FIGURE 65-1. Urinary cyclic adenosine monophosphate (cAMP) excretion in response to an infusion of bovine parathyroid extract (300 USP units). The peak response in normal subjects (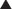 ) and those with pseudopseudohypoparathyroidism (PPHP) (not shown) is 50- to 100-fold times basal. Subjects with PHP type Ia (o) or PHP type Ib (•) show only a two- to fivefold increase. Urinary cAMP is expressed as nanomoles per 100 mL of glomerular filtrate (GF), UcAMP (nanomoles per 100 mL GF) = UcAMP (nanomoles/dL) × SCre (mg/dL)/UCre (mg/dL).
) and those with pseudopseudohypoparathyroidism (PPHP) (not shown) is 50- to 100-fold times basal. Subjects with PHP type Ia (o) or PHP type Ib (•) show only a two- to fivefold increase. Urinary cAMP is expressed as nanomoles per 100 mL of glomerular filtrate (GF), UcAMP (nanomoles per 100 mL GF) = UcAMP (nanomoles/dL) × SCre (mg/dL)/UCre (mg/dL).
(Data from Levine et al., 1986.65)
Signs and symptoms of decreased serum calcium often reflect increased neuromuscular excitability. Although the most common manifestations of hypocalcemia include muscle tetany and spasms, findings vary markedly among patients. In more severe cases, patients present with seizures. Other neurologic symptoms can arise from hypocalcemia, and some patients with PHP initially have been diagnosed with movement disorders.20–24 In one report,25 two sisters with PHP Ib (see below) presented with paroxysmal kinesigenic choreoathetosis, and the diagnosis of PTH resistance in one sister was made through genetic testing and biochemical evaluation only after approximately 4 years of antiepileptic oral treatment.25 Some patients presenting with seizures demonstrate epileptiform activity on electroencephalogram (EEG), and, because this activity typically responds to antiepileptic treatment, the diagnosis of PHP can be delayed.26,27 As another complication of changes in serum calcium and phosphorus, brain imaging studies in PHP patients frequently show intracranial calcifications.20,28–34
PHP is a congenital disorder, and few reports describe findings consistent with PTH resistance during the neonatal period.35,36 However, clinical manifestations of hypocalcemia typically occur only later in childhood. PTH resistance and resultant changes in serum calcium and phosphorus develop gradually.37–40 In a longitudinal study of a child with PHP Ia (see below), cAMP response to PTH was found to be normal at age 3 months, although it was blunted at age 2.6 years.38 In another PHP Ia case, a gradual decline in serum calcium, preceded by increasing serum phosphorus and PTH levels, was demonstrated.37 In addition, a PHP Ib patient diagnosed by genetic analysis (see below) at birth was shown to have normal serum PTH levels until age 18 months, when an elevation in PTH was first detected despite normal serum calcium and phosphorus.39 It thus appears that PTH responses are intact during the early postnatal period despite the existence of the molecular defect underlying PHP. The mechanisms that allow normal PTH signaling during this developmental stage remain unknown.
The primary goal of treatment entails correction of abnormal serum biochemistries that result from PTH and, in some cases, other hormone resistance. Regarding hypocalcemia, treatment involves oral calcium supplements and 1,25(OH)2D (calcitriol) preparations. Note that the active form of vitamin D is required because of the lowered capacity of the proximal tubule to convert 25(OH)D into the biologically active 1,25(OH)2D. In addition, treatment for patients with PTH resistance should be aimed at keeping the serum PTH level within or close to the normal range, rather than simply avoiding symptomatic hypocalcemia, because persistent elevation of serum PTH will increase bone resorption and eventually may lead to hyperparathyroid bone disease, including osteitis fibrosa cystica.41 PTH actions in the distal tubule, which are not impaired, provide sufficient calcium reabsorption from the glomerular filtrate; therefore, urinary calcium levels are usually low, and affected individuals do not have an increased risk of developing kidney stones and nephrocalcinosis. In fact, during the course of treatment, urinary calcium elevation typically does not occur. Nevertheless, blood chemistries and urinary calcium excretion in patients undergoing treatment should be monitored annually, but more frequently during pubertal development and once skeletal growth is completed, as the requirements for treatment with calcium and 1,25(OH)2D may need adjustment.
Pseudohypoparathyroidism Type Ia
Among the two main PHP types, PHP type I is much more common. Clinical variants of PHP type I have been described, based on the presence or absence of clinical manifestations that coexist with PTH resistance, diminished stimulatory G protein activity in easily accessible cells, and imprinting abnormalities of the GNAS gene encoding the α subunit of the stimulatory G protein (Gsα) (Table 65-1).
As was described originally by Albright et al.,1 some PHP patients exhibit characteristic physical features that may include obesity, round facies, short stature, brachydactyly, ectopic ossification, and mental impairment (Fig. 65-2). These features are now termed Albright’s hereditary osteodystrophy (AHO), and PHP patients who present with these features are classified as having pseudohypoparathyroidism type Ia (PHP Ia). The brachydactyly observed in patients with AHO typically involves the metacarpal and/or metatarsal bones; thus, the pattern of shortening of hand bones differs from that seen in other disorders with brachydactyly, such as familial brachydactyly and Turner’s syndrome.42 Because of shortened metacarpals, dimpling over the knuckles of a clenched fist (Archibald sign) is often observed.43 Shortening of the distal phalanx of the thumb, however, is the most common skeletal abnormality (called “murderer’s” or “potter’s” thumb), and some patients have shortening of all digits.44 Mental impairment is mild, often presenting as cognitive defects. It is possible that the cause of mental impairment is the deficiency of Gsα signaling in the brain. Although hypocalcemia and/or hypothyroidism may play a role in this phenotype, correction of these biochemical defects does not prevent mental impairment in all cases. Remarkable patient-to-patient variability is seen in AHO, even among patients who carry the same genetic mutation and belong to the same family (see below for discussion of the underlying genetic defect). Some patients may exhibit a single AHO feature only, such as obesity; others may present with multiple different AHO features. Furthermore, the severity of each feature differs vastly among patients. In addition, individual AHO features are not unique to PHP, as they can be observed in other unrelated disorders. The variable expressivity and the lack of specificity of individual features can make the AHO diagnosis challenging. Although the coexistence of hormone resistance in PHP Ia patients is often helpful, it also can be misleading. This is particularly important in differential diagnosis of different PHP forms characterized by the presence of AHO features alone or hormone resistance alone (see below).
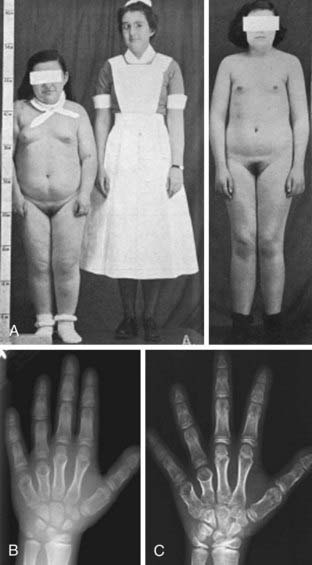
FIGURE 65-2. Albright’s hereditary osteodystrophy (AHO). A, Short stature and obesity are among the typical features of AHO.
B, Hand radiograph of a child with AHO demonstrating short fourth and fifth metacarpals at age 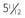 years. C, The same patient’s hand radiograph at age
years. C, The same patient’s hand radiograph at age 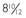 years.
years.
(A Adapted from Albright et al: Pseudohypoparathyroidism—an example of “Seabright-Bantam syndrome,” Endocrinology 30:922–932, 1942).
In addition to having PTH resistance and AHO, patients with PHP Ia show clinical evidence that is consistent with target organ resistance to other hormones. The most common additional hormone resistance involves the actions of thyroid-stimulating hormone (TSH) that lead to hypothyroidism.45,46 In fact, unlike PTH resistance, which typically develops later in life, TSH resistance can be present at birth.47–50 Resistance toward gonadotropins and growth hormone–releasing factor has been reported,51–53 whereas resistance toward other peptide hormones that also mediate their actions through Gsα-coupled receptors, such as vasopressin or adrenocorticotropic hormone (ACTH), does not appear to become clinically overt.52,54–58
The genetic mutation that causes PHP Ia is located within Gsα-coding GNAS exons.59,60 A protein that is essential for the actions of many hormones, Gsα primarily mediates agonist-induced generation of intracellular cAMP. Activation of a stimulatory G protein–coupled receptor by its agonist, such as PTH, leads to a guanosine diphosphate (GDP)-guanosine triphosphate (GTP) exchange on Gsα, causing dissociation of the latter from Gβγ subunits (Fig. 65-3). This allows both Gsα and Gβγ to stimulate their respective effectors. In its GTP-bound state, Gsα can directly activate several different effectors, such as Src tyrosine kinase,61 and certain Ca channels.62,63 Apart from these effectors, however, adenylyl cyclase is by far the most ubiquitous and the most extensively investigated effector molecule stimulated by Gsα. An integral membrane protein, adenylyl cyclase catalyzes the synthesis of the ubiquitous second messenger cAMP, which then triggers various cell-specific responses. Activation of adenylyl cyclase and other effectors by Gsα is regulated by the intrinsic GTP hydrolase (GTPase) activity of Gsα. Conversion of GTP into GDP results in reassembly of the G protein heterotrimer and thereby prevents further effector stimulation (see Fig. 65-3).
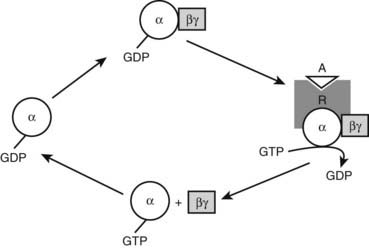
FIGURE 65-3. Heterotrimeric G protein activation cycle. The heterotrimeric complex is assembled at the basal state, with the α subunit associated with a guanosine diphosphate (GDP) molecule. Upon binding of an agonist (A) to its Gs-coupled receptor (R), the GDP molecule bound to the α subunit is replaced with a guanosine triphosphate (GTP) molecule, that is, the activated receptor acts as a guanine nucleotide exchange factor for the α subunit. The GTP-bound form of the α subunit dissociates from the βγ subunits and thereby stimulates its downstream effectors, which, in the case of Gsα, include adenylyl cyclase. Note that the free Gβγ dimer can also stimulate different downstream effectors. The intrinsic GTP hydrolase activity of the α subunit converts GTP into GDP, resulting in reassociation of the heterotrimer and, thus, termination of effector stimulation.
Mutations identified in PHP-Ia patients are heterozygous and scattered throughout all 13 GNAS exons that encode Gsα, including missense and nonsense amino acid changes, insertions/deletions that cause frameshift, and nucleotide alterations that disrupt pre-mRNA splicing (an extensive list of these mutations can be found at OMIM entry #139320 at http://www.ncbi.nlm.nih.gov). Constitutional deletions of the chromosomal arm containing GNAS have also been identified.64 Consistent with heterozygous mutations, Gsα level/activity is reduced by approximately 50% in easily accessible tissues from PHP Ia patients, such as erythrocytes, skin fibroblasts, and platelets.45,65–78 Deficiency of Gsα has been demonstrated in renal membranes from a patient with PHP.79 A complementation assay is typically used to examine Gsα activity, involving patient-derived erythrocyte membranes and membranes from turkey erythrocyte that lack endogenous Gsα (Fig. 65-4). The detection of reduced Gsα activity by this means is important for the establishment of PHP Ia diagnosis, particularly in cases where genetic analysis fails to reveal a GNAS mutation. Reduction of Gsα activity in PHP Ia is consistent with the fact that PTH and the other hormones with impaired actions in this disorder act via cAMP-mediated signaling pathways.
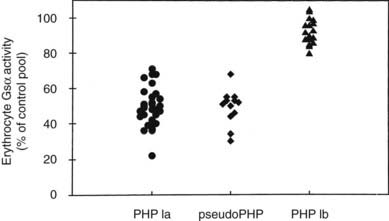
FIGURE 65-4. Gsα activity of erythrocyte membranes. Gsα is quantified in complementation assays with S49 cyc− membranes, which genetically lack Gsα but retain all other components necessary for hormone responsive adenylyl cyclase activity. Activity is reduced by approximately 50% in patients with Albright’s hereditary osteodystrophy (AHO) with pseudohypothyroidism (PHP) type Ia or pseudoPHP but is normal in patients with PHP type Ib.
Among the many different inactivating GNAS mutations, a 4 bp deletion in exon 7 has been identified in numerous families, representing a genetic “hot spot.” In addition, two different mutations are associated with additional phenotypes. A missense mutation in exon 13 (A366S) was identified in two unrelated boys who presented with PHP Ia and precocious puberty.80 This mutant Gsα protein is temperature sensitive and thus rapidly degrades at normal body temperature. The amino acid substitution, however, renders the protein constitutively active, resulting in elevated cAMP signaling in the cooler temperature of the testis. Recently, another mutant Gsα protein was described in a unique case of familial PHP Ia and transient neonatal diarrhea.81 The mutation, which entails a repeated AVDT sequence at residues 366 to 369, generates an unstable but constitutively active Gsα mutant as the result of enhanced GDP-GTP exchange and reduced GTPase activity. Although hormone resistance results from the instability of the Gsα-AVDT mutant, diarrhea is attributed to increased plasma membrane localization of the mutant protein in the small intestinal epithelium.
PHP Ic has been described as a distinct variant of PHP Ia,68 but the clinical features of patients with PHP Ic are indistinguishable from those with PHP Ia, in that they have both AHO and multihormone resistance. In contrast to PHP Ia, however, biochemical assays demonstrate no reduction in Gsα activity in erythrocytes obtained from PHP Ic patients, suggesting the absence of mutations within the Gsα gene. Nevertheless, recent molecular characterizations have revealed Gsα mutations in at least some PHP Ic patients. However, the Gsα mutants show impaired coupling to different G protein–coupled receptors, yet show normal Gsα activity in complementation assays that use nonhydrolyzable GTP analogues rather than a receptor agonist for stimulation of Gsα activity.44,82 Thus, the mutant Gsα protein that causes PHP Ic allows basal cAMP formation but is unable to trigger an increase in response to hormones. Hence, at least some PHP Ic cases are allelic variants of PHP Ia. Although it remains possible that some patients who match the description of PHP Ic develop hormone resistance and AHO as the result of a novel gene mutation that affects cAMP production without functionally impairing Gsα itself (e.g., inactivating mutations that affect one of the adenylyl cyclases, or activating mutations in the phosphodiesterase), it appears unlikely that such putative mutations affect only a few G protein–coupled receptors and thus result in limited hormonal resistance as observed in PHP Ia and PHP Ic.
The Complex GNAS Locus
GNAS is a complex locus giving rise to multiple different coding and noncoding transcripts that show monoallelic, parent-of-origin specific expression profiles (Fig. 65-5). GNAS maps to the telomeric end of the long arm of chromosome 20 (20q13.2-20q13.3).83–85 Gsα is encoded by 13 exons,86 but because of alternative pre-mRNA splicing, the Gsα transcript has several variants. The long and short Gsα variants (Gsα-L and Gsα-S, respectively) differ from each other by the inclusion or exclusion of exon 386–88; these Gsα variants are typically detected as 52 and 45 kDa protein bands on Western blots. Showing further complexity, each Gsα form either includes or excludes a CAG trinucleotide (encoding serine) at the start of exon 4. Small but potentially important differences have been reported between the activities of Gsα-L and Gsα-S. For example, Gsα-L has been shown to display a greater ability to mediate receptor signaling than Gsα-S when partially purified proteins from rabbit liver were examined,89 although the opposite finding was reported upon the use of cultured pancreatic islet cells.90 Furthermore, the GDP release rate from Gsα-L appears to be approximately twofold higher than that of Gsα-S,91 and, accordingly, fusion proteins involving the β2-adrenergic receptor and Gsα-L have shown higher constitutive activity than those involving the receptor and Gsα-S.92 Moreover, differences have been reported in the subcellular targeting of these two Gsα variants.93–95 Currently, it remains unclear whether these differences translate into biologically significant effects, such as divergence in the variety of effectors and/or the efficiency of effector activation. Nonetheless, a mutation in exon 3 has been identified recently in two siblings affected by a mild form of PHP Ia.96 The mildness of the phenotype is consistent with the disruption of only one of the two main Gsα variants—that is, Gsα-L. It remains unknown whether this exon 3 mutation impairs agonist responses in an effector- and tissue-specific manner; this possibility depends on the putative effector selectivity and relative expression levels of Gsα-L and Gsα-S in different tissues.
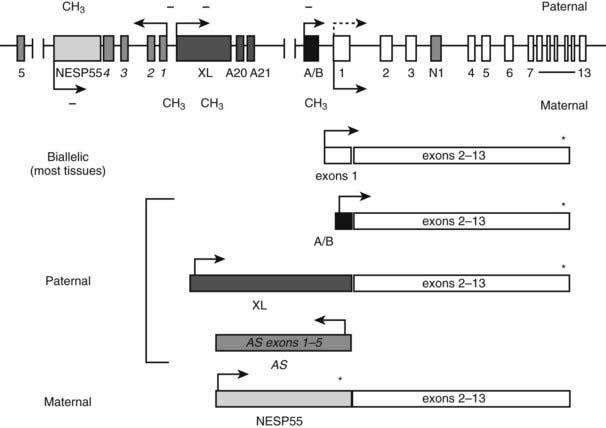
FIGURE 65-5. The GNAS locus. The complex GNAS locus gives rise to multiple transcripts. Boxes and connecting lines indicate exons and introns, respectively. Arrows show the direction of transcription for each transcript. The main transcriptions derived from GNAS and the utilized exons are depicted as rectangles below the gene schematic. Gsα is biallelically expressed, except in a small number of tissues, including renal proximal tubules, thyroid, gonads, and pituitary, in which expression from the paternal allele is silenced (dashed arrow). XLαs, A/B and antisense (AS) transcripts are derived from the paternal allele, and the NESP55 transcript from the maternal allele. Promoters of XLαs, A/B, antisense, and NESP55 transcripts are methylated on the silenced allele, as indicated by CH3 (methylated CpGs) and − (unmethylated CpGs).
Recent studies have revealed that GNAS gives rise to multiple additional gene products that show parent-of-origin specific, monoallelic expression. Besides Gsα, at least two translated GNAS transcripts exist, using distinct upstream promoters and alternative first exons that splice onto exons 2 to 13 encoding Gsα. The most upstream promoter relative to the Gsα promoter drives expression of a neuroendocrine secretory protein with an apparent molecular mass of 55,000 (NESP55).97 The paternal NESP55 promoter is methylated, and transcription occurs from the maternal allele.98,99 In humans, the NESP55 protein is encoded by a single exon, so that Gsα exons 2 to 13 make up the 3′ untranslated region.98,99 Expressed in neuroendocrine tissues, the peripheral and central nervous system, and some endocrine tissues,97,100–103 NESP55 is a chromogranin-like protein that is associated with the constitutive secretory pathway.104 Loss of NESP55 expression does not seem to have an overt clinical outcome in humans, as evidenced from patients with PHP type Ib (see below). However, its disruption in mice results in a subtle behavioral phenotype characterized by increased reactivity to novel environments.105
Another GNAS product is XLαs, which is expressed from the paternal allele.99,106,107 XLαs also uses a distinct upstream promoter and a unique first exon that splices onto Gsα exons 2 to 13.99,107 Unlike in NESP55 transcript, however, the latter portion of the XLαs transcript is included in the translated product, resulting in a protein that is partially identical to Gsα.106 Consistent with its structural similarity to Gsα, XLαs can mediate receptor-activated adenylyl cyclase stimulation in transfected cells.44,108,109 However, the phenotype of mice with targeted disruption of the XL exon suggests that XLαs has unique, albeit as yet undefined, cellular functions. These mice show high early postnatal mortality as the result of poor adaptation to feeding and impairment in glucose and energy metabolism,110 in contrast to Gsα knockout mice, which to a large extent recapitulate the findings in patients with PHP Ia.111,112
The paternal GNAS gives rise to two additional transcripts. From the sense strand originates the A/B transcript (also termed 1A or 1′), which, similar to NESP55 and XLαs, utilizes an upstream promoter and an alternative first exon (exon A/B) that splices onto Gsα exons 2 to 13.113,114 Exon A/B does not comprise a translation initiation codon, but, as demonstrated in vitro, translation can be started through the use of an AUG located within exon 2, thereby giving rise to an N-terminally truncated protein that localizes to the plasma membrane.114 However, no evidence currently supports the existence of endogenous A/B protein. It is thought instead that the A/B transcript is a noncoding RNA. Another paternal GNAS transcript is derived from the antisense strand.115,116 The GNAS antisense transcript, which is formed in humans by several primary exons that show multiple alternative splicing patterns,115,117 is also considered to be noncoding. The promoter of GNAS antisense transcript is immediately upstream of the promoter of XLαs. Although the promoters of XLαs and antisense transcript are located together within a large maternally methylated region, the female germline-specific imprint is established at the antisense promoter only.118 The A/B promoter likewise is methylated in the female, but not in the male, germline.119 Thus, the two germline imprint marks at the GNAS locus include promoters of the antisense and A/B transcripts. Accordingly, data from different genetically manipulated mouse models show that these noncoding transcripts are essential for the regulation of imprinted gene expression from GNAS.120–122 Imprinting of the A/B transcript is particularly important for the development of hormone resistance seen in patients with PHP Ib (see below).
Unlike the promoters of NESP55, antisense, XLαs, and A/B transcripts, the promoter of Gsα is not differentially methylated and, accordingly, Gsα expression is biallelic in most tissues.98,107,123,124 Biallelic Gsα expression has been shown specifically in human bone and adipose tissue.125 However, paternal Gsα expression is silenced in a small subset of tissues through as yet undefined mechanisms, so that the maternal allele is the predominant source of Gsα expression. These tissues include the renal proximal tubule, thyroid, pituitary, and gonads.112,126–128 Although devoid of differential methylation, the Gsα promoter exhibits parent-of-origin specific histone modifications in those tissues in which it is monoallelic. The active maternal Gsα promoter shows a greater ratio of trimethylated to dimethylated histone-3 Lys4 compared with the silenced paternal promoter in the proximal tubule, whereas the quantity of methylated histones is similar in maternal and paternal Gsα promoters in liver—a tissue in which Gsα is biallelic.129 As is discussed below, tissue-specific, paternal Gsα silencing has a key role in the development of PTH resistance in patients with PHP Ia and PHP Ib.
Clinically Distinct, Genetically Related PHP Ia Variants
PSEUDOPSEUDOHYPOPARATHYROIDISM
Physical abnormalities similar to those observed in patients with PHP Ia but without evidence of an abnormal regulation of calcium and phosphate homeostasis were first reported in 1952.130 Because of the lack of abnormal regulation of mineral ion homeostasis, the name pseudopseudohypoparathyroidism (PPHP) was coined to describe this disorder.130 It is interesting to note that patients with PPHP also carry GNAS mutations that lead to diminished Gsα function, and these mutations can be found in the same kindred as those with PHP Ia. However, both disorders are never seen in the same sibling kinship, and careful analysis of multiple families has revealed that the mode of inheritance of each disorder depends on the gender of the parent who transmits the Gsα mutations.131 Thus, an inactivating Gsα mutation causes PHP Ia (i.e., hormone resistance and AHO) after maternal inheritance, whereas the same mutation results in PPHP (AHO only). Because AHO appears to develop regardless of the parent of origin, it is primarily hormone resistance that displays an imprinted mode of inheritance. Recent findings regarding tissue-specific imprinting of Gsα expression (see above) now can explain the parent-of-origin specific inheritance of hormone resistance. In those tissues where Gsα expression is paternally silenced (i.e., Gsα is expressed exclusively or predominantly from the maternal allele), an inactivating mutation located on the paternal allele is not predicted to alter Gsα function, whereas the same mutation located on the maternal allele is predicted to abolish Gsα function completely (Fig. 65-6). Tissue-specific imprinting of Gsα expression also explains why the target organ resistance involves only a small subset of tissues despite the involvement of Gsα signaling in a multitude of physiologic responses. Hormone resistance is observed in those tissues where Gsα is imprinted, such as the proximal renal tubule and the thyroid; hormone responses are unimpaired in those tissues where Gsα is biallelic, such as the distal renal tubules. The role of tissue-specific Gsα imprinting in the development of PTH resistance has been demonstrated through the generation of mice heterozygous for maternal or paternal disruption of Gnas.126
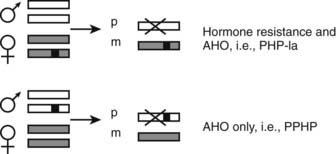
FIGURE 65-6. The effect of paternal Gsα silencing in the development of hormone resistance. Gsα is biallelic in most tissues; a heterozygous inactivating Gsα mutation therefore causes an approximately 50% reduction in Gsα activity/expression regardless of the parent of origin of the mutation. However, in some tissues, such as the proximal tubule and thyroid, the paternally inherited Gsα transcript is silenced (X). Thus, if a Gsα mutation (black square) is inherited from a female individual, this mutation nearly abolishes the expression or activity of Gsα in those tissues, thus leading to hormone resistance (PHP Ia). In contrast, upon paternal inheritance, the same mutation Gsα does not lead to a significant change in expression/activity, and thus, hormone responses are unimpaired. Paternal and maternal Gsα alleles are depicted by white or gray rectangles.
Most AHO features develop regardless of the parent transmitting a Gsα mutation; this observation has led to the hypothesis that inheritance of AHO is due to Gsα haploinsufficiency in various tissues, which appears to be true in certain settings. PTH-related protein-induced cAMP generation is critical for proper control of hypertrophic differentiation of growth plate chondrocytes,132 and Gsα haploinsufficiency has been demonstrated in this tissue through the study of mice chimeric for wild-type cells and mutant cells heterozygous for disruption of Gnas exon 2.133 Regardless of the parental origin of the Gnas exon 2 disruption, mutant cells displayed premature hypertrophy compared with their wild-type neighbors, although paternal disruption (i.e., loss of one Gsα allele combined with a complete loss of XLαs) resulted in significantly more premature hypertrophy than did maternal disruption (loss of one Gsα allele only). Thus, the brachydactyly and/or short stature observed in the context of AHO likely results from diminished Gsα signaling in growth plate chondrocytes. Although these data correlate well with the notion that AHO develops after both maternal and paternal inheritance of a Gsα mutation, recent evidence suggests that individual AHO features can be subject to imprinting. Careful analysis of multiple patients with PHP Ia and PPHP revealed that obesity is primarily a feature of PHP Ia patients, which develops after maternal inheritance.134 Given that Gsα is biallelic in white adipose tissue,125 it was proposed that Gsα may also be imprinted—predominantly by maternal expression—in areas of the central nervous system that control satiety and body weight.134 Recent reports have demonstrated that cognitive impairment is more prevalent in PHP Ia than PPHP, thus indicating that tissue-specific Gsα imprinting may involve additional brain regions.135 On the other hand, imprinted inheritance has not been reported for short stature, despite the finding that Gsα is imprinted in the pituitary,127,128 and that PHP Ia patients display growth hormone-releasing hormone (GHRH) resistance and growth hormone (GH) deficiency.52,53 Future analyses of patients with PHP Ia and PPHP will be helpful in determining the relative roles of genomic imprinting and haploinsufficiency in the development of individual AHO features.
PROGRESSIVE OSSEOUS HETEROPLASIA
A disorder termed progressive osseous heteroplasia (POH) has been described in patients with severe extraskeletal ossifications that involve deep connective tissue and skeletal muscle (Fig. 65-7).136,137 In POH, ectopic bone is primarily intramembranous, as opposed to a similar disease, termed fibrodysplasia ossificans progressiva (FOP), in which extraskeletal bone formation occurs via endochondral mechanisms and is accompanied by skeletal malformations.138,139 Few patients with POH demonstrate AHO features and, consistent with the occasional coexistence of these two sets of clinical defects, heterozygous inactivating Gsα mutations have been identified as a cause of POH.140–142 Several of the identified mutations are identical to those identified in PHP Ia/PPHP kindreds.141,142 Gsα activity and downstream signaling have been implicated in the control of osteogenic differentiation. Patients who are mosaic for heterozygous GNAS mutations that result in constitutive Gsα activity develop fibrous dyplasia of bone characterized by irregular woven bone disrupted by fibrous tissue.143 Moreover, in human mesenchymal stem cells, reduction of Gsα protein levels has been shown to cause osteogenic differentiation, while inhibiting the formation of adipocytes.144,145 In addition, Runx2, a key regulator of osteoblast-specific gene expression, appears to suppress Gsα expression.146 Thus, osteoprogenitor formation and early stages of osteoblastic differentiation require reduced levels of cAMP signaling, consistent with the association of inactivating Gsα mutations with the severe ectopic bone formation observed in POH.
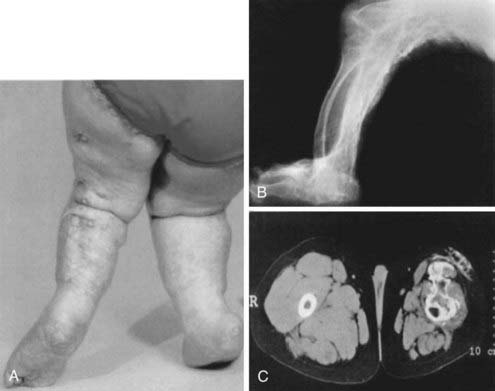
FIGURE 65-7. Clinical and radiographic appearance of progressive osseous heteroplasia (POH). A, Posterior view of the legs and feet of a 5-year-old girl with POH, showing severe maculopapular lesions caused by extensive dermal and subcutaneous ossification. B, A lateral radiogram of the right leg of an 11-year-old girl with POH demonstrating severe heterotopic ossification of the soft tissues. C, Computed tomographic image of the thighs of a 10-year-old boy with POH, showing atrophied soft tissues in the left leg and extensive ossification of the skin, subcutaneous fat, and quadriceps muscles.
(Adapted from Shore EM, et al.142)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree