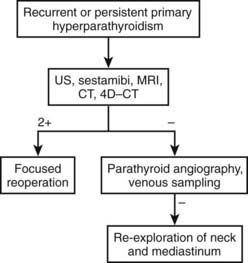
FIGURE 64-1. Flow diagram for localization strategy in previously unoperated patients. US, Ultrasound.
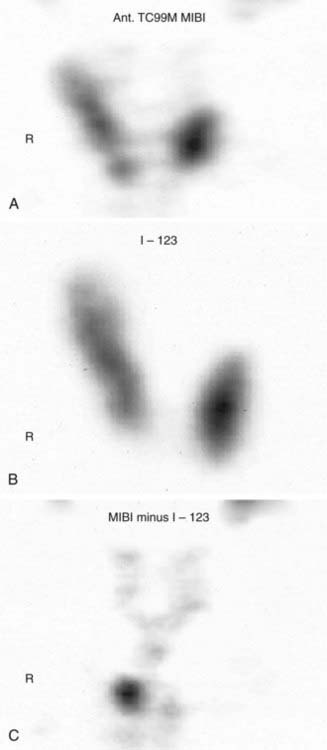
FIGURE 64-2. A, Sestamibi scan showing uptake in thyroid as well as parathyroid tissue. B, Thyroid uptake of I-123. C, Subtracted image of sestamibi without I-123.
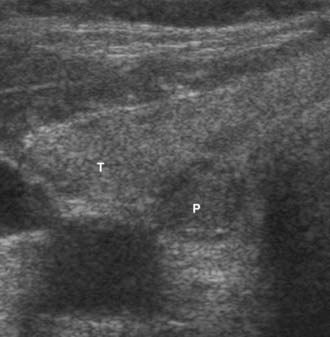
FIGURE 64-3. Hypoechoic mass (P) is an intrathyroidal parathyroid adenoma, with the more echogenic right superior thyroid lobe (T).
PARATHYROID LOCALIZATION BEFORE REPEAT SURGERY
In patients with persistent or recurrent (normocalcemia for 6 months or longer, then recurrent hypercalcemia) hyperparathyroidism, the chance of successful surgery is reduced,39,40 and the incidence of complications is greater.41–43 Therefore, maximum effort at parathyroid gland localization is made, commencing with the noninvasive procedures (US, computed tomography [CT], magnetic resonance imaging [MRI], and sestamibi scanning) and proceeding, if necessary, to the more invasive studies (Fig. 64-4). Currently, noninvasive techniques localize an abnormal gland in about 75% to 80% of patients requiring repeat surgery,44 whereas invasive studies, such as venous sampling, selective angiography, or percutaneous ultrasound/CT-guided fine-needle aspiration, help with the remainder.45,46
Ultrasound
US, using a 10-MHz probe, is readily available, noninvasive, and the least expensive technique to preoperatively image abnormal parathyroid glands. It is particularly effective for localizing enlarged parathyroid glands in the neck and can be used to identify 60% of the abnormal glands in patients requiring reoperation.44 US identifies juxtathyroidal parathyroid glands (see Fig. 64-3).
US has some disadvantages. It is operator-dependent, making the accuracy variable.35,37,38,47 It may fail to image posterior glands in the tracheoesophageal groove and glands in the anterior mediastinum. In multiple-gland hyperplasia, it generally demonstrates only the dominant gland.
Sestamibi Scintigraphy
Technetium 99m–labeled sestamibi scanning has superior resolution and sensitivity (80% to 90%) in detecting hypercellular parathyroid glands prior to reoperations.48 Both the thyroid and parathyroid will take up sestamibi, but the uptake will be stronger and the signal will persist longer in parathyroid adenomas or hyperplasia (see Fig. 64-2). The combination of single-photon emission CT (SPECT) with sestamibi has improved the sensitivity to about 85%, especially for deep cervical and mediastinal parathyroid tumors.25 Sestamibi has been combined with the gamma probe for hand-held intraoperative localization of abnormal parathyroid glands.27,29–34 Advocates suggest that this approach is less invasive and can be done under local anesthesia as an outpatient procedure through a smaller incision. Sestamibi scans facilitate the dissection and make the surgery easier, but they have not been shown to affect the outcome in previously unoperated patients. One study demonstrated that there was no significant difference in cure rate between patients who had preoperative sestamibi scan and those who did not. The cure rate was 97.5% and 99%, respectively. However, there was a significant difference in cure rate between the negative sestamibi scan group (92.7%) and both the no-scan group (99.3%) and the positive-scan group (100%). Thus sestamibi scan can be used to identify those patients who are less likely to have successful surgery.49
Computed Tomography
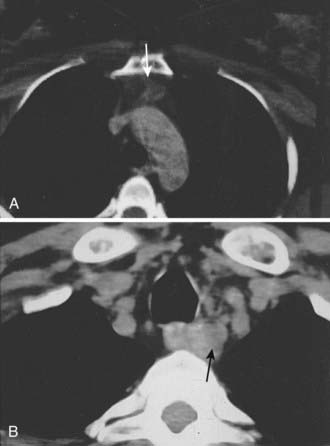
CT is particularly effective for identifying ectopic glands in the anterior mediastinum and enlarged glands in the tracheoesophageal groove. Ectopic glands in the anterior mediastinum often lie within the fat-replaced thymus, and even small adenomas are readily visualized (Fig. 64-5A). Ectopic glands in the tracheoesophageal groove are detected as a solid mass adjacent to the esophagus (Fig. 64-5B). Undescended glands near the carotid bifurcation are also identified by CT, provided that the examination is carried up to the level of the hyoid bone. On the other hand, CT is poor at detecting intrathyroid or juxtathyroid tumors and exposes the patient to risks associated with contrast media and radiation.
Magnetic Resonance Imaging
Initial experience with MRI of abnormal parathyroid glands has been successful for large parathyroid adenomas, which on T2-weighted or stir-pulse sequences produce a bright signal.50 In the mediastinum, this signal may be confused with fat, and a T1-weighted image is required to specifically identify the pathology. With gadolinium-enhanced MRI and T1- and T2-weighted images, MRI can now provide higher sensitivity than CT for identifying ectopic parathyroid tumors. It can be a useful study, and it may have more sensitivity than CT.
Angiography
The potential for morbidity associated with angiographic procedures to localize parathyroid glands limits its use to patients with symptomatic persistent or recurrent hyperparathyroidism requiring reoperation. Intraarterial digital techniques have greatly simplified angiographic localization of parathyroid pathology. Because digital examination does not require highly selective catheter positioning, it can be accomplished expeditiously. The improved sensitivity of digital subtraction arteriography makes it possible to significantly reduce the total dose of water-soluble contrast material, thereby decreasing adverse effects on the kidney in a group of patients who may already have compromised renal function.
Selective Venous Sampling for Parathyroid Hormone
Selective venous sampling requires the greatest experience and is the most variably performed of all the localizing procedures in nonreferral centers. Contrast load, radiation exposure, and cost (15 to 20 PTH determinations), in addition to radiography costs, are all significant. Moreover, gradients determined by selective catheterization identify only the region of pathology (e.g., right side of the neck, mediastinum) but do not image the elusive gland. A new technique is to add the rapid PTH assay to selective venous sampling to provide a short turnaround time and allow the radiologist to obtain more selective samples in regions in which high concentrations of PTH are found. This combination of venous sampling and rapid PTH assay localized the abnormal parathyroid gland correctly in 6 of 7 patients who had negative noninvasive imaging and required reoperation for prior unsuccessful parathyroid surgery.51 It is indicated in only a small proportion of reoperative patients who have significant primary hyperparathyroidism and no apparent localizing information after completing all noninvasive studies and angiography.
Positron Emission Tomography
Regional body fluorodeoxyglucose positron emission tomography (FDG-PET) has been evaluated as a means of localizing pathology in recurrent hyperparathyroidism. Regional PET imaging of the neck and upper chest was able to identify 79% of parathyroid adenomas in 20 patients.52 PET appears to have potential; however, is relatively expensive compared to other more commonly utilized localization techniques. It is not used frequently for abnormal parathyroid gland localization.
Four-Dimensional Computed Tomography
4D-CT is the latest described technology for the preoperative evaluation of patients with parathyroid disease. 4D-CT adds to existing CT by detecting the perfusion characteristics of parathyroid and is able to differentiate between normal and hyperfunctioning glands (which have rapid uptake and quick washout). In a way, this is similar to CT angiography. This modality combines anatomic and functional localization in a single study, with preliminary data suggesting greater accuracy than sestamibi scan. Perhaps the greatest yield with 4D-CT imaging will be in localization of hyperfunctioning glands in the setting of persistent or recurrent parathyroid disease.53
Summary of Radiographic Localization
We suggest that sestamibi and US be used in patients undergoing initial exploration for pHPT. Accurate preoperative localization studies allow a minimally invasive parathyroidectomy that shortens hospital stay, minimizes scar, and provides a successful outcome. Furthermore, in patients undergoing reoperations, preoperative radiologic localization studies are necessary and helpful to plan the operative approach (see Fig. 64-4). We recommend liberal use of each of the noninvasive imaging studies (US, CT, 99mTc-sestamibi, and MRI) as an initial imaging cluster. If two studies identify the abnormal parathyroid gland in the same location, we proceed with surgery. Our institution is currently establishing 4D-CT technology, but it is not yet available. As such, if the noninvasive studies are equivocal, we then perform arteriography. If that study is positive, we perform surgery; if negative, we recommend selective venous sampling for rapid PTH measurements (as discussed above).51
Intraoperative Determination of Parathyroid Hormone
Intraoperative determination of PTH allows rapid monitoring of parathyroid status during parathyroid surgery.54 Generally, after successful removal of a single parathyroid adenoma or adequate resection of hyperplastic glands, serum PTH levels begin to fall immediately and reach a 50% drop from the baseline level or normal range within 10 to 15 minutes.55 Studies demonstrate that serum levels of intact PTH decline rapidly, only 5 minutes after resection of a parathyroid adenoma (Fig. 64-6).56,57 Furthermore, the rate of decline is less in patients with hyperplasia and may provide an additional intraoperative means of diagnosing hyperplasia (Fig. 64-7).58 A serum sample for PTH should be obtained just after the induction of anesthesia. Repeated serum samples are obtained intraoperatively immediately following resection of an enlarged gland, and then 10 minutes following removal. This protocol has been designed to take into account the half-life of PTH, which is 1 to 4 minutes, and avoid misleading results from a spike in concentration that may occur during handling and removal of the adenoma.57 A 50% reduction in the PTH level from the median baseline level indicates a successful outcome (see Fig. 64-6).56 Some also recommend a second criterion of a normal serum level of PTH. The operation can be terminated on this result without identification of other parathyroid glands. Furthermore, the assay can be used to diagnose an abnormal parathyroid gland by performing fine needle aspiration (FNA) of a mass lesion and then diluting the sample with heparinized saline and measuring PTH levels—which will be very high if the mass is parathyroid tissue.58
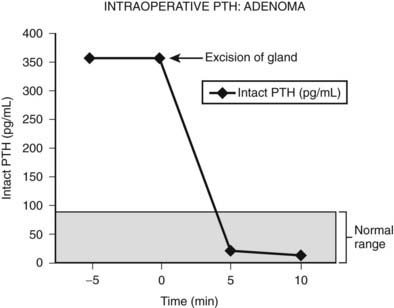
FIGURE 64-6. Intraoperative measurement of intact PTH in a patient undergoing surgery for an adenoma. After the parathyroid adenoma is removed, the serum PTH levels decrease more than 50% from the median baseline level, indicating a successful outcome, and the surgery is terminated.
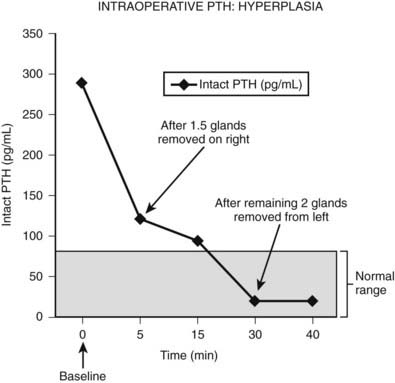
FIGURE 64-7. Intraoperative measurement of intact PTH in a patient who underwent neck exploration for pHPT. The right upper gland and half of the right lower gland were excised. Both appeared abnormally large, and frozen section analysis showed hypercellular tissue. The PTH decreased to less than 50% baseline, but it did not return to the normal range (<80 pg/mL). The left neck was explored, and both parathyroid glands were excised. The PTH level subsequently dropped within normal range.
Intraoperative determination of PTH levels appears to complement surgical skill and histopathologic information and has the potential to provide additional guidance regarding the extent and degree of neck exploration. However, false-negative results57 or technical difficulties may be encountered, and this information may be difficult to interpret in the case of double adenoma or hyperplasia. Nevertheless, its use has greatly facilitated minimally invasive parathyroidectomy, and operative failure rates with the use of intraoperative PTH assay have decreased from 6% to 1.5% for initial operations59 and from 24% to 6% for reoperations.60
Surgical Management of Primary Hyperparathyroidism
Surgery is the mainstay for treatment of pHPT. The methods of surgery are becoming less invasive. The possible causes of pHPT are adenoma (83%), hyperplasia (15%), double adenoma (1% to 2%), and carcinoma (1%).61 Some argue that double adenomas might represent undetected hyperplasia. However, studies have shown that recurrent disease does not develop in patients with removed double adenomas after long follow-up.62,63 This suggests that the diagnosis of double adenoma is real. A family history of parathyroid disease or associated endocrinopathies is associated with hyperplasia. A history of neck irradiation is associated with adenoma.64
Before performing surgery for pHPT, the surgeon must obtain informed consent, which requires careful discussion of the outcome and complications. A successful initial parathyroid surgery, using either minimally invasive techniques or bilateral cervical exploration, is expected in greater than 95% of patients undergoing initial operations21,22,64 and in 78% to 90% of reoperations.65,66 Udelsman et al.42 recently reported a success rate for reoperative surgery for pHPT as high as 96%. Recurrent laryngeal nerve injury occurs in less than 1% of initial operations64 and more than 5% of repeat operations.41 Fortunately, the symptoms in many of these nerve injuries are temporary, and full recovery may be seen at the 3- to 6-month follow-up. Hypoparathyroidism rarely occurs after initial explorations but may occur in 2.7% to 16% of reoperations.65,66
ANATOMY
Facility in the surgical identification of normal and abnormal parathyroid glands is essential. Parathyroid glands vary in color from light yellow to reddish brown, and the consistency is usually soft and pliable.67 A reddish color and dense consistency reflect a high parenchymal cell content (abnormal gland); a yellowish white color is found with a high fat content (normal gland).67 Typically, four parathyroid glands are present.
Parathyroid glands differ in shape and size. Eighty-three percent are oval or bean-shaped, 11% are elongated, 5% are bilobate, and 1% are multilobated.67 Normal glands tend to be flat and ovoid; with enlargement, they become globular. Normal measurements are 3 × 5 × 7 mm. The combined weight of all parathyroid glands is 90 to 130 mg, and the superior glands are usually smaller than the inferior glands.64 Most parathyroid glands are suspended by a small vascular pedicle and enveloped by a pad of fatty tissue.68
Autopsy series demonstrate that four glands are found in 91% of subjects, five glands in 4%, and three glands in 5%.69 In studies done by serial sectioning of embryos, at least four parathyroid glands are found in every specimen.69 Approximately 5% of humans have supernumerary (more than four) parathyroid glands.70 Supernumerary glands and fragments of parathyroid glands are most commonly found within the thymus.
Although gland distribution may deviate widely, the location of parathyroid glands is predictable from knowledge of embryology.68 Originating from the fourth pharyngeal pouch,71 the superior parathyroid glands are commonly found along the posterior surface of the upper two-thirds of the thyroid gland (92%)67 (Fig. 64-8). Frequently (40%), superior parathyroid gland adenomas migrate posteriorly, behind the inferior thyroid artery to a position along the esophagus.61 Division of the superior thyroid artery and mobilization of the superior thyroid pole are usually unnecessary to expose the superior parathyroid glands, but the fascia connecting the lateral portion of the thyroid lobe to the carotid sheath must be incised. The location of the superior glands is relatively constant, and these glands can generally be identified quickly and easily. Superior parathyroid adenomas may have a unique relationship to the recurrent laryngeal nerve such that the nerve is embedded in the anterior medial capsule of the adenoma, or the gland can be rounded and tucked into the exact spot where the recurrent nerve enters the larynx.
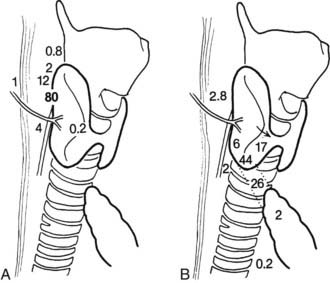
FIGURE 64-8. Diagram of potential locations of superior (A) and inferior (B) parathyroid glands. Numbers refer to the percentage of glands found at each location.
(From Akerstrom G, Malmaeus J, Bergstrom R: Surgical anatomy of human parathyroid glands. Surgery 95:14, 1984.)
The inferior parathyroid glands have a more variable distribution than the superior ones (see Fig. 64-8). With the thymus, they originate from the third pharyngeal pouch.71 As the thymus migrates caudally, the lower glands migrate until they reach the lower pole of the thyroid gland. Seventeen percent of inferior parathyroid glands touch the inferior border of the thyroid, 44% are within 1 cm of the inferior border of the thyroid (also known as the thyrothymic ligament), 26% are within the superior horn of the thymus, and 2% are in the mediastinal thymus.67,68 The remainder are either within the thyroid or are undescended in the upper portion of the neck near the carotid bifurcation72 (see Fig. 64-8). This variable anatomic distribution makes the inferior glands more difficult to locate than the superior ones. An inferior parathyroid adenoma is generally bordered posteriorly and laterally by the recurrent laryngeal nerve and is inferior to the inferior thyroid artery.
GENERAL TECHNIQUE OF EXPLORATION
It appears that some form of minimally invasive parathyroid surgery has replaced standard bilateral neck exploration for pHPT. This is still controversial, and some recent studies suggest that standard bilateral neck exploration is just as good and less expensive.73 However, most surgeons, endocrinologists, and patients now think that MIP is preferable because it is associated with similar excellent results, less pain, better cosmesis, and more rapid recovery.74 We recommend the use of intraoperative rapid PTH assay to assess outcome intraoperatively without extensive exploration, but this has been controversial; one group has demonstrated that this is unnecessary if sestamibi demonstrates a single abnormal gland.75 General endotracheal anesthesia is used, although regional block anesthesia has been advocated by some and is equally effective (Fig. 64-9).76,77 Local anesthesia or regional block has been advocated for the elderly undergoing targeted parathyroidectomy.76
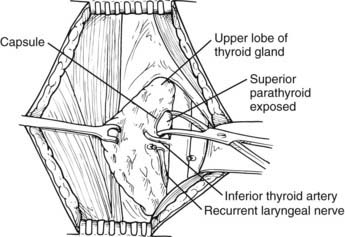
FIGURE 64-9. Identification of a left superior parathyroid adenoma. The thyroid gland is elevated with a Babcock clamp. The investing thyroid fascia is opened posterior to the upper pole of the left lobe. A left upper parathyroid adenoma is identified superior to the inferior thyroid artery and posterolateral to the recurrent laryngeal nerve. The left recurrent laryngeal nerve is shown in its usual location within the tracheoesophageal groove and posterior to the inferior thyroid artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree