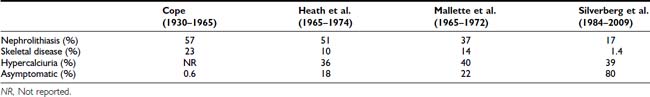
FIGURE 62-1. Radiologic representation of osteitis fibrosa cystica in classic primary hyperparathyroidism. A, Salt-and-pepper skull. B, Cystic bone disease of the clavicle. C, Subperiosteal bone resorption of the digits. D, Cortical erosions.
Pathology
PARATHYROID ADENOMAS
By far the most common lesion found in patients with PHPT is the solitary parathyroid adenoma, occurring in 80% of patients.7,14 Several risk factors have been identified in the development of PHPT. These include a history of neck irradiation16 and prolonged use of lithium therapy for affective disorders.17–18 While in most cases a single adenoma is found, multiple parathyroid adenomas have been reported in 2% to 4% of cases.19 These may be familial or sporadic. Parathyroid adenomas can be discovered in many unexpected anatomic locations (see Chapter 60). Embryonal migration patterns of parathyroid tissue account for a plethora of possible sites of ectopic parathyroid adenomas. The most common sites are within the thyroid gland, the superior mediastinum, and within the thymus.20 Occasionally, the adenoma may ultimately be identified in the retroesophageal space, the pharynx, the lateral neck, and even the alimentary submucosa of the esophagus.21–23 On histologic examination, most parathyroid adenomas are encapsulated and composed of parathyroid chief cells. Adenomas containing mainly oxyphilic or oncocytic cells are rare but can give rise to clinical PHPT.
MULTIGLANDULAR PARATHYROID DISEASE
In approximately 15% of patients with PHPT, all four parathyroid glands are involved. There are no clinical features that differentiate single versus multiglandular disease. The etiology of four-gland parathyroid hyperplasia is multifactorial. In nearly half of cases, it is associated with a familial hereditary syndrome such as multiple endocrine neoplasia (MEN) type 1 or type 2a. These syndromes are discussed in detail in Chapters 150 and 151, respectively.
Clinical Presentation
INCIDENCE
The incidence of PHPT has changed dramatically.8,9,24,25 Prior to the advent of the multichannel autoanalyzer in the early 1970s, Heath et al.8 reported an incidence of 7.8 cases per 100,000 persons in Rochester, Minnesota. With the introduction of routine calcium measurements in the mid-1970s, this rate rose precipitously to 51.1 cases per 100,000 in the same community. Once prevalent cases were diagnosed (the “sweeping” effect), the incidence declined to approximately 27 per 100,000 persons per year. A report from Rochester, Minnesota, suggested that newly diagnosed cases of PHPT have been declining continuously since the mid-1970s.24 The decline in incidence is not explained by a change in the use of multichannel chemical screening because, in the United States, it is only in the very late 1990s that use of this technique became limited. Moreover, such declines in incidence are not apparent at other medical centers. It is possible that the special demographics of Rochester, Minnesota, combined with the rather complete discovery of PHPT in a population that receives virtually all of its care in one system (ideal epidemiologic surveillance) would naturally be associated with declining numbers for years thereafter. The analogy here might be to overfishing a small pond. Unrelated to the Rochester, Minnesota, experience, the United States may soon experience a decline in new cases of PHPT because multichannel screening is now more limited. On the other hand, greater appreciation of the potential of parathyroid hormone to be a catabolic force in postmenopausal women with osteoporosis has led to measurement of PTH even in subjects who do not have hypercalcemia. This trend has led to the emergence of a new entity, normocalcemic PHPT. A number of factors and forces are thus likely to influence the incidence of PHPT in the future (see later discussion).
CLINICAL FEATURES
PHPT occurs predominantly in individuals in their middle years, with a peak incidence between ages 50 and 60 years. However, the disease is seen at all ages. Women are affected more frequently than men, in a ratio of approximately 3 : 1. At the time of diagnosis, most patients with PHPT do not have classic symptoms or signs associated with disease. Kidney stones are uncommon, and fractures are rare.14 Diseases associated epidemiologically with PHPT have included hypertension,26–28 peptic ulcer disease, gout, or pseudogout.29,30 Some concomitant disorders such as hypertension are commonly seen, but it is not established that any of these associated disorders are etiologically linked to the disease. The only exception is the MEN syndromes, in which MEN1 is often seen with peptic ulcer disease, and MEN2 may be associated with a pheochromocytoma. Constitutional complaints such as weakness, easy fatigability, depression, and intellectual weariness are seen with some regularity (see later discussion).31
The physical examination is generally unremarkable. Band keratopathy, a hallmark of classic PHPT, occurs because of deposition of calcium phosphate crystals in the cornea but is virtually never seen grossly. Even by slit-lamp examination, this finding is rare. The neck shows no masses. The neuromuscular system is normal.
DIFFERENTIAL DIAGNOSIS
The diagnosis of PHPT is made when hypercalcemia and elevated PTH levels are present. The other major cause of hypercalcemia, malignancy, is readily distinguished from PHPT. Patients with hypercalcemia of malignancy typically have advanced disease that has already been diagnosed. An exception is multiple myeloma, in which hypercalcemia can be the initial manifestation. These diseases can be easily distinguished; PTH levels are suppressed in multiple myeloma. The differential diagnosis of hypercalcemia, however, includes a number of other etiologies, including rare ones.32
Improved testing for PTH, especially immunoradiometric (IRMA) and immunochemiluminometric (ICMA) assay, has facilitated the distinction between PHPT and hypercalcemia of malignancy. In recent years, it has become clear that the “intact” immunoradiometric assay for PTH (“intact” IRMA) may significantly overestimate the concentration of biologically active parathyroid hormone. In 1998, Lepage et al.33 demonstrated a large non-(1–84) PTH fragment that comigrated with a large amino-terminally truncated fragment (PTH[7–84]) and had substantial cross-reactivity in commercially available IRMAs. This large, inactive moiety constituted as much as 50% (20% to 90%) of immunoreactivity by IRMA for PTH in individuals with chronic renal failure.34 Recognition of this molecule led to the development of a new IRMA utilizing affinity purified polyclonal antibodies to PTH(39–84) and to the extreme N-terminal amino acid regions, PTH(1–4).35,36 This assay detects only the full-length PTH molecule, PTH(1–84). It does not detect the large inactive fragment the normally circulates This assay has clear utility in uremic patients, in whom the “intact” IRMA has been shown to considerably overestimate elevations in biologically active hormone concentration.33,37,38 In PHPT, it is less clear that this assay will aid in the diagnostic evaluation.
A small percentage of patients with PHPT have PTH levels that are within the normal reference range as measured either by the classic IRMA or the newer PTH(1–84) assay. In these patients, levels tend to be in the upper range of normal. In PHPT, such values, although within the normal range, are clearly abnormal in a hypercalcemic setting. This is even more evident in patients younger than 45 years of age. Because PTH levels normally rise with age, the broad normal range for the older IRMA (10 to 65 pg/mL) reflects values for the entire population. In the younger-than-45 individual, one expects a narrower, lower normal range (10 to 45 pg/mL), so hypercalcemia and a PTH level of 50 pg/mL is distinctly abnormal. Occasionally, in either a younger or older patient, the PTH level as measured by the established IRMA will not even be in the upper end of the normal range but as low as 30 pg/mL. Such unusual examples generally require a more careful consideration of other causes of hypercalcemia. But in the end, these individuals are likely to have PHPT also because non–PTH dependent hypercalcemia should suppress the PTH concentration to levels that are either undetectable or at the lower limits of the reference range. Souberbielle et al.39 have illustrated that the normal range is very much a function of whether or not the reference population is or is not vitamin D deficient. When vitamin D deficient individuals were excluded, the upper limit of the PTH reference interval decreased from 65 to 46 pg/mL. When vitamin D–deficient individuals were excluded from the subjects used to establish a reference interval for “whole PTH,” the upper limit decreased from 44 to 34 ng/L.
Ninety percent of patients with hypercalcemia will be shown either to have PHPT or malignancy. Although there are many other causes of hypercalcemia (Table 62-2), they constitute only approximately 10% of the hypercalcemic population. Here also, the PTH assay is useful. With the exception of lithium and thiazide use and familial hypocalciuric hypercalcemia (FHH), virtually all other causes of hypercalcemia are associated with suppressed levels of PTH. If the patient can be safely withdrawn from lithium or thiazide, this should be attempted. Serum calcium and PTH levels are then reassessed 3 months later. If the serum calcium and PTH levels continue to be elevated, the diagnosis of PHPT is made. While patients can generally be readily withdrawn from a thiazide diuretic, patient safety must be the first consideration in any decision to withdraw lithium therapy. The recent emergence of a number of alternative therapeutic approaches may make this option realistic. FHH is differentiated from PHPT by (1) family history, (2) markedly lowered urinary calcium excretion, and (3) the specific gene abnormality (see Chapter 63).
Table 62-2. Differential Diagnosis of Hypercalcemia
Rarely, a patient with malignancy will be shown to have elevated PTH levels due to ectopic secretion of native PTH from the tumor itself.32 Much more commonly, the malignancy is associated with the secretion of parathyroid hormone–related protein (PTHrP), a molecule that does not cross-react in the IRMA and ICMA assay. Finally, it is possible that a malignancy is present in association with PHPT. When the PTH level is elevated in someone with a malignancy, this is more likely to be the case than a true ectopic PTH syndrome.
Using the third-generation assay for PTH(1–84), a second molecular form of PTH(1–84) that is immunologically intact at both extremes has been identified. This molecule reacts only poorly in second-generation PTH assays. This molecular species represents less than 10% of the immunoreactivity in normal individuals and up to 15% in renal failure patients. It has been shown to be overexpressed, however, in a limited number of patients with a severe form of PHPT or with parathyroid cancer.41
Occasionally a patient with PHPT has normal calcium levels. Although the term “normocalcemic PHPT” has been in use for decades, there has been considerable controversy concerning the accuracy of this designation. In many cases, the increases in PTH levels were more apparent than real and attributable to the limitations of available assay technology. The midmolecule radioimmunoassay for PTH, previously in common use, measured hormone fragments in addition to the intact molecule. The latter are retained, leading to spuriously elevated PTH levels, particularly in those with renal insufficiency, in whom clearance of hormone fragments is impaired. Alternative explanations for hyperparathyroidism in so-called normocalcemic PHPT patients have been discovered, including hypercalciuria, renal insufficiency, and certain forms of liver and gastrointestinal disease. In recent years, it has become clear that many patients designated as having normocalcemic PHPT in fact were vitamin D deficient. Vitamin D deficiency with coexisting PHPT can give the semblance of normal calcium levels when in fact they would have been hypercalcemic if the vitamin D levels were normal. Furthermore, we now appreciate that the normal circulating physiologic range of 25-hydroxyvitamin D (25[OH]D) may be significantly higher than the levels once used to diagnose vitamin D deficiency. The diagnosis of normocalcemic PHPT requires that the patient have levels of 25(OH)D within the normal minimum physiologic range of 30 ng/mL.
With these points in mind, patients with elevated PTH levels and normal calcium levels have been reported. These subjects do not have serum calcium levels that extend occasionally into the abnormal range nor do they have any of the known secondary causes for an elevated PTH level. An explanation for why this entity is being seen today may reside in the fact that endocrinologists and other osteoporosis specialists currently evaluate the skeletal status of women at risk for osteoporosis not only with determination of bone density but also with calciotropic hormone measurements. These patients may represent the earliest manifestations of PHPT, a “forme fruste” of the disease. That these patients should exist is not surprising, insofar as clinical manifestations of PHPT are already present when the disorder is commonly diagnosed with hypercalcemia.42,43 One would expect the earliest phase of this disease to be characterized by elevated PTH levels in the absence of hypercalcemia. During this clinically silent period, the patient would not come to medical attention because the serum calcium is normal. However, if these patients were to have PTH levels measured, one might expect to discover them. Several reports describing these individuals have recently been reported, with several patients progressing to overt hypercalcemia while under observation.44–46 Frankly low or low-normal serum calcium concentrations suggest an adaptive response to hypocalcemia with high PTH levels. Secondary hyperparathyroidism can be seen in patients with renal insufficiency, malabsorption, or any of the other vitamin D–deficiency states. Rarely, patients with PHPT and coexisting vitamin D deficiency will present with low calcium concentration. PTH levels are high. In such patients, correction of the vitamin D deficiency is associated with a rise in serum calcium concentration into the hypercalcemic range.47
OTHER BIOCHEMICAL FEATURES
In PHPT, serum phosphorus tends to be in the lower range of normal, but frank hypophosphatemia is present in less than a fourth of patients. The hypophosphatemia, when present, represents the phosphaturic actions of PTH. Average total urinary calcium excretion is at the upper end of the normal range, with about 40% of all patients having hypercalciuria. Serum 25(OH)D levels tend to be in the lower end of the normal range. While mean values of 1,25(OH)2D3 are in the high-normal range, approximately a third of patients have frankly elevated levels of 1,25(OH)2D3.48 This pattern is due to the actions of PTH to facilitate the conversion of 25(OH)D to 1,25(OH)2D. A mild hyperchloremia is seen occasionally, due to the effect of PTH on renal acid-base balance. A typical biochemical profile is shown in Table 62-3.
Table 62-3. Biochemical Profile in Primary Hyperparathyroidism (n = 137)
| Patients (Mean ± SEM) | Normal Range | |
|---|---|---|
| Serum calcium | 10.7 ± 0.1 mg/dL | 8.2–10.2 mg/dL |
| Serum phosphorus | 2.8 ± 0.1 mg/dL | 2.5–4.5 mg/dL |
| Total alkaline phosphatase | 114 ± 5 IU/L | <100 IU/L |
| Serum magnesium | 2.0 ± 0.1 mg/dL | 1.8–2.4 mg/dL |
| PTH (IRMA) | 119 ± 7 pg/mL | 10–65 pg/mL |
| 25(OH)D | 19 ± 1 ng/mL | 30–100 ng/mL |
| 1,25(OH)2D | 54 ± 2 pg/mL | 15–60 pg/mL |
| Urinary calcium | 240 ± 11 mg/g creatinine | |
| Urine DPD | 17.6 ± 1.3 nmol/mmol creatinine | <14.6 nmol/mmol creatinine |
| Urine PYD | 46.8 ± 2.7 nmol/mmol creatinine | <51.8 nmol/mmol creatinine |
DPD, Deoxypyridinoline; PTH (IRMA), parathyroid hormone (immunoradiometric assay); PYD, pyridinoline.
The Skeleton
The classic radiologic bone disease of PHPT, osteitis fibrosa cystica, is rarely seen today in the United States. Most series place the incidence of osteitis fibrosa cystica at less than 2% of patients with PHPT. The absence of classic radiographic features (salt-and-pepper skull, tapering of the distal third of the clavicle, brown tumors) does not mean that the skeleton is not involved in the metabolic processes associated with hyperparathyroid bone disease. With more sensitive techniques, it has become clear that skeletal involvement in the hyperparathyroid process is actually quite common. This section reviews the profile of the skeleton in PHPT as it is reflected in assays for bone markers, bone densitometry, and bone histomorphometry.
BONE MARKERS
Both bone resorption and bone formation are stimulated by PTH. Markers of bone turnover, which reflect those dynamics, provide clues to the extent of skeletal involvement in PHPT.12 The study of bone markers in PHPT has been of considerable interest for several reasons. First, this inquiry sheds light on which markers accurately reflect skeletal activity in the patient with PHPT. Second, the evaluation of markers of bone turnover in PHPT has provided insight into the hyperparathyroid process in bone. Finally, clues to the extent of postoperative improvements in bone mineral density (BMD) might be provided by markers of bone turnover.
Bone Formation Markers
Bone formation is reflected by osteoblast products, including bone-specific alkaline phosphatase activity, osteocalcin, and type 1 procollagen peptide.12 Despite the availability of these sensitive measurements of bone formation, the total alkaline phosphatase activity—part of the multichannel biochemical screening profile—is still widely assessed in PHPT. In PHPT, levels can be mildly elevated, but in many patients, total alkaline phosphatase values are within normal limits.49,50 In a small study from our group,51 bone-specific alkaline phosphatase activity correlated with PTH levels and BMD at the lumbar spine and femoral neck. Osteocalcin is also generally increased in patients with PHPT.51–53 Osteocalcin correlates with other indices of bone formation. Assays for procollagen extension peptides reflect osteoblast activation and bone formation but have not been shown to have significant predictive or clinical utility in PHPT.53 In a small study of patients with PHPT, C-terminal propeptide of human type 1 procollagen (PICP) levels were higher than in control subjects,54 but distinct elevations were much less impressive than those seen for alkaline phosphatase, osteocalcin, or even hydroxyproline (see later).
Bone Resorption Markers
Markers of bone resorption include the osteoclast product, tartrate-resistant acid phosphatase (TRAP), and collagen breakdown products such as hydroxyproline, hydroxypyridinium cross-links of collagen, and N- and C-telopeptides of type 1 collagen.12 Urinary hydroxyproline, once the only available marker of bone resorption, no longer offers sufficient sensitivity or specificity to make it a useful tool in the assessment of patients with PHPT. Although urinary hydroxyproline was frankly elevated in patients with osteitis fibrosa cystica, in mild asymptomatic PHPT, it is now generally normal. Hydroxypyridinium cross-links of collagen, pyridinoline (PYD), and deoxypyridinoline (DPD), on the other hand, are often elevated in PHPT. They return to normal after parathyroidectomy.55 DPD and PYD both correlate positively with PTH concentrations. N- and C-terminal peptides of type I collagen (NTX and CTX) are likely to have utility, but they have not been studied extensively in PHPT. Other markers of bone resorption have also been limited in their application to bone turnover in PHPT. For example, studies of TRAP are limited, although levels have been shown to be elevated.47 In the case of the PYD cross-linked telopeptide domain of type I collagen (ICTP), pooled data from patients with high turnover diseases (i.e., PHPT as well as hyperthyroidism) suggest that this marker may reflect calcium kinetics and histomorphometric indices.12 Data specifically relevant to PHPT are not yet available. Bone sialoprotein, a phosphorylated glycoprotein which makes up approximately 5% to 10% of the noncollagenous bone protein, appears to reflect processes associated with both bone formation and bone resorption. In PHPT, bone sialoprotein levels are elevated and correlate with urinary PYD and DPD.55 Thus, sensitive assays of bone formation and bone resorption are both elevated in mild PHPT.
Longitudinal Bone Marker Studies
Studies of bone markers in the longitudinal follow-up of patients with PHPT are limited but indicate a reduction in these turnover markers following parathyroidectomy. Information from our group,55,56 Guo et al.,57 and Tanaka et al.58 all report declining levels of bone markers following surgery, although the choice of markers in the individual studies differed. Data are also emerging concerning the kinetics of change in bone resorption versus bone formation following parathyroidectomy. We have found that markers of bone resorption decline rapidly following cure of PHPT, while indices of bone formation follow a more gradual decrease.55 Urinary PYD and DPD decreased significantly as early as 2 weeks following parathyroidectomy, preceding reductions in alkaline phosphatase. Similar data were reported from Tanaka et al.,58 who demonstrated a discrepancy between changes in NTX (reflecting bone resorption) and osteocalcin (reflecting bone formation) following parathyroidectomy, and Minisola et al.,54 who reported a drop in bone resorptive markers and no significant change in alkaline phosphatase or osteocalcin. Short-term studies reported a brief increase in PICP immediately following parathyroidectomy, while bone resorptive markers fell promptly. The persistence of elevated bone formation markers coupled with rapid declines in bone resorption markers indicates a shift in the coupling between bone formation and bone resorption toward an anabolic buildup of bone mineral postoperatively. Increases in bone density postoperatively provide support for this idea.
CYTOKINES
Although studies of bone markers shed light on the skeletal manifestations of PHPT, other molecules have been studied to elucidate the mechanism underlying the effects of PTH excess on bone. These factors, or cytokines, released in response to PTH, lead to important direct and indirect effects on bone cells. Some, such as interleukins (ILs)-1, -6, and -11, transforming growth factor β (TGF-β), epidermal growth factor (EGF), and tumor necrosis factor (TNF), stimulate bone resorption. Others, including IL-4, insulin-like growth factor 1 (IGF-1), TGF-β, and interferons, may be anabolic for bone. Alterations in the levels of some or all of these cytokines may account for the mechanism of accelerated bone turnover and selective bone loss in PHPT.
IL-6 and TNF-α have been studied as possible mediators of bone resorption in PHPT. In vitro and in vivo data support an effect of PTH in stimulating production of IL-6, which in turn leads to increased osteoclastogenesis.59,60 Furthermore, antibodies to IL-6 prevent PTH-mediated bone resorption. In PHPT, serum levels of TNF-α and IL-6 are increased and fall following parathyroidectomy.61 Importantly, TNF and IL-6 concentrations correlate with the level of PTH in patients with PHPT. Bone turnover markers correlate with levels of these cytokines as well, supporting an important role of the cytokines in mediating the skeletal effects of PTH excess in PHPT.62,63 Furthermore, IL-6 soluble receptor has recently been reported to predict rates of bone loss in mild PHPT. Although cytokine levels did not correlate significantly with bone density measurements in the report of Grey et al.,61 it should be noted that bone density was not assessed at the site containing mostly cortical bone (the radius) where the catabolic effects of excess PTH would be expected to be seen most clearly. More information is needed to confirm these observations, especially with appropriate control subjects, and to test for potential involvement of other bone-resorptive cytokines in PHPT.
The involvement of other factors in the anabolic effect of PHPT on bone is also being studied. Levels of IGF-1, which is well documented to be a direct mediator of PTH action in bone, and insulin-like growth factor–binding protein 3 (IGFBP-3), the major binding protein for IGF-1, change following parathyroidectomy in PHPT.64 The alteration in the ratio of IGF-1 to IGFBP-3 supports enhanced delivery of IGF-1 to tissues following surgery, an increase inversely proportional to the observed rise in lumbar spine and femoral neck bone density. It is not known whether the anabolic properties of PTH at cancellous sites (e.g., lumbar spine) can be explained by IGF-1 prior to surgery.
BONE DENSOMETRY
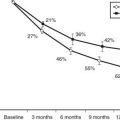
The advent of bone mineral densitometry as a major diagnostic tool for osteoporosis occurred at a time when the clinical profile of PHPT was changing from a symptomatic to an asymptomatic disease. This fortuitous timing allowed questions about skeletal involvement in PHPT to be addressed when specific radiologic features of PHPT had all but disappeared. Bone mass measurements could provide information about the actual state of bone mineral with great accuracy and precision. The known physiologic proclivity of PTH to be catabolic at sites of cortical bone make a cortical site essential to any complete densitometric study of PHPT. By convention, the distal third of the radius is the site used. The early densitometric studies in PHPT also revealed another physiologic property of PTH, namely, to be anabolic at cancellous sites. The lumbar spine became an important site to measure not only because it is predominantly cancellous bone, but also because postmenopausal women are at risk for cancellous bone loss. In PHPT, bone density at the distal third of the radius is diminished.65,66 Bone density at the lumbar spine is only minimally reduced. The hip region, containing a relatively equal mixture of cortical and cancellous elements, shows bone density intermediate between the cortical and cancellous sites (Fig. 62-2). The results support not only the notion that PTH is catabolic for cortical bone but also the view that PTH is anabolic in cancellous bone.67–69 In postmenopausal women, the same pattern was observed.66 Postmenopausal women with PHPT, therefore, show a reversal of the pattern typically associated with postmenopausal bone loss: preferential loss of cancellous bone. The reduced bone density at the distal radius (cortical bone) and preserved density at the lumbar spine (cancellous bone) suggest that PHPT helps protect postmenopausal women from bone loss due to estrogen deficiency.
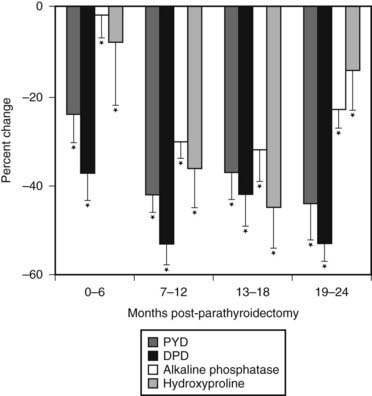
FIGURE 62-2. Bone densitometry in primary hyperparathyroidism. Data are shown in comparison to age- and sex-matched normal subjects. Divergence from expected values is different at each site (P < 0.0001).
(Data from Silverberg SJ, Shane E, de la Cruz L et al: Skeletal disease in primary hyperparathyroidism, J Bone Miner Res 4:283–291, 1989.)
Observations of skeletal health in PHPT made by bone densitometry have established the importance of this technology in the evaluation of all patients with PHPT. Without this information, the data set leading to surgical recommendations is incomplete. The Consensus Development Conference on Asymptomatic Primary Hyperparathyroidism in 1990 implicitly acknowledged this point when bone mineral densitometry was included as a separate criterion for clinical decision making.70 Since that time, bone densitometry has become an indispensable component of both evaluating the patient and establishing clinical guidelines for management and monitoring.
The bone density profile in which there is relative preservation of skeletal mass at the vertebrae and diminution at the more cortical distal radius is not always seen in PHPT. While this pattern is evident in the vast majority of patients, a small group of patients have evidence of vertebral osteopenia at the time of presentation. In our natural history study, approximately 15% of patients had a lumbar spine Z score of less than −1.5 at the time of diagnosis.72 Only half of these patients were postmenopausal women, so not all vetebral bone loss could be attributed entirely to estrogen deficiency. These patients are of interest with regard to changes in bone density following parathyroidectomy and are discussed in further detail later. The extent of vertebral bone involvement will vary as a function of disease severity. In the typical mild form of the disease, the pattern described earlier is seen. When PHPT is more advanced, there will be more generalized involvement, and the lumbar spine will not appear to be protected. When PHPT is severe or more symptomatic, all bones can be extensively involved.
BONE HISTOMORPHOMETRY
Analyses of percutaneous bone biopsies from patients with PHPT have provided direct information that could only be indirectly surmised by bone densitometry and by bone markers. Both static and dynamic parameters present a picture of cortical thinning, maintenance of cancellous bone volume (Fig. 62-3), and a very dynamic process associated with high turnover and accelerated bone remodeling.
FIGURE 62-3. Scanning electron micrograph of bone biopsy specimens in a normal subject (top) and an age- and sex-matched patient with primary hyperparathyroidism (bottom). The cortices of the hyperparathyroid sample are markedly thinned, but cancellous bone and trabecular connectivity appear to be well preserved. (Magnification ×31.25.)
(From Parisien MV, Silverberg SJ, Shane E et al: Bone disease in primary hyperparathyroidism, Endocrinol Metab Clin North Am 19:19–34, 1990.)
Cortical thinning, inferred by bone mineral densitometry, is clearly documented in a quantitative manner by iliac crest bone biopsy.72–74 Van Doorn et al.75 demonstrated a positive correlation between PTH levels and cortical porosity. These findings are consistent with the known effect of PTH to be catabolic at endocortical surfaces of bone. Osteoclasts are thought to erode more deeply along the corticomedullary junction under the influence of PTH.
Histomorphometric studies have contributed most by elucidating the nature of cancellous bone preservation in PHPT.75 Again, as suggested by bone densitometry, cancellous bone volume is clearly well preserved in PHPT. This is seen as well among postmenopausal women with PHPT. Several studies have shown that cancellous bone is actually increased in PHPT as compared to normal subjects.76,77 When cancellous bone volume is compared among age- and sex-matched subjects with PHPT or postmenopausal osteoporosis, a dramatic difference is evident (Fig. 62-4). Whereas postmenopausal women with osteoporosis have reduced cancellous bone volume, women with PHPT have higher cancellous bone volume.77 This observation suggests that while PHPT is said to be a risk factor for postmenopausal osteoporosis, it is a syndrome of bone loss. The region(s) of bone loss in PHPT is directed toward the cortical bone compartment, with good maintenance of cancellous bone volume unless the PHPT is unusually active.
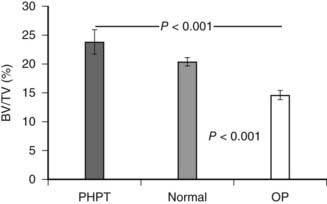
FIGURE 62-4. Cancellous bone volume in primary hyperparathyroidism. Cancellous bone volume was analyzed from bone biopsy specimens of the iliac crest. Comparisons are between 16 women with primary hyperparathyroidism, 17 women with postmenopausal osteoporosis, and 31 women with no known disorder of bone metabolism. Subjects were matched for age and other indices.
(Data from Parisien M, Cosman F, Mellish RW et al: Bone structure in postmenopausal hyperparathyroid, osteoporotic and normal women, J Bone Miner Res 10:1393–1399, 1995.)
Preservation of cancellous bone volume even extends to comparisons with the expected losses associated with the effects of aging on cancellous bone physiology. In a study of 27 patients with PHPT (10 men and 17 women), static parameters of bone turnover (osteoid surface, osteoid volume, and eroded surface) were increased, as expected, in patients relative to control subjects.78 However, in control subjects, trabecular number varied inversely with age, while trabecular separation increased with advancing age. Both of these observations are expected concomitants of aging. In marked contrast, in the patients with PHPT, no such age dependency was seen. There was no relationship between trabecular number or separation and age in PHPT, suggesting that the actual plates and their connections were being maintained over time more effectively than one would have expected through the aging process. Thus, PHPT seems to retard the normal age-related processes associated with trabecular loss.
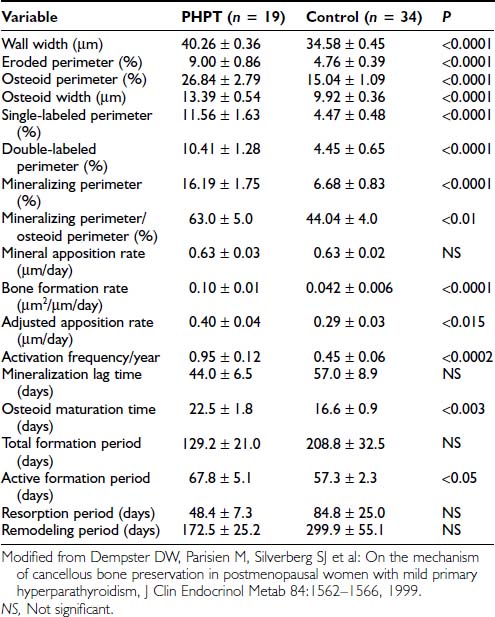
In PHPT, indices of trabecular connectivity are greater than expected, while indices of disconnectivity are decreased. When three matched groups of postmenopausal women were assessed (a normal group, a group with postmenopausal osteoporosis, and a group with PHPT), women with PHPT were shown to have trabeculae with less evidence of disconnectivity compared with normals, despite increased levels of bone turnover,76,78 so cancellous bone is preserved in PHPT through the maintenance of well-connected trabecular plates. In order to determine the mechanism of cancellous bone preservation in PHPT, static and dynamic histomorphometric indices were compared between normal and hyperparathyroid postmenopausal women. In normal postmenopausal women, there is an imbalance in bone formation and resorption which favors excess bone resorption. In postmenopausal women with PHPT, on the other hand, the adjusted apposition rate is increased. Bone formation, thus favored, may explain the efficacy of PTH at cancellous sites in patients with osteoporosis.67,79–81 Assessment of bone remodeling variables in patients with PHPT shows increases in the active bone-formation period77 (Table 62-4). The increased bone formation rate and total formation period may explain the preservation of cancellous bone seen in this disease.
Table 62-4. Wall Width and Remodeling Variables in Primary Hyperparathyroidism (PHPT) and Control Groups (Mean ± SEM)
Recently, further analysis of trabecular microarchitecture has taken advantage of newer technologies that have largely been confirmatory. In a three-dimensional analysis of transiliac bone biopsies using microCT technology, a highly significant correlation was observed with the conventional histomorphometry82 described earlier. In comparison to age-matched control subjects without PHPT, postmenopausal women with PHPT had higher bone volume (BV/TV), higher bone surface area (BS/TV), higher connectivity density (Conn.D), and lower trabecular separation (Tb.Sp.). There were also less marked age-related declines in BV/TV and Conn.D as compared to controls, with no decline in BS/TV. Using the technique of backscattered electron imaging (qBEI) to evaluate trabecular BMD distribution (BMDD) in iliac crest bone biopsies, Roschger et al.83 showed reduced average mineralization density and increase in the heterogeneity of the degree of mineralization, consistent with reduced mean age of bone tissue. Studies of collagen maturity using Fourier Transform Infrared Spectroscopy provide further support for these observations.84 Bone strength, therefore, in PHPT has to take into account a number of factors related to skeletal properties of bone besides BMD.85
FRACTURES
Fractures were an integral element of classic PHPT, but their importance in modern-day disease is unclear. In a case-control study published in 1975, Dauphine et al.86 suggested that back pain and vertebral crush fractures might be part of the presenting clinical profile of PHPT. Since that time, reports on fracture incidence have been conflicting. A retrospective review of lateral chest radiographs of patients who underwent parathyroidectomy showed an increased incidence of vertebral fractures in one study,87 while Wilson et al.88 found no increase in such fractures in a cohort of 174 consecutive patients who had mild asymptomatic PHPT.
In a study that focused on hip fracture, a population-based prospective analysis (mean of 17 years’ duration; 23,341 person years) showed women with PHPT in Sweden not to be at increased risk.89 In a much smaller study (46 patients, 44 controls), fractures at any site were increased in hyperparathyroidism.90 This study is flawed not only by its small sample size but also by the unusually high fracture incidence in both patients (48%) and control subjects (28%) and by the use of thyroid medication in a significantly greater number of patients (28%) relative to control subjects.
The Mayo Clinic experience with PHPT and risk of fracture reviewed 407 cases of PHPT recognized during the 28-year period between 1965 and 1992.91 Fracture risk was assessed by comparing fractures at a number of sites with numbers of fractures expected on the basis of sex and age from the general population. The clinical presentation of these patients with PHPT was typical of the mild form of the disease, with the serum calcium being only modestly elevated at 10.9 ± 0.6 mg/dL. The data from this retrospective epidemiologic study indicate that overall fracture risk was significantly increased at many sites such as the vertebral spine, the distal forearm, the ribs, and the pelvis. There was no increase in hip fractures. After multivariate analysis, age and female sex remained significant independent predictors of fracture risk. These data, however, are subject to potential ascertainment bias. Patients with PHPT are typically followed more conscientiously, and thus fractures at some of these sites may have been recognized by greater surveillance. This may certainly be true of the vertebral spine and the ribs but unlikely in the case of fractures of the forearm. One might expect to see an increased incidence of distal forearm fractures, since the hyperparathyroid process tends to lead to reduction of cortical bone (distal forearm) in preference to cancellous bone (vertebral spine). Unfortunately, there were no densitometric data provided in this study, so one could not relate bone density to fracture incidence. It is difficult to know whether this study in fact confirms an expectation of preferential distal forearm fractures in PHPT or whether some other process is at work conferring universally greater fracture risk in these patients. In a more recent study, Vignali et al.92 studied the incidence of vertebral fractures in PHPT as determined by DXA-based vertebral fracture assessment (VFA). In this case control study, 150 consecutive patients and 300 healthy women matched for age and menopausal age were studied. Vertebral fractures were detected in more subjects with PHPT (24.6%) than the control subjects (4.0%; P < 0.001). Among asymptomatic PHPT patients, only those who met surgical guidelines showed a higher incidence of vertebral fractures compared with controls. We still lack prospective, controlled studies to determine fracture incidence in PHPT.
Even the expectation of an increased fracture risk at a cortical site, like the forearm, in PHPT is fraught with uncertainties. The expectation is based upon bone-density data and the presumption that in this disease, it is as predictive of fracture as it is in postmenopausal women who do not have PHPT. By analogy with osteoporosis, it is reasonable to consider bone density as a risk factor for fracture in PHPT, but other issues could lead to a different relationship between bone density and fracture risk in PHPT. It is now known that bone density is only one of several important qualities of bone. These other qualities are influential in the overall assessment of fracture risk. Bone size, for example, influences fracture risk. From the clinical trials of parathyroid hormone in the treatment of osteoporosis, as well as in observations of PHPT per se, it is likely that bone size is affected by PTH. Cortical thinning through PTH-mediated endosteal resorption is compensated for by PTH-mediated periosteal apposition, leading to bone that may be increased in cross-sectional diameter. This increase in bone size provides biomechanical protection for the skeleton. In PHPT, an interesting paradigm is set up: cortical thinning tends to increase fracture risk, whereas increased bone size tends to reduce fracture risk. In addition, as noted, microarchitecture of bone does not show the same kind of deterioration that is commonly seen in postmenopausal osteoporosis. The relative preservation of microarchitecture in PHPT is another factor that may protect bone. More recent studies in which hyperparathyroid bone has been studied with regard to bone mineralization density83 and collagen quality84 may also be relevant to this discussion (see earlier). These considerations emphasize the need for prospective studies of site-specific fracture incidence in PHPT.85,92–94
Nephrolithiasis
In the past, classic clinical descriptions of PHPT emphasized skeletal involvement and kidney stones as principal complications of the disease.95 The cause of nephrolithiasis in PHPT is probably multifactorial. An increase in the amount of calcium filtered at the glomerulus due to the hypercalcemia of hyperparathyroidism may lead to hypercalciuria despite the physiologic actions of PTH to facilitate calcium reabsorption. A component of absorptive hypercalciuria exists in this disorder. The enhanced intestinal calcium absorption is believed to be due to increased production of 1,25(OH)2D, a consequence of another physiologic action of PTH, namely to increase the synthesis of this active metabolite.96,97 Urinary calcium excretion is correlated with 1,25(OH)2D levels.97,98 In addition, increased intestinal calcium absorption seen in nephrolithiasis99 may also occur in PHPT. The skeleton provides yet another possible source for the increased levels of calcium in the glomerular filtrate. Hyperparathyroid bone resorption might contribute to hypercalciuria, and subsequently to nephrolithiasis, even though there is no convincing evidence to support this hypothesis.100 Finally, alteration in local urinary factors, such as a reduction in inhibitor activity or an increase in stone-promoting factors, may predispose some patients with PHPT to nephrolithiasis.100,101 It remains unclear whether the urine of patients with hyperparathyroid stone disease is different in this regard from that of other stone formers.
Studies in the 1970s and 1980s documented a higher incidence of renal stone disease than do reports of more recent experience. With the decreased incidence of osteitis fibrosa cystica, studies in the modern era have tended to focus on patients with kidney stones. Conflicting results have emerged, with one group providing evidence that 1,25(OH)2D3 plays an etiologic role in the development of nephrolithiasis in PHPT and other groups unable to document differences in 1,25(OH)2D3 levels between those with and without renal stone disease.95,100–102
Although the incidence of nephrolithiasis is much less common than the incidence in the classic, older presentation of PHPT, kidney stones remain the most common manifestation of symptomatic PHPT (see Table 62-1). Estimates in recent studies place the incidence of kidney stones at 15% to 20% of all patients.103 Other renal manifestations of PHPT include hypercalciuria, which is seen in approximately 40% of patients, and nephrocalcinosis, the frequency of which is unknown. It is important to note that in patients with PHPT who do not have renal stone disease, there is no relationship between extent of hypercalciuria and the development of kidney stones.104
In the 1930s, it was generally accepted that bone and stone disease did not coexist in the same patient with classic PHPT.1,6 Albright and Reifenstein1 postulated that low dietary calcium intake would lead to bone disease, while adequate or high dietary calcium levels would be associated with stone disease. Dent et al.,105 who provided convincing evidence against this construct, proposed the existence of two forms of circulating PTH, one causing renal stones and the other causing bone disease. A host of mechanisms, including differences in dietary calcium, calcium absorption, forms of circulating PTH, and levels of 1,25(OH)2D3, were proposed to account for the clinical distinction between bone and stone disease in PHPT.100,105 Today, there is no clear evidence for two distinct subtypes of PHPT. In our patients with PHPT, we could not identify a distinctive set of biochemical data for patients with stone disease.95 Furthermore, although our population did not include patients with classic hyperparathyroid bone disease, we found no evidence to support the notion that the processes affecting the skeleton and kidneys in hyperparathyroidism occur in different subsets of patients. Urinary calcium excretion per gram of creatinine, levels of 1,25(OH)2D, and BMD at all sites were indistinguishable among patients with and without nephrolithiasis. Cortical bone demineralization is as common and as extensive in those with and without nephrolithiasis.95,100
Other Organ Involvement
NEUROCOGNITIVE AND NEUROPSYCHOLOGICAL FEATURES
Over the years, PHPT has been associated with complaints referable to many different organ systems. Perhaps the most common complaints have been those of weakness and easy fatigability.31 Classic PHPT used to be associated with a distinct neuromuscular syndrome characterized by type II muscle cell atrophy.106,107 Originally described by Vicale in 1949,108 the syndrome consisted of easy fatigability, symmetric proximal muscle weakness, and muscle atrophy. Both the clinical and electromyographic features of this disorder were reversible after parathyroid surgery.109,110 In the milder, less symptomatic form of the disease that is common today, this disorder is rarely seen.111 In a group of 42 patients with mild disease, none had complaints consistent with the classic neuromuscular dysfunction described above. Although over half of all patients had nonspecific complaints of paresthesias and muscle cramps, electromyographic studies did not confirm the picture of past observations.
The “psychic groans” described by early observers of patients with classic PHPT remain a source of controversy today. Patients with PHPT often report some degree of behavioral and/or psychiatric symptomatology. A retrospective look at patients with more severe disease revealed a 23% incidence of psychiatric symptomatology (n = 441).112 More recent studies have shown some abnormalities on psychological tests preoperatively, with no improvement after surgery.113,114 The study of Solomon et al.115 studied psychiatric rather than neuropsychological symptoms.115 Using the SCL-90-R rating scale, they observed a constellation of abnormalities, most of which improved after successful surgery. The control group, who underwent neck surgery for nodular thyroid disease, had similar postoperative improvement. This study documented psychiatric symptoms in the patients with PHPT but could not distinguish the improvement in symptomatology to cure of hyperparathyroidism or to the effects of a successful surgical procedure.
The surgical literature provides further data supporting postoperative improvements. Data from Clark et al.116 on 152 patients (thyroid surgery control subjects) found 40% of patients reporting less fatigue after cure. Similar findings were reported by Pasieka and Parsons117 and Burney et al.118
The subject of neurocognitive and neuropsychological impairment was reviewed in detail by Silverberg et al.119 The more recent literature continues to be unclear on whether the symptomatology is specific to PHPT and whether it is improved following successful parathyroid surgery.119–124 Silverberg et al.119 have pointed out key limitations in experimental design in many of the published studies. The lack of adequate controls and the value of some of the quantitative instruments have been problematic. There are now three randomized, prospective trials in which this issue has been addressed.125–127 Despite the rigorous experimental design, with control built into these more recent studies, there was great variability in which features of PHPT if any were improved or not following successful parathyroid surgery. Some of the randomized clinical trials are not yet complete. It is clear that the issue remains unsettled.
CARDIOVASCULAR SYSTEM
Interest in the effect of PHPT on cardiovascular function is rooted in pathophysiologic observations of the hypercalcemic state. Hypercalcemia has been associated with increases in blood pressure, left ventricular hypertrophy, heart muscle hypercontractility, and arrhythmias.128–130 Furthermore, evidence of calcium deposition has been documented in the form of calcifications in the myocardium, heart valves, and coronary arteries. The association of overt cardiovascular symptomatology with modern-day PHPT is unclear. Hypertension, a common feature of PHPT when it is part of a MEN syndrome with pheochromocytoma or hyperaldosteronism, has also been reported to be more prevalent in sporadic, asymptomatic PHPT than in appropriately matched control groups. The mechanism of this association is unknown, and the condition does not clearly remit following cure of the hyperparathyroid state.131,132
An explanation for the inconsistent results reported in the literature on the cardiovascular manifestations of PHPT relates to the fact that the clinical profile of the disease has changed. As a result, the cohorts that have been studied have varied greatly in the severity of their underlying disease. This is particularly true in terms of the serum calcium and parathyroid hormone concentrations, with data from cohorts with marked hypercalcemia and hyperparathyroidism showing the most cardiovascular involvement. Since it is known that both calcium and PTH can independently affect the cardiovascular system, such variability among cohorts can give rise to the inconsistent results that have been reported in the literature. Nevertheless, one can consider this topic usefully in the following terms: mortality, hypertension, cardiac, and noncardiac vascular abnormalities, as well as more subtle functional changes in the cardiovascular system.
Cardiovascular Mortality
There is little doubt that in very active PHPT, cardiovascular mortality is increased.133–136 Of some interest are the postoperative observations in which the higher cardiovascular mortality rate persists for years after cure.137 These observations differ markedly from those in which asymptomatic PHPT have been studied. Although limited, the studies have not shown any increase in mortality.138–139 The Mayo Clinic studies help to bring these observations together. In the mildly hypercalcemic individuals, overall and cardiovascular mortality was reduced, but in those whose serum calcium was in the highest quartile, cardiovascular mortality was increased.139 The idea that the more common asymptomatic form of PHPT is not associated with increased mortality is supported by data from Nilsson et al.140 and by other studies141–142 in which more recently enrolled subjects had better survival than those who had been entered earlier and presumably had more active disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree