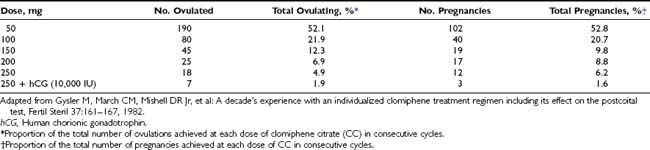
FIGURE 133-1. Distribution of normogonadotrophic oligomenorrheic and amenorrheic infertile women who do or do not ovulate after clomiphene citrate (CC) treatment in incremental daily doses of 50, 100, and 150 mg for 5 subsequent days.
(Data from Imani B, Eijkemans MJ, te Velde ER, et al: Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility, J Clin Endocrinol Metab 83:2361–2365, 1998.)
Results of Treatment With CC
A significant improvement in the occurrence of ovulation has been observed in oligo-ovulatory women subjected to CC treatment (common odds ratio [OR]: 6.82; 95% confidence interval [CI]: 3.92 to 11.85).10 However, although 70% to 85% of patients will ovulate with CC, only 40% to 45% of patients will achieve pregnancy.11 This discrepancy between ovulation and conception rates might be attributed to unreliable confirmation of ovulation, the presence of additional infertility factors, and early discontinuation of treatment. The cumulative conception rate in couples with no other factors contributing to subfertility rises with six to nine successive cycles to 70% to 75%,12,13 approaching the pregnancy rate observed in the normal population.
Resistance to CC
A discernible difference in describing CC-treated populations is seen between CC resistance (failure to ovulate under the maximum allowable dose of CC) and CC failure (failure to conceive after a certain number of ovulatory cycles). CC resistance occurs in about 15% to 30% of patients and is associated with obesity, hyperandrogenemia, amenorrhea, and increased ovarian volume.7 Administration of gonadotrophins in these patients will result in ovulation in about 50% of cases; however, it remains an expensive approach accompanied by potentially serious complications.
Alternatively, in CC-resistant patients, the addition of dexamethasone to the CC regimen has been shown to result in high rates of ovulation and pregnancy.14 In addition, the co-administration of metformin with CC in PCOS patients results in a significantly higher ovulation rate as compared with CC alone (odds ratio: 4.41; 95% CI: 2.37 to 8.22).15 A recent meta-analysis evaluating the role of metformin in PCOS showed that in CC-resistant women, metformin plus CC led to higher live birth rates than CC alone (relative risk: 6.4; 95% CI: 1.2 to 35).16 Opioid receptor blockers alone or in combination with CC have been used successfully in anovulatory patients resistant to CC, indicating the inhibitory action of endogenous opioids to GnRH secretion.17 Adequate monitoring of a CC-stimulated cycle will identify patients who remain anovulatory despite follicular maturation and progressive increments in estradiol levels. In such cases, accurately timed human chorionic gonadotrophin (hCG) administration may confer benefit in inducing ovulation.
Effects of CC at the Periphery
The effects of CC on cervical mucus and on endometrium quality are debatable. Inadequate endometrial development, as reflected by endometrial thickness assessed by ultrasound, may adversely affect implantation and result in biochemical pregnancy in patients undergoing ovulation induction with CC.18 Ultrasonographic findings from the endometrium do not correlate directly with histologic findings, however, and the association between endometrial thickness and pregnancy achievement in CC-treated cycles is not uniformly accepted.19
Side Effects of CC
CC may result in multiple follicular development and thus multiple pregnancies not related to zygotic cleavage. Multiple follicular development resulting from increased gonadotrophin levels in the early follicular phase occurs in 35% to 60% of CC cycles compared to a 5% to 10% occurrence in the general population, whereas the reported multiple pregnancy rate in the literature ranges from 8% to 13%, of which the majority are twins. The need for monitoring CC cycles using ultrasound scans and serum endocrinology should therefore be emphasized. Although the occurrence of ovarian hyperstimulation syndrome (OHSS) is very rare, mild ovarian enlargement and cyst formation may occur with CC treatment. In addition, CC treatment has been associated with infrequent side effects such as hot flushes, visual side effects, abdominal distension, breast discomfort, nausea, and vomiting.
Alternate Medications for Ovulation Induction
Tamoxifen
Both CC and tamoxifen are nonsteroidal estrogen receptor modulators that share a structural homology. Tamoxifen can induce ovulation with efficiency comparable with that of CC.20 Tamoxifen is administered as a 20 mg dose in the early follicular phase of the cycle and, in contrast with CC, has been reported to improve cervical mucus scores in women with an abnormal postcoital test.21
Aromatase Inhibitors
Letrozole, the best known of the aromatase inhibitors, appears to be a promising alternative for ovulation induction.22 Aromatase enzyme is a cytochrome P-450 protein that can catalyze the aromatization of C19 androgens into estrogens.
Induction of ovulation by letrozole is thought to be mediated through increased secretion of gonadotrophins resulting from inhibition of estrogen negative feedback on the hypothalamus. Enhancement of the sensitivity of granulosa cells to FSH through an increase in androgen production may also play a role. The dose of letrozole given for ovulation induction is 2.5 mg daily from day 3 to day 7 of the cycle. Letrozole is as effective as other methods of ovulation induction23; however, concern has been expressed about its safety in terms of neonatal outcome.24
Ovulation Induction Using GnRH
Women with hypogonadotropic hypogonadism (HH) are characterized by reduced hypothalamic or pituitary function. If pituitary function is intact, GnRH treatment can lead to ovulation and establishment of pregnancy. Stimulation with GnRH maintains the normal pituitary-ovarian feedback mechanisms, as rising levels of estradiol inhibit endogenous FSH secretion, allowing the development of a single dominant follicle.
GnRH is administered intravenously (IV) or subcutaneously (SC) in pulses every 60 to 90 minutes at a dose of 15 to 20 µg SC or 5 to 10 µg IV. If the patient fails to respond, the dose may be increased by 5 µg increments. hCG can be used to trigger final oocyte maturation and follicular rupture. The luteal phase can be supported with progesterone/hCG, or, alternatively, the ensuing corpus luteum can be stimulated by continuing the GnRH infusion after ovulation.
GnRH is an effective treatment, with an 80% to 93% cumulative pregnancy rate reported after six treatment cycles.25 Moreover, it is associated more frequently with monofollicular development compared with gonadotrophins; thus its use leads to a low multiple pregnancy rate (4% to 5%).
Although safe and inexpensive, ovulation induction with GnRH requires administration of the medication through an infusion pump for several weeks, resulting in decreased patient compliance and acceptance. Side effects are minimal and are due mainly to local irritation from the pump needle or thrombophlebitis in cases of IV administration.
Ovulation Induction With Gonadotrophins
Gonadotrophins are used for ovulation induction in patients with hypogonadotrophic hypogonadism, in whom the HPO axis cannot be stimulated to produce endogenous gonadotrophins. In addition, they are used for women in the WHO II category, for whom ovulation induction with CC or aromatase inhibitors has failed.
Currently available gonadotrophin preparations are derived from the urine of postmenopausal women or are synthesized by recombinant technology, having replaced those originating from pregnant mares and human pituitaries.
Conventional Step-Up Protocol
Gonadotrophins can be administered according to a conventional step-up protocol, in which stimulation is initiated with 75 IU/day and dose is increased by 75 IU if no response occurs after 7 days. The response to treatment is judged by monitoring estradiol levels. Homburg and colleagues26 reported a cumulative pregnancy rate of 82% after six treatment cycles. This approach, although effective, is associated with an increased chance for multiple follicular development, increasing the probability of multiple pregnancy to 34% and that of hyperstimulation syndrome to 4.6%.27
Low-Dose Step-Up Protocol
Alternatively, a low-dose step-up protocol can be used, especially in patients prone to multifollicular development, as in PCOS. Stimulation starts with 75 IU/day, although lower starting doses have been applied, and remains unchanged for 14 days. If no response is observed, the dose can be increased by 37.5 IU/day. After each increase, the dose remains constant for 7 days.
Several studies have examined the use of a low-dose step-up protocol to avoid the occurrence of multiple pregnancies; the collective results appear in Table 133-2.28 Comparison of a low-dose step-up protocol versus a conventional step-up protocol has shown that pregnancy rates are at least equal, and the probability of multifollicular development and multiple pregnancies appears to be decreased.26
Table 133-2. A Summary of Results of Published Series of Low Starting Dose FSH Therapy for Women With PCOS
| Number (%) | Range (%) | |
|---|---|---|
| Patients | 717 | |
| Cycles completed | 1391 | |
| Clinical pregnancies | 280 (40) | 21-45 |
| Fecundity/cycle | (20) | 12-24 |
| Uniovulatory cycle | (69) | 54-88 |
| OHSS | 0.14 | 0-2.4 |
| Multiple pregnancies | 5.7 | 0-14.1 |
FSH, Follicle-stimulating hormone; OHSS, ovarian hyperstimulation syndrome; PCOS, polycystic ovarian syndrome.
Adapted from Homburg R, Howles CM: Low-dose FSH therapy for anovulatory infertility associated with polycystic ovary syndrome: rationale, results, reflections and refinements, Hum Reprod Update 5:493–499, 1999.
Step-Down Protocol
The step-down protocol is a more physiologic approach that simulates the events of a normal follicular phase, in which FSH levels decline as estradiol levels rise. An initial dose of 150 IU/day is followed by two reducing steps (37.5 IU each) based on sonographic criteria, to a final daily dose of 75 IU, which is sustained until hCG administration.
A comparison between the low-dose step-up and step-down protocols showed that monofollicular development is present in a significantly higher proportion of cases in the step-down protocol (88% vs. 56%, respectively) after a shorter duration of stimulation (9 days vs. 18 days, respectively).29
Which Type of Gonadotrophin Should Be Used for Ovulation Induction?
No conclusive data currently exist on the type of gonadotrophin to be used for ovulation induction.30–32 In hypogonadotropic patients, it appears that luteinizing hormone (LH) is necessary for optimal stimulation of follicle development and estradiol synthesis.33 Utilization of human menopausal gonadotrophins (hMG) in HH patients results in 90% conception rates after 6 months of treatment.34 These are far higher than the success rates of ovulation induction with gonadotrophins in women in the WHO II category. It should be noted, however, that HH patients represent a minority of the patients seeking treatment for anovulation.
Chance of Singleton Live Birth After Ovulation Induction Using CC or in CC-Resistant Patients Using Gonadotrophins
Eijkemans and colleagues35 prospectively studied 240 consecutive anovulatory patients (WHO II) who had not been treated previously. Patients underwent six cycles with CC before switching to gonadotrophins if no pregnancy ensued, or three cycles of CC if they remained anovulatory. Stimulation with gonadotrophins was performed according to a step-down protocol. Singleton live birth occurred in 56% of women, and the cumulative pregnancy rate leading to singleton live birth was 50% and 71% after 12 and 24 months, respectively. The cumulative chance of pregnancy increased to 74% when multiple pregnancies were included (7% of all births).
GnRH Analogues in Ovulation Induction
A Cochrane review36 was performed to assess the value of GnRH agonist pretreatment in combination with FSH/hMG for ovulation induction in WHO II women with ovulatory dysfunction. No evidence suggesting a benefit of GnRH agonist pretreatment in terms of pregnancy rates could be demonstrated, although a tendency toward increased risk for ovarian hyperstimulation syndrome (OHSS) was associated with the use of GnRH agonists. Pretreatment with GnRH analogues for ovulation induction probably should not be recommended as standard treatment for WHO II patients.
A recent meta-analysis suggests that the use of GnRH antagonists in patients treated with gonadotrophins for intrauterine insemination results in increased pregnancy rates. However, multiple pregnancy rates under this approach require further evaluation.37
OVULATION INDUCTION AND MULTIPLE PREGNANCIES
Several studies have shown38,39 that at least one third of twin pregnancies and most triplet or high-order gestations can be attributed to ovulation induction. This is probably an underestimation of its true contribution to the occurrence of multiple pregnancies, as no reporting system on the use of ovulation-inducing drugs not associated with IVF is available.
Options to Prevent the Occurrence of Multiple Pregnancies From Ovulation Induction
Currently, the positions of professional societies (Society for Assisted Reproductive Technology [SART], American Society for Reproductive Medicine [ASRM], and Society for Reproductive Endocrinology and Infertility [SREI]) coincide in that data are insufficient to apply regulations such as practice guidelines with the goal of avoiding multiple pregnancies from ovulation induction. Multifollicular development during ovulation induction has been treated by converting the cycle to in vitro fertilization (IVF). This allows control of the number of embryos to be transferred. The current availability of GnRH antagonists increases the odds of successful conversion to IVF,40 although a proportion of patients are reluctant to follow such an approach. In these patients, preovulatory follicular reduction may have a role.41 Multifetal reduction is acceptable only if the physician has done all that is possible to prevent the occurrence of multiple pregnancies; it certainly is not preventive in nature, and it is associated with its own risks.
Assisted Reproduction
Assisted reproduction, or assisted reproductive technology (ART), refers to a spectrum of techniques that are aimed at enhancing human reproductive potential, resulting in the delivery of a healthy child. The previous section of this chapter dealt with induction of ovulation, which is used in couples with dysovulation problems. Subfertility due to a cervical factor, a male factor, or unidentified causes can be managed by intrauterine insemination (IUI) alone or in combination with enhancement of ovulation. If these approaches fail to result in pregnancy, the alternative is IVF.
After the first delivery from IVF in 1978, a wide armamentarium of techniques was developed by investigators, who altered or expanded the concept of the original method.42 In parallel, several alternative stimulation protocols (use of GnRH agonists or GnRH antagonists) and newer, safer medications (recombinant FSH [r-FSH], recombinant LH [r-LH], and recombinant hCG [r-hCG]) were introduced for ovarian superovulation, to ensure the availability of female gametes for IVF.
INTRAUTERINE INSEMINATION
In IUI, sperm is transferred within the uterus around the time of ovulation. Facilitation of fertilization is achieved by enhancing the number and the quality of the spermatozoa reaching the fallopian tubes and may be combined with an increase in the number of oocytes ovulated. This is achieved by semen preparation, which removes seminal fluid and selects spermatozoa with progressive motility, and by the use of ovarian stimulation, respectively. Alternative methods of insemination include intravaginal, intracervical, pericervical using a cap, intratubal, and direct intraperitoneal insemination. Intrauterine insemination, however, appears to be the preferred method in most studies.43,44
Timing of IUI is an important factor that may affect its success. This is due to the fact that spermatozoa probably survive for a shorter period after IUI, because they are not deposited in the cervical crypts, as is the case after intercourse. Moreover, data from in vitro studies suggest that the lifespan of the oocyte in vivo during which fertilization can occur is probably short. When ovulation is timed, onset of the LH rise is a more accurate criterion than the LH peak itself. Insemination is performed on the day after initiation of the LH surge or 36 hours after administration of hCG.
IUI can be performed with the husband’s or the donor’s sperm. IUI with donor sperm is used not only in cases of severe male factor but also in single women or lesbian couples.45
A significantly higher fecundity rate at 1 month is present when IUI is compared with sexual intercourse (21.2% vs. 3.9%) in women with cervical factor infertility during a natural cycle.46 On the other hand, in couples with male subfertility, IUI significantly improves the probability of conception compared with timed intercourse, both in natural cycles and in cycles in which ovulation is enhanced.47 Currently, the minimum number of spermatozoa in the ejaculate in the presence of which IUI should be attempted is not known, although IUI success seems to be impaired, with <5% normal spermatozoa and an inseminating motile count of <1 × 106.
Performing double intrauterine insemination in the same cycle48 has not been shown to result in an increased probability of conception as compared with single intrauterine insemination.49
Semen Preparation for Assisted Reproduction
Semen preparation for assisted reproduction is aimed at separating motile sperm from nonmotile, dead sperm, cell debris, and prostaglandins, as well as at initiating capacitation and concentrating motile sperm in a small volume of culture medium. Methods of semen preparation include straight centrifugation washing, glass wool filtration, swim-up, use of Sephadex columns, and discontinuous or continuous gradients.50
IN VITRO FERTILIZATION
Collection of Female Gametes for IVF
Currently, the method of choice for collecting female gametes is the transvaginal route via ultrasound visualization.51 Local anesthesia can be used successfully in most cases. Light vaginal hemorrhage may occur in 8.6% of patients. More severe and rare complications may result from inadvertent puncture of iliac vessels and from pelvic infection by inoculation of vaginal flora intraperitoneally or by bowel injury.
Laparoscopic retrieval of oocytes, used originally by Steptoe and Edwards, is still performed in cases in which the ovaries are inaccessible vaginally, or in cases of gamete intrafallopian transfer (GIFT), wherein laparoscopy is necessary for placing the gametes in the fallopian tube. Other methods for oocyte collection, such as through the abdominal wall and the bladder or via the urethral route, are no longer used.
Male Gametes
The standard procedure for collecting semen involves masturbation into a sterile glass or a plastic flask. In cases of retrograde ejaculation, spermatozoa may be collected in urine that is pre-processed with sodium bicarbonate to adjust urinary pH to 7. In patients suffering from impotence caused by spinal paralysis in the segments T11-L2, ejaculation can be induced by vibration or by electroejaculation, which is effective in 90% of patients.
In cases of azoospermia, several collection techniques, including microsurgical sperm aspiration from the epididymis (MESA), percutaneous sperm aspiration from the epididymis (PESA), biopsy and sperm extraction from the testicle (TESE), and testicular fine-needle sperm aspiration (TEFNA), have been used.
In MESA, several incisions into the epididymis may be required before sperm is found. During TESE, the number of tissue samples removed depends on the origin of azoospermia. Usually in obstructive azoospermia, one tissue sample is enough, whereas in nonobstructive azoospermia, multiple tissue samples may be required. In nonobstructive azoospermia, focal spermatogenesis appears to be present, and sperm can be retrieved in about 50% of cases.
PESA and TEFNA are less invasive techniques than TESE and MESA and are performed more rapidly and without the costly equipment required for TESE and MESA. The numbers of recovered spermatozoa appear to be lower than the number of those present after the use of more invasive procedures; however, at present, evidence is insufficient to recommend any specific sperm retrieval technique for azoospermic men undergoing intracytoplasmic sperm injection (ICSI).52
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree