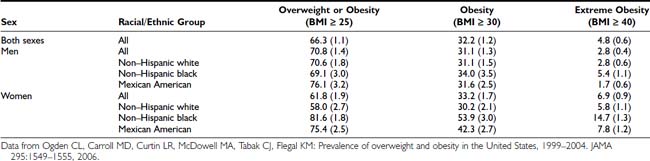
Using the definitions in Table 28-1, the prevalence of obesity in U.S. adults doubled between 1980 and 2002.8 Findings from the most recent National Health and Nutrition Examination Survey (NHANES 2003–2004) indicate that 66.3% of the total U.S. population (men and women) was overweight, and 32.2% of the population was obese.3 The prevalence of overweight and obesity were greater for non-Hispanic blacks (76.1% and 45.0%) and Mexican Americans (75.8% and 36.8%). As illustrated in Table 28-2, minority women (non-Hispanic black and Mexican) were markedly more overweight and obese than Caucasian women or minority men.
Table 28-2. Prevalence of Overweight, Obesity, and Extreme Obesity in the U.S. Population for 2003-2004
Excess body weight is also prevalent in children and adolescents (Fig. 28-1). Among children 2 to 19 years, 17.1% were overweight (defined as a BMI for age at or above the sex-specific 95th percentile) in NHANES 2003–2004. This was a significant increase compared to data from 1999–2000 and 2001–2002. Using a cutoff of BMI for age at or above the sex-specific 85th percentile, 34.8% of children and adolescents were at risk for overweight or were overweight. The prevalence of overweight in children and adolescents suggests that the need for medical management of obesity and associated comorbidities will not decline in the future.
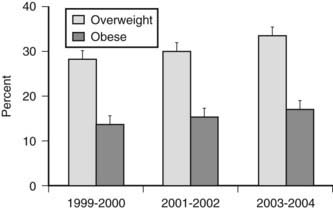
FIGURE 28-1. Prevalence of overweight (BMI for age at 85th percentile or higher) and obesity (BMI for age at 95th percentile or higher) in children and adolescents ages 2 to 19 years.
(Data presented as mean ± SEM from Ogden CL, Carroll MD, Curtin LR, et al: Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555, 2006.)
Medical expenses for overweight and obesity accounted for 9.1% of total U.S. medical expenditures in 1998 and may have reached as high as $78.5 billion ($92.6 billion in 2002 dollars). Approximately half of these costs were paid by Medicaid and Medicare.9 It has also been estimated that annual medical expenditures incurred by obese adults are 37.4% higher ($732) than for normal weight adults.10 Additional economic costs of obesity include lost productivity and wages due to illness and absence from work.10
Diseases Associated With Obesity
Overweight and obesity are associated with the development of a number of comorbidities, many of which contribute to the higher rates of mortality observed in this population. A number of factors modify the relationship between obesity and disease.11 For example, the relative risk of mortality from obesity is significantly increased in elderly men and women (≥65 years) compared to younger age groups. However, in contrast to younger subjects, overweight does not confer increased risk of mortality in elderly individuals.12 Gender and ethnicity further modify the relationship of adiposity to disease.
The distribution of body fat into intraabdominal (visceral) depots versus that in peripheral subcutaneous depots is often a better predictor of disease in obesity than is total adiposity measured by BMI. Increased visceral adipose tissue is a significant determinant of risk for type 2 diabetes mellitus13 and cardiovascular disease.14
CARDIOVASCULAR AND CEREBROVASCULAR DISEASE
Overweight, obesity, and abdominal fat deposition increase the risk of both cardiovascular and cerebrovascular diseases in the United States11 and worldwide.15 Obesity results in significantly greater rates of cardiovascular mortality.11 The reasons for the increased risk for cardiovascular and cerebrovascular diseases include elevations of blood pressure, low-density lipoprotein cholesterol, triglycerides, small dense low-density lipoprotein cholesterol, total cholesterol, fibrinogen, plasminogen activator inhibitor-1, and insulin, together with decreases in high-density lipoprotein cholesterol. The cluster of three abnormalities is diagnostic of the metabolic syndrome.16 The metabolic syndrome is defined by the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) as having three or more of the following criteria: waist circumference greater than 102 cm in men and 88 cm in women; serum triglycerides of at least 150 mg/dL (1.69 mmol/L in men) and 50 mg/dL (1.29 mmol/L) in women; blood pressure of at least 130/85 mm Hg; or serum glucose level of at least 100 mg/dL (6.1 mmol/L). The age-adjusted prevalence of the metabolic syndrome is 25% in the U.S. population.17 Not only is this disorder highly prevalent, it also increases cardiovascular and all-cause mortality as demonstrated in an 11-year follow-up study.18
Hypertension
The INTERSALT study involving more than 10,000 men and women reported that a 10-kg increase in weight was associated with a 3 mm Hg rise in systolic blood pressure and a 2.3 mm Hg rise in diastolic blood pressure.19 This degree of blood pressure elevation has been associated with a 12% increase in coronary heart disease (CHD) and a 24% increase in stroke. The precise mechanism by which changes in weight alter blood pressure has not been established. Increased sympathetic activity due to elevated serum leptin appears to be an important component of obesity-related hypertension in rodent models.20
Dyslipidemia
Increases in BMI are associated with increases in total cholesterol, triglycerides, total low-density lipoprotein (LDL), and small dense LDL and with decreases in high-density lipoproteins.21 The risk of CHD is primarily due to increases in LDL. An increase in BMI of 10 units, starting from a level between 20 and 30 kg/m2, will raise LDL cholesterol levels between 10 and 20 mg/dL. Changes of this magnitude can be expected to increase the risk of CHD by 10% over a 5- to 10-year period. The risk may be particularly great for individuals with more prominent upper body obesity, in whom triglyceride, small dense LDL, and apolipoprotein B levels are high.
Coronary Artery Disease
In the Determinants of Atherosclerosis in Youth (PDAY) study, fatty streaks and advanced lesions in coronary arteries postmortem from subjects 15 to 34 years old were positively associated with obesity and abdominal fat deposition.22 Subjects undergoing cardiac catheterization today are more frequently obese, younger with more comorbidities, and present with more single-vessel disease.23 However, obesity is not associated with increased mortality or postoperative cerebrovascular events after cardiac surgery.24
Congestive Heart Failure
Both overweight and obesity have been shown to be independent risk factors for the development of congestive heart failure.25,26 Furthermore, because hypertension and diabetes are also associated with congestive heart failure, the overall risk when these dependent factors are taken into account is proportionally increased.27
Stroke
The risk of stroke is nearly twofold higher in women with a BMI greater than 32 kg/m2 than in women with a BMI less than 21 kg/m2.28 In men, each unit increase of BMI over 23 kg/m2 was associated with a significant 6% increase in the adjusted relative risks of total, ischemic, and hemorrhagic stroke.29 Adjustment for hypertension, diabetes, and hypercholesterolemia slightly attenuated the risk for stroke in this study.
DIABETES MELLITUS
An association between increased weight and the development of type 2 diabetes mellitus is well established.30 In fact, the risk for diabetes increases at BMI levels below that established for the diagnosis of overweight. In the Nurses’ Health Study, BMI values above 22 kg/m2 were associated with an increased risk of diabetes.31 It has been estimated that the relative risk for diabetes increases by 25% for each unit of BMI above 22 kg/m. It has also been estimated that more than a quarter of all newly diagnosed cases of diabetes in the United States are due to weight gain of more than 5 kg.32 See Chapter 41 for additional information on the etiology of diabetes and obesity.
NONALCOHOLIC FATTY LIVER DISEASE
Nonalcoholic fatty liver disease (NAFLD) describes a range of liver abnormalities that include hepatomegaly, abnormal liver biochemistry, steatosis, steatohepatitis, fibrosis, and cirrhosis.33 Estimates of the prevalence of NAFLD in obese subjects range from 30% to 100%, and there is a strong association with abdominal obesity and metabolic syndrome. The disease is equally represented in men and women and is also known to affect children. The progression from simple steatosis (for the most part considered benign) to steatohepatitis (necroinflammatory change and hepatocellular injury) has been suggested to follow a “two-hit” model.34 The first hit results from any interference in metabolism of free fatty acids, which then accumulate in the liver. The second hit may be due to oxidative stress resulting from metabolism of excess fatty acids or could result from proinflammatory cytokines. Thus the insulin resistance and subclinical inflammation present in obesity support the development of NAFLD. Weight loss and improved insulin sensitivity appear to reduce hepatic steatosis,35,36 although additional study is needed to fully understand the effects of various therapeutic interventions.
CANCER
There is a strong association between adiposity and increased risk of mortality from cancer.37 In reviewing the evidence in 2007, the World Cancer Research Fund (WCRF) concluded that body fatness is associated with increased risk of esophageal adenocarcinoma and with cancers of the pancreas, colorectum, postmenopausal breast, endometrium, and kidney.38 In a very recent meta-analysis,39 it was determined that in men, a 5 kg/m2 increase in BMI was strongly associated with esophageal adenocarcinoma and with thyroid, colon, and renal cancers. In women, strong associations were found between a 5 kg/m2 increase in BMI and endometrial, gallbladder, esophageal adenocarcinoma, and renal cancers. Weaker positive associations were also found between increased BMI and rectal cancer and malignant melanoma in men; postmenopausal breast, pancreatic, thyroid, and colon cancers in women; and leukemia, multiple myeloma, and non-Hodgkin lymphoma in both sexes. Possible mechanisms linking cancer with excess body weight could be increased insulin/IGF (which may alter the balance between cell proliferation and apoptosis), altered adipokines, localized inflammation, and oxidative stress.40
GYENECOLOGIC ABNORMALITIES
Polycystic ovarian syndrome, a disorder that includes hirsutism, obesity, ovulatory and menstrual dysfunction, and insulin resistance, is among the most common causes of infertility in women who are overweight.41 Even modest increases in weight in young women can adversely affect reproductive function.42
Obesity during pregnancy is also associated with excessive morbidity. Pregnant women with obesity have nearly a 10-fold excess risk of hypertension and a significant increase in the risk of gestational diabetes. Furthermore, the risk of congenital malformations, primarily neural tube defects, is increased in the pregnancy of obese women.43 Finally, increased weight before pregnancy has been shown to result in an increased risk of adverse fetal outcomes.44
OBSTRUCTIVE SLEEP APNEA
Episodes of apnea and hypopnea during sleep occur due to partial or complete airway obstruction in obese subjects. A conservative estimate of the prevalence of obstructive sleep apnea in obesity is 30%.45 Diagnosis and treatment of sleep apnea in obese patients is particularly important because of the sequelae of hypoxia, hypertension, myocardial infarction, and cardiac arrhythmias associated with this condition. Obstructive sleep apnea is also independently associated with alterations in glucose metabolism which put obese subjects at greater risk for development of type 2 diabetes.46
GALLSTONES
For women with a BMI greater than 40 kg/m2, the risk of gallstones is nearly seven times higher than for women with a BMI less than 24 kg/m2.47 The risk of gallstones is increased with rapid weight loss such as with very-low-calorie diets or weight loss surgery.48
OSTEOARTHRITIS
Obesity is likely the single most important risk factor for development of severe osteoarthritis of the knee due to the additional load on this joint.49 A study in twins estimated that for every 1-kg rise in body weight, the risk of osteoarthritis increases by approximately 10%.50 Obesity is also a cofactor in development of osteoarthritis in non-weight-bearing joints, with hyperinsulinemia and proinflammatory cytokines suggested to contribute to disease mechanisms.49
Regulation of Energy Balance
Changes in body weight follow the laws of physics which dictate that if energy intake is greater than energy expenditure, energy will be stored in the body, and weight gain will occur. The regulation of body weight by the central nervous system (CNS) is the result of a complex integration of genetic, social, behavioral, and physiologic signals, many of which have yet to be fully understood. Further, the systems that regulate body weight and energy homeostasis developed over an evolutionary time scale. Thus the biological processes responsible for the prevalence of obesity today were initiated in our early ancestors and most likely developed to defend against significant loss of lean mass during times of food scarcity. In an environment of plenitude, these “survival genes” continue to function but now have an adverse effect on well-being and longevity. The following sections provide a brief overview of the regulation of energy intake and expenditure to facilitate understanding of the basis for current and developing therapies for obesity.
ENERGY EXPENDITURE
Total daily energy expenditure (TEE) can be divided into four major components, including resting metabolic rate (RMR), the thermic effect of food, physical energy expenditure, and non-exercise activity thermogenesis (see Chapter 27 for details). Fat-free mass (primarily skeletal muscle) is the most important contributor to TEE, so the greater an individual’s muscle mass, the greater their TEE.51 Fat-free mass is also the main determinant of RMR, which accounts for 60% to 70% of the total daily energy expenditure. After adjusting for fat-free mass, age and gender are also important predictors of TEE and RMR. The significant contribution of muscle mass to TEE underlies the recommended use of exercise in weight loss programs.
Energy expenditure has been rigorously examined in an attempt to detect defects that could contribute to the development of obesity. In adult Pima Indians, a population prone to the development of obesity, low relative RMR was associated with greater risk of becoming obese, compared to subjects with the highest RMR.51 However, low RMR as a significant cause of obesity has not been born out in other populations, and in most studies, obese subjects actually have a higher RMR than the lean subjects, owing to their greater skeletal muscle mass.52 TEE is also increased in obese subjects secondary to the greater energy expenditure required to move a larger body mass, although during non-weight-bearing exercise, obese individuals expend the same amount of energy as lean subjects doing equivalent work.53 Despite these physiologic observations, epidemiologic studies suggest that obese subjects are more likely to engage in sedentary behaviors than lean individuals.53,54 Thus defects in energy expenditure other than reduced physical activity are likely to contribute little to the development of obesity in humans.
Under highly controlled experimental conditions, it has been demonstrated that changes in body weight result in compensatory changes in energy expenditure which attempt to restore the body to the original starting weight. For example, a 10% reduction in body weight in either lean or obese subjects is associated with a significant reduction in both resting and non-resting metabolic rate beyond that which would be predicted to result from the decrease in fat mass and fat-free mass.55 This greater-than-expected reduction in TEE, termed adaptive thermogenesis, likely contributes to a degree to the failure of weight loss programs. The environmental pollutant organochlorine and oxygen desaturation due to obstructive sleep apnea have recently been proposed to increase adaptive thermogenesis.56
Spontaneous physical activity or non-exercise-activity thermogenesis (NEAT—all activity other than volitional exercise) defends against body weight gain in certain individuals.57,58 Individuals who are resistant to weight gain have a greater activation of NEAT than do individuals who gain weight easily when an experimental increase in caloric intake is imposed. It has also been shown that lean individuals stand and walk 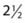 hours per day more than obese subjects.59 Imposing a weight gain in lean subjects does not alter the time standing or walking. In contrast, weight loss does not decrease the sitting time of obese subjects. These observations have prompted the theoretical model that individuals are genetically determined to be either NEAT activators who remain lean or NEAT conservers who become obese in the current environment.60 It has thus been suggested that increasing NEAT through both individual approaches (get up from the chair) and environmental re-engineering (remove the chair) are needed to reduce obesity.60
hours per day more than obese subjects.59 Imposing a weight gain in lean subjects does not alter the time standing or walking. In contrast, weight loss does not decrease the sitting time of obese subjects. These observations have prompted the theoretical model that individuals are genetically determined to be either NEAT activators who remain lean or NEAT conservers who become obese in the current environment.60 It has thus been suggested that increasing NEAT through both individual approaches (get up from the chair) and environmental re-engineering (remove the chair) are needed to reduce obesity.60
ENERGY INTAKE
In humans, the decision to eat is the result of the complex integration of environmental cues and higher cognitive function with internal physiologic signals regarding nutrient status and energy stores.61 The gastrointestinal tract, liver, pancreas, and adipose tissue provide information concerning the presence of nutrients and amount of energy stored in the body via humoral and neural signals to the CNS (Fig. 28-2). The most important targets within the CNS for the internal physiologic signals are thought to be the hypothalamus and brainstem (nucleus of solitary tract, area postrema, parabrachial nucleus). In addition, circulating free fatty acids and glucose also regulate hypothalamic function.62,63
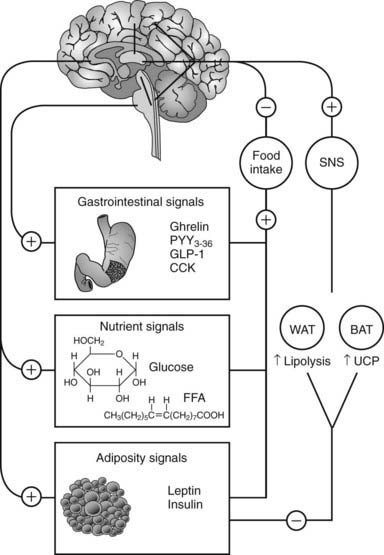
FIGURE 28-2. Integration between feeding-related signals to the brain and food intake and energy expenditure. Food intake initiates a series of signals that reach the hypothalamus (nutrient and adiposity signals) or the brainstem (gastrointestinal signals). The hypothalamus integrates these signals with other sensory, cognitive, and environmental information from the cerebral cortex. This integrated information, when sent back to the periphery, results in a decrease in food intake and activation of the sympathetic nervous system (SNS). The SNS stimulates lipolysis in white adipose tissue (WAT) and thermogenesis in brown adipose tissue of rodents (BAT) via activation of uncoupling protein-1 (UCP). CCK, Cholecystokinin; FFA, free fatty acids; GLP-1, glucagon-like peptide-1; PYY3-36, peptide YY3-36.
Adiposity Signals
Leptin and insulin convey information to the hypothalamus about the amount of energy stored within the body and satisfy the criteria for a negative-feedback system.64 These hormones circulate in the blood at concentrations proportional to body fat content. Leptin and insulin promote weight loss when administered to the CNS, and blocking the central neural activity of these hormones increases food intake and weight gain.
Gastrointestinal Signals
Both mechanical signals, such as distension of the esophagus and stomach, and peptide hormones signal the presence of food in the gastrointestinal tract. The majority of hormones released by the gut are satiety factors that function to terminate a meal.
Cholecystokinin (CCK) was the first gut peptide demonstrated to regulate meal size in a dose-dependent manner.65 Whereas CCK reduced meal intake by nearly one half in rodents, a selective antagonist completely reversed this suppression. By itself, the CCK antagonist was able to increase food intake by approximately one third.66
Peptide YY (PYY) is a 36-amino-acid peptide structurally related to neuropeptide Y and pancreatic polypeptide. The major form of PYY released by L cells in the gut is the N-terminal truncated form PYY3-36. Peripheral administration of PYY3-36 at physiologic doses has been shown to reduce food intake in both rodents and humans.67
Glucagon-like peptide-1 (GLP-1) is a neuropeptide hormone produced by posttranslational processing of the preproglucagon gene by L cells in the gut. This incretin is of significant current interest for its ability to stimulate insulin release; a number of pharmaceutical compounds that prolong the lifetime of this hormone in the circulation are currently in use.68 The GLP-1 analog exendin-4 reduced body weight in clinical trials testing the compound’s ability to enhance insulin secretion.69
Oxyntomodulin is also a posttranslational product of the preproglucagon gene released into the circulation postprandially. Oxyntomodulin inhibits gastric acid secretion and reduces food intake in rodents and humans.70
Ghrelin, a 28-amino-acid peptide made in the stomach, is a ligand for the growth hormone–secretagogue receptor present in the hypothalamus and brainstem.71 In contrast to all other known gut peptides, ghrelin stimulates food intake in rodents72 and humans.73 Plasma levels of ghrelin increase during fasting and fall after eating, which has been interpreted to suggest that ghrelin is a hunger hormone.72 Plasma ghrelin concentrations are increased with diet-induced weight loss,74 and postprandial fall in circulating levels is attenuated or absent75 in obese subjects, suggesting that ghrelin may be involved in the pathophysiology of obesity. The active form of ghrelin contains a fatty acyl side chain attached to serine 3.71 The enzyme responsible for acylating ghrelin has recently been identified, providing a new therapeutic target to regulate the actions of this orexigenic hormone.76,77
CENTRAL INTEGRATION OF ENERGY HOMEOSTASIS
As illustrated in Fig. 28-2, the signals of nutrient status and energy stores are communicated to the hypothalamus and brainstem via humoral and neural pathways. Within the arcuate nucleus of the hypothalamus, neurons co-expressing neuropeptide Y (NPY) and agouti-related protein (AgRP) stimulate food intake. Other neurons co-express α-melanocyte-stimulating hormone (α-MSH) and cocaine- and amphetamine-regulated transcript (CART) and activate melanocortin receptors to decrease food intake (see Chapter 27 for details). Leptin and insulin activate α-MSH/CART signaling and inhibit NPY/AgRP neurons to reduce food intake.62,78 Cross-talk exists between leptin/insulin-responsive arcuate neurons and brainstem neurons that receive input from the gastrointestinal satiety hormones.62 The importance of this interaction underlies current efforts to develop dual pharmacotherapy for weight loss.79
The reward value of food intake is mediated by dopamine and µ-opioid signaling in forebrain structures. Neurons from the ventral tegmental area and nucleus accumbens project to the hypothalamus, where reward is integrated with the signals of nutrient status.62
Efferent neural signals arising within the hypothalamus communicate with peripheral tissues to regulate food intake and energy expenditure. Activation of sympathetic neural activity increases lipolysis in white adipose tissue and energy expenditure in brown adipose tissue of rodents. Uncoupling protein (UCP-1) in brown adipose tissue functions to produce proton leak across the mitochondrial membrane, dissociating substrate oxidation from ATP synthesis.80 As humans have little brown fat, this mechanism for activation of energy expenditure may appear to be limited. However, a family of novel uncoupling proteins is expressed in human tissue including muscle. These proteins do not appear to mediate inducible mitochondrial proton leak in a manner similar to that of UCP-1, although UCP-3 has been suggested to facilitate fatty acid oxidation.81 In addition, recent findings using fluorodeoxyglucose positron emission tomography challenge the notion that adult humans lack brown fat.82 Distinct symmetrical areas of brown adipose tissue have been detected in the upper body and appear to be of sufficient quantity to influence energy expenditure, thus justifying investigation into the role of this tissue in regulating body weight.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








