, the possibility that a drug that activates or inhibits AMPK activity in peripheral tissues has the opposite effect in the hypothalamus or elsewhere in the central nervous system needs to be considered in evaluating its clinical efficacy and side effects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree