FIGURE 113-1. Effects of gravity on arterial and venous blood pressures in an erect, motionless man. Arterial and venous pressures in the lower part of the body are increased, and in the upper part of the body are decreased. Note that cerebral arterial pressure is about 15 mm Hg lower than aortic root pressure. Because the brain is enclosed by the rigid skull, venous pressures may be below atmospheric pressure. This results in a relatively constant arterial-venous pressure difference in different parts of the brain.
(From Hainsworth R: Arterial blood pressure. In Henderby GEH, ed: Hypotensive anaesthesia, Edinburgh, 1985, Churchill Livingstone, pp 3–29.1)
Comparative biology has provided information on how our forebears have developed means to circumvent the effects of gravitational stress. This is necessary even in quadrupeds, who can rapidly become hypotensive when suspended with their legs dependent.2 Adaptive mechanisms, even in the same species, provide the ability to cope with differences in morphology, activities, and terrain, as observed in the snake.3 Aquatic (sea) snakes can cope with vertical pressure gradients as they are unaffected by gravity despite their low level of blood pressure (15 to 39 mm Hg), because the density of their blood is similar to that of sea water. Terrestrial (land) snakes have a higher level of blood pressure and by the use of body coiling and tail movements maintain cerebral perfusion pressure, for instance when poised to attack when the head is raised. Arboreal (tree) snakes have a higher level of pressure (50 to 90 mm Hg) caused by increased vascular tone; they are slimmer with a thinner tail to prevent vascular pooling, and they have their heart closer to the head, thus aiding cerebral perfusion. The morphology of certain mammals, such as the giraffe whose head is 20 feet above the heart, poses specific challenges. The giraffe has a markedly elevated systemic blood pressure (almost twice that of humans), which is helped by a large and muscular heart, a series of one-way valves in the jugular vein to prevent blood backflow, and thick-walled arteries in the lower limbs beneath an extremely tight skin that prevents pooling.
In humans, the ability to overcome the effects of gravitational stress is dependent on a variety of factors that include cardiac output, tone in resistance vessels, the state of capacitance vessels, vasoactive hormones that are circulating (renin-angiotensin-aldosterone system) and are locally produced (nitric oxide and endothelin), intravascular volume and overall fluid status, and the baroreceptor reflex, which is part of a highly developed autonomic nervous system. Afferent pathways in the carotid sinus, heart, and major cardiopulmonary vessels relay information to the brain through the vagus and glossopharyngeal nerves. Within the brain are numerous cerebral connections. The efferent pathways consist of the sympathetic outflow to blood vessels and heart, along with the parasympathetic (vagus) nerves to the heart. The baroreflex enables beat-by-beat control of blood pressure through vasoconstriction induced by sympathetic activation, as has been demonstrated in humans using the technique of sympathetic microneurography (Fig. 113-2).4 These factors maintain blood pressure and heart rate when upright. Impairment of one or more vascular, endocrine, volume, or neurogenic factors can result in orthostatic intolerance when standing upright, although this may occur even while sitting. Orthostatic or postural hypotension is the term used to describe the fall in blood pressure that may occur when upright. It is defined as a fall in systolic blood pressure of 20 mm Hg or in diastolic blood pressure of 10 mm Hg, or greater, while standing or with tilted head up for 3 minutes. Orthostatic intolerance may occur independently of orthostatic hypotension, for instance in the postural tachycardia syndrome (PoTS).
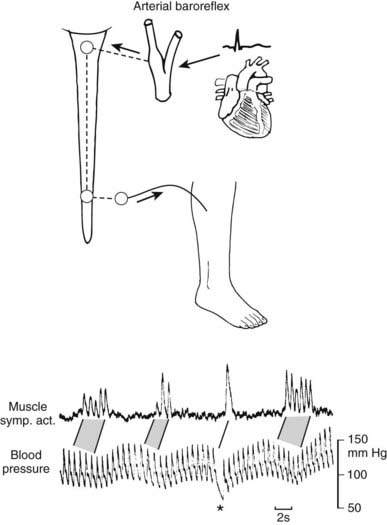
FIGURE 113-2. Relationship between spontaneous fluctuations of blood pressure and muscle nerve sympathetic activity (left) recorded in right peroneal nerve. The baroreceptor reflex accounts for the pulse synchrony of nerve activity and the inverse relationship to blood pressure fluctuations. Asterisk indicates diastolic blood pressure fall due to sudden atrioventricular block. Stippling indicates corresponding sequences of bursts and heartbeats.
(From Wallin BG, Linblad L-E: Baroreflex mechanisms controlling sympathetic outflow to the muscles. In Sleight P, ed: Arterial baroreceptors and hypertension, Oxford, 1988, Oxford University Press, p 101.4)
Classification
Orthostatic hypotension has many causes. Non-neurogenic causes, which are encountered more frequently (Table 113-1), include disorders that reduce intravascular fluid volume, impair function of the heart, or dilate blood vessels.5 Neurogenic causes result from disease affecting the autonomic nervous system. This may occur within the brain, spinal cord, or periphery or at multiple sites.6 Primary disorders have no clear cause or association, as in pure autonomic failure (PAF) and multiple system atrophy (MSA) (Table 113-2). Secondary causes include genetic disorders (familial dysautonomia), enzyme deficiencies (dopamine β-hydroxylase deficiency), and an association with clearly defined disease (diabetes mellitus and spinal cord injury). A variety of drugs can cause orthostatic hypotension by exerting direct pharmacologic effects on the autonomic nervous system, or by causing an autonomic neuropathy (Table 113-3).
Table 113-1. Examples of Non-Neurogenic Causes of Orthostatic Hypotension
| Low Intravascular Volume | |
| Blood/plasma loss | Hemorrhage, burns, hemodialysis |
| Fluid/electrolyte deficiency | |
| Diminished intake | Anorexia nervosa |
| Loss from gut | Vomiting, ileostomy losses, diarrhea |
| Loss from kidney | Salt-losing nephropathy, diuretics |
| Endocrine deficiency | Adrenal insufficiency (Addison’s disease) |
| Cardiac Insufficiency | |
| Myocardial | Myocarditis |
| Impaired ventricular filling | Atrial myxoma, constrictive pericarditis |
| Impaired output | Aortic stenosis |
| Vasodilatation | |
| Endogenous | |
| Exogenous | |
Adapted from Mathias CJ: Autonomic diseases: clinical features and laboratory evaluation, J Neurol Neurosurg Psychiatry 74:iii31–iii41, 2003.
Table 113-2. Neurogenic Causes of Orthostatic Hypotension
Drugs, Chemicals, Poisons, Toxins (See Table 113-3 also) |
Adapted from Mathias CJ: Autonomic diseases: clinical features and laboratory evaluation, J Neurol Neurosurg Psychiatry 74:iii31–iii41, 2003.
Table 113-3. Drugs, Chemicals, Poisons, and Toxins Causing Neurogenic Orthostatic Hypotension
Decreasing Sympathetic Activity |
Adapted from Mathias CJ: Autonomic diseases: clinical features and laboratory evaluation, J Neurol Neurosurg Psychiatry 74:iii31–iii41, 2003.
Orthostatic hypotension usually results in orthostatic intolerance, with difficulties upon standing and occasionally even when sitting. This may be a consistent feature, as often is seen in disorders with “fixed” damage to autonomic nerves. However, some autonomic disorders are “intermittent,” such as neurally mediated syncope, in which transient autonomic dysfunction results in a fall in blood pressure, heart rate, or both, with effects that are more substantial while upright (Table 113-4). In PoTS, symptoms occur predominantly while standing upright or during exertion, but often without a fall in blood pressure. Orthostatic intolerance may occur even in healthy subjects, as in astronauts exposed to negative gravity for a prolonged period.7
Table 113-4. Intermittent Autonomic Disorders That Cause Orthostatic Intolerance
Clinical Features
Orthostatic hypotension causes a variety of symptoms that arise mainly from underperfusion of organs, especially those above the level of the heart, such as the brain (Table 113-5).8 Similar features may be seen in non-neurogenic and neurogenic orthostatic hypotension, although certain aspects differentiate the two when blood pressure falls. Neurogenic causes of orthostatic hypotension often also involve the cardiac parasympathetic nerves, and in such cases the fall in blood pressure is not accompanied by an appropriate rise in heart rate, as may occur otherwise in an attempt to correct hypotension. Thus, in non-neurogenic orthostatic hypotension, tachycardia and palpitations are more likely to accompany the fall in blood pressure. In such subjects, additional compensatory mechanisms to raise blood pressure usually are triggered, causing peripheral vasoconstriction with symptoms of clamminess and sweating. This is less likely to occur in widespread autonomic failure. In orthostatic intolerance due to PoTS, a marked rise in heart rate of over 30 beats per minute (bpm) occurs, without a fall in blood pressure (Fig. 113-3).9
Table 113-5. Some of the Symptoms Resulting From Orthostatic Hypotension and Impaired Perfusion of Various Organs
Adapted from Mathias CJ: Autonomic diseases: clinical features and laboratory evaluation, J Neurol Neurosurg Psychiatry 74:iii31–iii41, 2003.
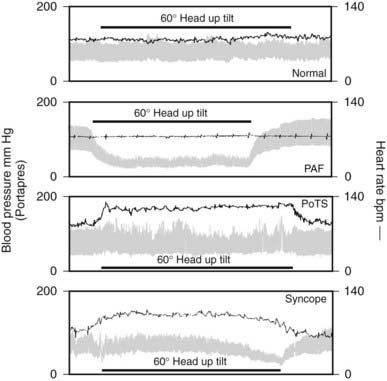
FIGURE 113-3. Blood pressure and heart rate measured continuously before, during, and after 60 degree head-up tilt by the Portapres II in a normal subject and in subjects with three different autonomic disorders: pure autonomic failure (PAF), postural tachycardia syndrome (PoTS), and vasovagal syncope.
(Data from Mathias CJ, Mallipeddi R, Bleasdale-Barr K: Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple system atrophy, J Neurol 246:893–898, 1999.9)
Symptoms arising from orthostatic hypotension can be considered with those arising from hypoperfusion of specific organs (see Table 113-5). The brain is at particular risk, and transient loss of consciousness (syncope, fainting, blackouts) may occur. Symptoms that precede syncope include dizziness, visual disturbances, and, in some, transient cognitive deficits. Several disorders can result in similar symptoms, but the hallmark in orthostatic hypotension is the onset of symptoms on assumption of the upright posture, and regression of symptoms, usually fairly rapidly, on return to the horizontal. In orthostatic hypotension associated with or caused by chronic disorders, many recognize the association with head-up postural change, either before or during the initial onset of symptoms, and introduce corrective means, such as sitting down, lying flat, or assuming curious postures such as squatting or stooping. The adaptive mechanisms depend in part upon the rapidity with which blood pressure may fall. In some, there may be no time as the blood pressure falls precipitously and syncope may occur rapidly, similar to a drop attack, but often with syncope. Loss of consciousness may result in injury. In some, seizures may result from cerebral hypoxia. The fall in blood pressure and symptoms may vary considerably even within the same patient, as there is dependence on a number of factors, including tolerance to a low cerebral perfusion pressure. Symptomatic tolerance may develop over time, probably as the result of improved cerebrovascular autoregulation, which may explain why some are at their worst in the early stages of the disorder, but later tolerate head-up postural change despite a similar fall in blood pressure. Some hyperventilate when blood pressure falls; this should be discouraged, as it has the potential to further reduce cerebral perfusion through cerebral vasoconstriction. In certain disorders, such as in high spinal cord injuries, subjects learn techniques to increase blood pressure, such as by initiation of autonomic dysreflexia (caused by activation of skeletal muscle spasms or urinary bladder contraction), that can trigger spinal reflexes, raise blood pressure, and thus prevent or reduce the postural fall in blood pressure.10 In subjects in whom adaptive mechanisms have not been or cannot be induced, the reduction in blood pressure may result in falling to the ground with the potential for trauma that in some may result in fractures and even death. Patients with cerebrovascular insufficiency due to atheroma and stenosis are at risk, as a relatively small fall in blood pressure can induce cerebral ischemia more readily.
Numerous symptoms are due to organ hypoperfusion, some of which are more common in chronic orthostatic hypotension. Hypoperfusion of tonically active suboccipital and paracervical muscles may result in neck and shoulder ache in a “coat-hanger” distribution, in which the pain is related to the head-up position when blood pressure is low.11 Unlike other causes of neck pain, such as that due to cervical spondylitis, it tends to resolve rapidly when the blood pressure recovers while sitting or lying flat. Chest pain may occur even in the young with apparently normal coronary arteries; mechanisms include chest wall ischemia. Symptoms of low back pain and occasionally even calf claudication may occur; whether this is due to impaired perfusion of muscles or to spinal cord ischemia during standing is difficult to ascertain. Some give a clear history of worsening symptoms and loss of consciousness while activating arm muscles, such as while putting out the washing, reaching upward, or using a lawn mower. This may result in increased blood supply to the pectoral girdle musculature and a subclavian “steal,” further impairing a low vertebrobasilar blood flow.
Orthostatic hypotension can reduce renal hyperperfusion and result in oliguria while upright. The reverse, polyuria, may occur, especially at night, when the subject is supine.12
Some may experience few of the symptoms listed previously, and instead may describe nonspecific symptoms such as weakness, lethargy, and fatigue.8 In the elderly, the key symptom may be falls of otherwise unknown cause.
Many factors can influence the postural fall in blood pressure, especially in patients with autonomic failure (Table 113-6). Some are worse in the morning, probably because of nocturia, which reduces intravascular fluid volume; this also has been observed after prolonged recumbency. The speed of positional change is of importance as rapid movement may lower blood pressure further. Cutaneous vasodilatation, in hot weather and after a warm bath, increases the tendency to orthostatic symptoms. Patients with widespread autonomic failure often have impairment of micturition and defecation, and straining can induce a Valsalva-like maneuver with a further fall in blood pressure. Similar changes may occur during coughing, and even during laughing. Ingestion of water alone, for reasons that remain unclear, can raise blood pressure.13 This differs from the ingestion of food and alcohol that can lower blood pressure substantially, presumably because of splanchnic vasodilatation that in autonomic failure is not opposed by vasoconstriction in other vascular regions, as occurs normally14–16 (Fig. 113-4). Physical exertion, even of a modest nature, also can lower blood pressure substantially, because of vasodilatation in exercising skeletal muscle17–19 (Fig. 113-5). Of importance are the vasodilatory actions of drugs, to which patients with autonomic failure are supersensitive because of their inability to compensate adequately.20 Examples are Parkinsonian patients, in whom the drug l-dopa, in part through the formation of dopamine, can unmask or aggravate orthostatic hypotension. In diabetic patients with an autonomic neuropathy, administration of insulin, by reducing blood volume through increasing transcapillary albumin escape or through vasodilatatory effects, occasionally may exacerbate orthostatic hypotension.
Table 113-6. Factors That May Influence Orthostatic Hypotension
Physical maneuvers and positions (bending forward, abdominal compression, leg crossing, squatting, activating calf muscle pump)† |
* This raises blood pressure in autonomic failure.
† These maneuvers, unlike the others, usually reduce the postural fall in blood pressure in neurogenic causes of orthostatic hypotension.
Adapted from Mathias CJ: Autonomic diseases: clinical features and laboratory evaluation, J Neurol Neurosurg Psychiatry 74:iii31–iii41, 2003.
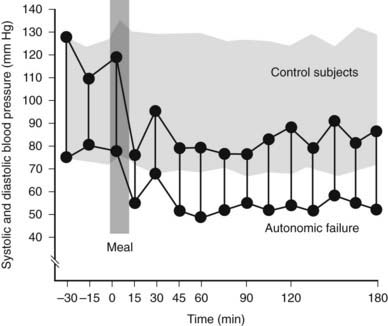
FIGURE 113-4. Supine systolic and diastolic blood pressure before and after a standard meal in a group of normal subjects (stippled area) and in a patient with autonomic failure. Blood pressure does not change in normal subjects after a meal taken while lying flat. A rapid fall in blood pressure to levels around 80/50 mm Hg occurs in the patient; this remains low while in the supine position over the 3 hour observational period.
(Data from Mathias CJ, Bannister R: Postprandial hypotension in autonomic disorders. In Mathias CJ, Bannister R, eds: Autonomic failure: a textbook of clinical disorders of the autonomic nervous system, ed 4, Oxford, 2002, Oxford University Press, pp 283–295.14)
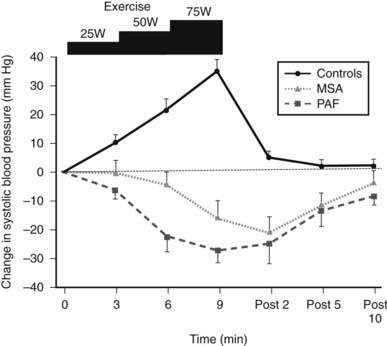
FIGURE 113-5. Changes in systolic blood pressure during supine bicycle exercise at three incremental levels (25, 50, and 75 watts) in normal subjects (controls) and patients with multiple system atrophy (MSA) and pure autonomic failure (PAF). Bars indicate standard error of the mean. Unlike controls, in whom a rise occurs, a fall in blood pressure is noted in both MSA and PAF. Blood pressure returns rapidly to baseline in controls, unlike in the two patient groups in whom it takes almost 10 minutes. All remained horizontal during and for 10 minutes post exercise.
(Data from Smith GDP, Watson LP, Pavitt DV, Mathias CJ: Abnormal cardiovascular and catecholamine responses to supine exercise in human subjects with sympathetic dysfunction, J Physiol (London) 485:255–265, 1995.17)
In the intermittent autonomic disorders, such as neurally mediated syncope, the fall in blood pressure and heart rate is transient and often is related to specific events, although it may occur without a recognized provoking cause. This may cause symptoms of dizziness, palpitations, and clamminess (pre-syncope) that may be followed by syncope. The warning signs vary between individuals and even between episodes in the same subject. In the cardio-inhibitory form, heart rate falls because of increased vagal activity; in the vasodepressor form, blood pressure falls as the result of withdrawal of sympathetic nerve activity to blood vessels; the mixed form involves a varying combination of the two. Neurally mediated syncope has three major causes: vasovagal, carotid sinus hypersensitivity, and miscellaneous causes (see Table 113-4). Most subjects with neurally mediated syncope are otherwise healthy individuals in whom autonomic screening normally shows no abnormalities.21 A variety of factors that include fear and pain may induce a vasovagal episode, hence the term “emotional syncope.” In carotid sinus hypersensitivity, there may be a history of syncope while turning the head, tightening the collar, or shaving. In the elderly, such associations often are not evident. A variety of miscellaneous causes include micturition-induced syncope, as is observed mainly in the male. Choking on objects also may induce syncope. Neurally mediated syncope is more likely to occur in the upright position.
In another intermittent autonomic disorder, the postural tachycardia syndrome (PoTS), orthostatic intolerance often is accompanied by palpitations with a heart rate rise of over 30 bpm, during head-up tilt or standing, without a fall in blood pressure. Many find that the tachycardia is worse during exercise; decompensation due to lack of physical activity may complicate the disorder. In some, there may be features of hyperventilation and panic attacks may occur, although whether this is primary or secondary to the problem remains difficult to determine.
Investigation of Orthostatic Hypotension and Orthostatic Intolerance
In non-neurogenic orthostatic hypotension, investigation will depend on the suspected cause, underlying deficit, and associated disorder. Key reasons for investigation are to confirm diagnosis, aid prognosis, and enable investigation-based management.
In neurogenic causes of orthostatic hypotension, a multi-pronged approach often is needed for evaluation, ideally in an autonomic laboratory. Outlined are screening tests to evaluate cardiovascular autonomic function (Table 113-7); additional tests may be needed. Laboratory investigation is needed for at least three purposes:
Table 113-7. Investigation of Neurogenic Orthostatic Hypotension
* Indicates screening autonomic tests used in our London Units. Gravitational stress (head-up tilt and standing) may be combined with measurement of plasma norepinephrine and epinephrine, as a marker of sympathetic neural and humoral activation.
Adapted from Mathias CJ, Bannister R: Investigation of autonomic disorders. In Mathias CJ, Bannister R, eds: Autonomic failure: a textbook of clinical disorders of the autonomic nervous system, ed 4, Oxford, 2002, Oxford University Press, pp 169–195.
In neurally mediated syncope, testing may have to be designed around the individual patient and circumstances. In generalized autonomic disease, extensive investigation of the nervous system may be required.
In the clinic, orthostatic hypotension can be measured while lying down and then sitting or standing. In the laboratory, head-up tilt (60 degrees) too often is used as the postural stimulus, especially when the neurologic deficit or severe hypotension makes it difficult for the patient to stand. Blood pressure and heart rate can be measured accurately through noninvasive techniques, many of which are automated and provide a printout at preset intervals. In autonomic failure, considerable variability in basal supine levels and postural fall in blood pressure may occur; the greatest changes often occur in the morning, after a meal, and following physical exertion. In such patients, non-neurogenic causes also must be considered, especially as they worsen neurogenic orthostatic hypotension.
Autonomic screening tests, in addition to head-up tilt testing, help to determine the site and extent of the cardiovascular autonomic abnormality. Responses to the Valsalva maneuver, during which intrathoracic pressure is raised to a maximum of 40 mm Hg, depend on the integrity of the entire baroreflex pathway. Changes in heart rate alone may provide a useful guide. Some patients, however, may raise mouth pressure without necessarily raising intrathoracic pressure, resulting in a falsely abnormal heart rate response. Stimuli that raise blood pressure, such as isometric exercise (by sustained hand grip for 3 minutes), the cold pressor test (immersing the hand in ice slush for 90 seconds), and mental arithmetic (using serial-7 or -17 subtraction), activate different afferent or central pathways, which then stimulate the sympathetic outflow. Heart rate responses to postural change, deep breathing (sinus arrhythmia), and hyperventilation assess the cardiac parasympathetic (vagus).
Additional investigations may be needed to determine factors that cause or contribute to orthostatic hypotension and syncope. These include responses to food ingestion, exercise, and carotid sinus massage. To assess postprandial hypotension, cardiovascular responses to a balanced liquid meal containing carbohydrate, protein, and fat are measured while supine, with comparisons of the blood pressure response to head-up tilt performed before the meal and 45 minutes later. To evaluate exercise-induced hypotension, responses are obtained during graded incremental supine exercise using a bicycle ergometer with measurement of postural responses before and after exercise. In suspected carotid sinus hypersensitivity, resuscitation facilities should be available because carotid massage may cause profound bradycardia or cardiac arrest. Massage also should be performed during head-up tilt as hypotension may occur only in this position, because of the greater dependence on sympathetic tone. Intermittent ambulatory blood pressure and heart rate recordings over a 24-hour period using small computerized lightweight devices are of particular value, especially at home, in determining the effects of various stimuli in daily life. However, it is essential in autonomic disease, in contrast to hypertension, that appropriate protocols are followed and an accurate diary of events maintained to determine the effects of postural change, food, and exercise (Fig. 113-6). The information obtained from such recordings is also of value in determining the beneficial effects of therapy.
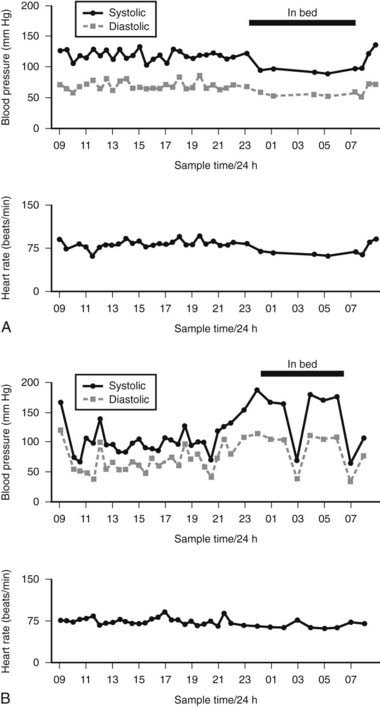
FIGURE 113-6. 24-Hour noninvasive ambulatory blood pressure profile, showing systolic O—–O and diastolic (ρ—–ρ) blood pressure and heart rate at intervals throughout the day and night. A, Changes in a normal subject with no postural fall in blood pressure; a fall in blood pressure is seen at night while asleep, with a rise in blood pressure on waking. B, Marked falls in blood pressure are usually the result of postural changes caused by sitting or standing. Supine blood pressure, particularly at night, is elevated. Getting up to micturate causes a marked fall in blood pressure (at 0300 hours). Reversal of diurnal changes in blood pressure is evident. Relatively small changes in heart rate occur, given the marked changes in blood pressure.
(Data from Mathias CJ, Bannister R: Investigation of autonomic disorders. In Mathias CJ, Bannister R, eds: Autonomic failure: a textbook of clinical disorders of the autonomic nervous system, ed 4, Oxford, 2002, Oxford University Press, pp 169–195.22)
Plasma catecholamine measurements are available in specialized laboratories and may be of value (Fig. 113-7). Plasma norepinephrine provides a measure of sympathetic neural activity and plasma epinephrine of adrenal medullary activity. In PAF, supine basal levels of plasma norepinephrine are low and suggest a distal lesion, compared with MSA, in which supine levels are often within the normal range. In both groups, an attenuation or lack of rise in plasma norepinephrine is seen during head-up tilt, indicating impairment of sympathetic neural activity. In high spinal cord lesions, basal plasma norepinephrine and epinephrine levels are low and do not rise with postural change. However, a rise (but only moderately above the basal levels of normal subjects) is noted during severe hypertension accompanying autonomic dysreflexia, which differs with paroxysmal hypertension due to a pheochromocytoma, in which plasma norepinephrine or epinephrine levels usually are greatly elevated (Fig. 113-8). Extremely low or undetectable levels of plasma norepinephrine and epinephrine with elevated plasma dopamine levels occur in sympathetic failure caused by deficiency of the enzyme dopamine β-hydroxylase (DBH), which converts dopamine into norepinephrine.
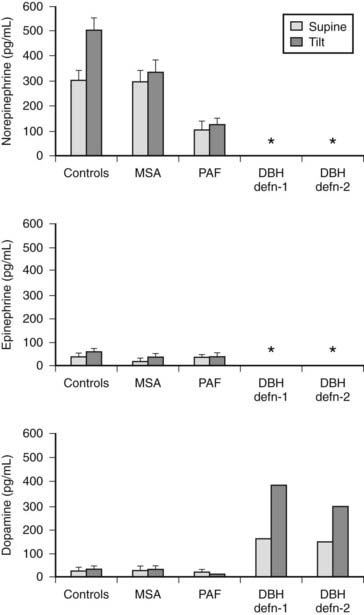
FIGURE 113-7. Plasma norepinephrine, epinephrine, and dopamine levels (measured by high-pressure liquid chromatography) in normal subjects (controls), in patients with multiple system atrophy (MSA) or pure autonomic failure (PAF), and in two individual patients with dopamine β-hydroxylase (DBH) deficiency while supine and after head-up tilt to 45 degrees for 10 minutes. Asterisk indicates levels below the detection limits for the assay, which are less than 5 pg/mL for norepinephrine and epinephrine, and less than 20 pg/mL for dopamine. Bars indicate ± SEM.
(Data from Mathias CJ, Bannister R: Investigation of autonomic disorders. In Mathias CJ, Bannister R, eds: Autonomic failure: a textbook of clinical disorders of the autonomic nervous system, ed 4, Oxford, 2002, Oxford University Press, pp 169–195.22)
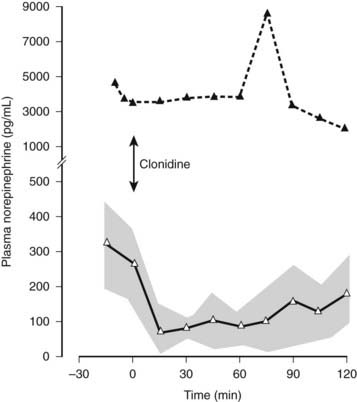
FIGURE 113-8. Plasma norepinephrine levels in a patient with a pheochromocytoma (black triangles) and in a group of patients with essential hypertension (open triangles) before and after intravenous clonidine, indicated by an arrow (2 µg/kg over 10 min). Plasma norepinephrine levels fall rapidly in individuals with essential hypertension after clonidine and remain low over the period of observation. Stippled area indicates ± SEM. Plasma norepinephrine levels are considerably higher in the patient with pheochromocytoma and are not affected by clonidine.
(Data from Mathias CJ, Bannister R: Investigation of autonomic disorders. In Mathias CJ, Bannister R, eds: Autonomic failure: a textbook of clinical disorders of the autonomic nervous system, ed 4, Oxford, 2002, Oxford University Press, pp 169–195, 2002.22)
Muscle and skin sympathetic nervous activity can be recorded directly by percutaneous insertion of tungsten microelectrodes into the peroneal or median nerves (see Fig. 113-1). Muscle sympathetic activity is closely linked to the baroreceptor reflex, with a clear relationship to blood pressure. In high spinal cord lesions, baseline neural activity is reduced, consistent with low basal plasma norepinephrine and blood pressure levels, because of the lack of transmission of tonic brain stem sympathetic activity. Increased nerve firing occurs in the Guillain-Barré syndrome, with hypertension and tachycardia. These microneurographic approaches have enhanced our understanding of the pathophysiologic processes but are of limited clinical application, especially in the investigation of autonomic failure.
Pharmacologic approaches determine the degree of sensitivity of different receptors and the functional integrity of sympathetic nerves and cardiac vagi. Some have value in the clinical situation. Repeat head-up tilt after stepwise intravenous atropine (to a maximum of 1800 µg), when the rate rises to 120 bpm, helps to determine the role of maintaining heart rate, such as by cardiac pacing in the cardio-inhibitory forms of vasovagal syncope. A vasodepressor response while heart rate is maintained indicates that pacing is unlikely to be effective.
Certain pharmacologic challenges, often combined with hormonal measurement, provide information in different disorders. Basal plasma norepinephrine levels may be elevated as the result of stress and other factors; in these situations, the central sympatholytic actions of clonidine suppress plasma norepinephrine levels; this does not occur with autonomous secretion in pheochromocytoma. Another central action of clonidine, through the hypothalamus and the anterior pituitary, is stimulation of growth hormone release. Serum growth hormone levels rise in normal subjects and in PAF with distal autonomic lesions; no response is seen in MSA, in which the lesions are central.
Advances in modern technology enable especially noninvasive measurement of autonomic cardiac function and blood flow in various regions. A variety of spectral analytic techniques are used to assess cardiovascular function. Invasive techniques measure total body and regional norepinephrine spillover in the heart, splanchnic, and renal circulations and in the brain. Radionuclide 123I-metaiodobenzylguanidine imaging assesses cardiac sympathetic innervation. These techniques have a role in the clinical research setting, and in due course, some may be applied to the clinical investigation of cardiovascular autonomic function.
Details of investigations in neurogenic orthostatic hypotension are described in major textbooks.22,23
Brief Descriptions of Key Disorders
NON-NEUROGENIC CAUSES OF ORTHOSTATIC HYPOTENSION
Many of the causes of orthostatic hypotension outlined in Table 113-1 do not warrant further description, other than brief mention of certain endocrine deficiencies.
Endocrine
Adrenal Failure
A reduction in cortisol or aldosterone levels or a combination of the two that occurs in Addison’s disease can cause orthostatic hypotension.
Hypocortisolism
Low cortisol levels may result from a primary adrenocorticol defect, a deficiency of adrenocorticotropic hormone (ACTH), or a reduction in corticotropin-releasing hormone (CRH), as may occur in intracranial disorders and pituitary tumors. Prolonged glucocorticoid therapy may suppress ACTH, with effects when the steroids are withdrawn rapidly or when associated with severe stress. Normal recumbent levels of blood pressure may be seen even when there is a marked reduction in cortisol levels. Supine hypotension often is an indication of severe adrenocortical failure. Tachycardia often is present, as autonomic responses are not impaired. With low cortisol levels, plasma volume is reduced,24 and this probably plays an important role in the orthostatic hypotension of adrenal insufficiency.
Hypoaldosteronism
Low levels of plasma aldosterone may be the result of low plasma renin levels, as may occur in some forms of autonomic failure25 or from a secretory defect primarily in the adrenal zona glomerulosa. When it results from hyporeninism, the consequent decrease in angiotensin II formation may reduce sympathetic function through loss of its normal actions on the area postrema, as well as in the potentiation of sympathetic ganglionic transmission.26 Hypoaldosteronism may contribute to, or be the sole cause of, orthostatic hypotension that is correctable with fludrocortisone replacement therapy. This effect of hypoaldosteronism presumably results from diminished reabsorption of sodium in the distal nephron and consequent reduction in plasma volume. In addition, patients may develop orthostatic hypotension through loss of the mineralocorticoid action that enhances the normal vasoconstrictive effect of norepinephrine on human arterioles27 and veins.28 Whether the effects of aldosterone on intracellular concentrations of sodium29 contribute is not known.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








