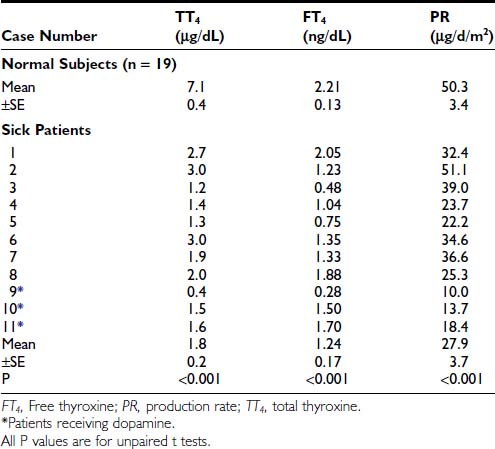
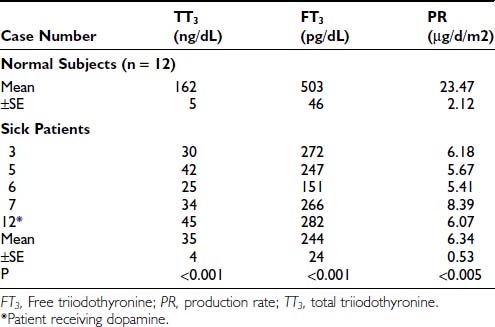
FIGURE 86-1. Free T3 concentrations in different groups of patients, as reported by Chopra et al.31 In this report, patients with NTIS have significantly lowered free T3 levels than normal subjects. NTIS, Nonthyroidal illness syndrome; T3, triiodothyronine.
Serum rT3 may be reduced, normal, or elevated and is not a reliable indicator of abnormal thyroid hormone supply. While it may be expected that rT3 should always be elevated, this is not true, and often it is within the normal range. Peeters et al.35 found in patients with NTIS, serum TSH, T4, T3, and the T3/rT3 ratio were lower, whereas serum rT3 was higher than in normal subjects (P < 0.0001). Liver D1 is down-regulated, and D3 (which is not evident in liver and skeletal muscle of healthy individuals) is induced, particularly in disease states associated with poor tissue perfusion. The level of rT3 reflects the action of several enzymes and presumably, as well, tissue metabolic function. Induction of D3 would tend to increase rT3. Degradation of rT3 is reduced by decreased function of the same D1 enzyme that generates T3. Moreover, formation of rT3 is limited by the low level of substrate (T4) in serum and in tissues and perhaps by inhibition of T4 entry into cells. Personal experience treating patients with NTIS (unpublished) shows that when T4 is given and repletes serum hormone levels, generation of rT3 rapidly increases, and levels often become significantly elevated.
Serum T4
Serum T4 levels are reduced in NTIS in proportion to the severity and, probably, length of the illness.24–35 In acute, short-term trauma such as cardiac bypass36 or in short-term starvation,37 there is no drop in serum T4. However, with increasing severity of trauma, illness, or infection, there is a drop in T4 which may become extreme. As indicated, serum T4 levels below 4 µg/dL are associated with a marked increased risk of death (up to 50%), and once T4 is below 2, prognosis becomes extremely guarded. In neonates, low total T4 and TSH are associated with a greater risk of death and severe intraventricular hemorrhage. It is suggested that thyroid hormone supplementation might be a potential benefit in infants with the lowest T4 values.27
Total serum T4 is reduced in part because of a reduction in TBG. One reason for this reduction appears to be because of cleavage of TBG. Schussler’s group recognized a rapid drop in TBG to 60% of baseline within 12 hours after bypass surgery, and their data suggest that this is due to cleavage of TBG by protease, which causes TBG to lose its T4-binding activity.38 Further studies by this group demonstrated the presence of a cleaved form of TBG present in serum of patients with sepsis.39
The impact of meningococcal sepsis on peripheral thyroid hormone metabolism and binding proteins was studied in 69 children with meningococcal sepsis. All children had decreased total T3 and total T3/rT3 ratios without elevated TSH. Lower total T4 levels were related to increased turnover of TBG by elastase. Lowered TBG is a partial explanation for lower total T4 and T3 in NTIS.40
Serum Free Thyroxine
A major problem in understanding NTIS is in analyzing data on the level of free T4. Free T4 is believed by most workers to represent hormone availability to tissues, although it is in fact intracellular T3 that binds to the receptors. The results of free T4 assays in NTIS are definitely method dependent. They may be influenced by a variety of variables, including (alleged) inhibitors present in serum or the effect of agents such as drugs, metabolites, or free fatty acids in the serum or assay. Assays which include an estimate of TBG capacity to estimate free hormone typically return low values for calculated free thyroxine in NTIS. Methods using T3 analogs in the assay also give levels that are depressed. The free T4 level determined by dialysis varies widely, as does T4 measured by ultrafiltration25–29; the majority of reports are of normal or low values but in some samples, elevated values.25,26,41–43
In theory, methods utilizing equilibrium dialysis may allow dilution of dialyzable inhibitors. Compounds such as 3-carboxy-4-methyl-5-propyl-2-furan-propanoic acid, indoxyl sulfate, and hippuric acid, can accumulate in severe renal failure.44 However, these compounds probably do not interfere with serum hormone assays. Free fatty acids, if elevated to 2 to 5 mmol/L, can displace T4 binding to TBG and elevate free T4. Free fatty acids almost never reach such levels in vivo.45,46 However, even small quantities of heparin (0.08 units/kg given IV, or 5000 units given SC), commonly given to patients in an ICU, can lead to in vitro generation of free fatty acids during extended serum dialysis for “free T4” assay and falsely augment apparent free hormone levels.47 This is probably a widespread and serious problem, which explains many instances of apparently elevated free T4 levels in patients with acute illness.
Results obtained using ultrafiltration also are variable. Wang et al.48 found that in patients with NTIS, free T4 measured by ultrafiltration was uniformly low (average of 11.7 ng/L), but when measured by equilibrium dialysis, free T4 was near normal, at 18 ng/L. By ultrafiltration, free T3 was also (not surprisingly) found to be low and similar to free T3 by radioimmune assay. Chopra32 found levels below the normal mean, ±2 SD, when measured by dialysis; 6 of 9 were low when measured by ultrafiltration, and 7 of 9 were low when measured by standard resin-uptake-corrected free T4. The means of the NTIS patients in this study were clearly below the mean of normals.
Thus, although free T4 is low in most assays that involve a correction for TBG levels, there is still some question as to the true free T4 in patients with NTIS. It is of interest that this problem does not carry over to estimates of free T3, which are depressed in most studies. There might be two reasons for this difference. Firstly, the depression of total T3 is proportionately greater than of total T4. Secondly, factors which affect thyroid hormone binding are more apt to alter T4 assays than T3, since T4 is normally more tightly bound to TBG than is T3.
IS THERE EVIDENCE FOR SUBSTANCES IN SERUM WHICH CAN AFFECT T4 BINDING TO PROTEINS?
Mendel et al.49 carefully review the studies that have claimed the presence of dialyzable inhibitors of binding and point out that many of these studies must be viewed with caution.44,45,50–53 Numerous artifacts are present in both dialysis assays and ultrafiltration assays. They also point out that while the low free T4 by resin uptake assays found in NTIS generally do not agree with the clinical status of the patient, it is equally true that clinical assessment generally does not fit with the high free T4 results found by some equilibrium dialysis assays in NTIS.
An argument that completely refutes the importance of factors in serum inhibiting binding of thyroid hormone is provided in the clinical study of Brent and Hershman (Fig. 86-2).54 These researchers gave 1.5 µg of T4 per kg body weight daily to 12 of 24 patients with severe NTIS and followed serum hormone levels over 14 days. T4 levels returned to the normal range within 3 days of therapy. Thus the thyroxine pool was easily replenished, and T4 levels reached normal values. Not surprisingly, because of reduced T4>T3 deiodination, T3 levels did not return to the normal range until the end of the study period in the few patients who survived. However, the ability of intravenous thyroxine in replacement doses to promptly restore the plasma pool to normal clearly shows that neither a loss of serum TBG nor an inhibitor of binding could be the main cause of low serum T4 in this group of severely ill patients.
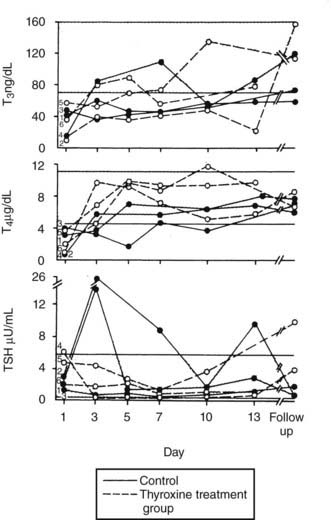
FIGURE 86-2. Patients with severe NTIS were randomized and left untreated (control, solid lines) or given IV T4 (thyroxine-treated group, dashed lines) over 2 weeks.54 Serum T3, T4, and TSH concentrations are shown for the survivors of the control (filled circles) and T4-treated groups (open circles) during the study period and at the time of follow-up. NTIS, Nonthyroidal illness syndrome; IV, intravenous; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
TSH LEVELS
Serum TSH in NTIS is typically normal or reduced and may be markedly low, although usually not less than 0.05 µU/mL.16,24,25,28,29,31,55 However, to use usual endocrinology logic, these TSH levels are almost always inappropriately low for the observed serum T4 and T3. Third-generation assays with sensitivity down to 0.001 U/mL may allow differentiation of patients with hyperthyroidism from those with NTIS, although there can be overlap in these very disparate conditions.56 Serum TSH in patients with NTIS may have reduced biological activity, perhaps because of reduced thyrotropin-releasing hormone (TRH) secretion and reduced glycosylation. Some patients are found with a TSH level above normal, and elevation of TSH above normal commonly occurs transiently if patients recover from NTIS (Fig. 86-3).16,29,54 This elevation of TSH strongly suggests that the patients are recovering from a hypothyroid state, during which the ability of the pituitary to respond had been temporarily inhibited.
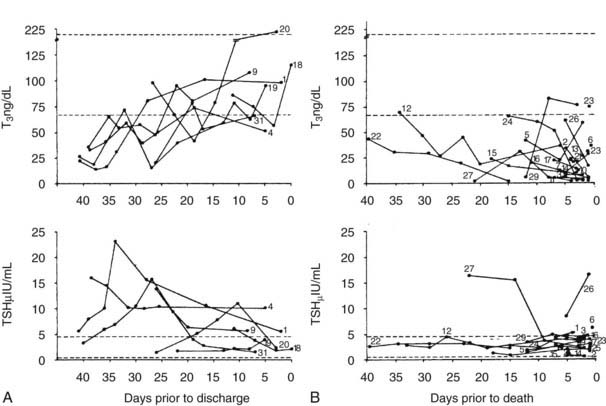
FIGURE 86-3. T3 and TSH concentrations are shown in patients with nonthyroidal illness who were eventually discharged from hospital (left panels).29 The broken line indicates ±2 SD of the mean value in the normal subjects. The right panel displays T3 and TSH concentrations in patients with NTIS who died. Subjects are indicated by numbers. Note the elevated TSH in some patients who recovered and the generally dropping T3 and low TSH levels in patients who died.29 NTIS, Nonthyroidal illness syndrome; SD, standard deviation; T3, triiodothyronine; TSH, thyroid-stimulating hormone.
Responsiveness of the pituitary to TRH during NTIS is variable: many patients respond less than normal,57 and others respond normally.58 “Normal” responsiveness in the presence of low TSH may suggest that there is a hypothalamic abnormality as a cause of the low TSH and low T4. There is also a diminution or loss of the diurnal rhythm of TSH,59 and in some studies, there is evidence for reduction of TSH glycosylation, with lower TSH bioactivity.60 A logical alternative explanation is that the low TSH is in fact the proximate cause of the low thyroid hormone levels. Hypothalamic function is impaired in patients with NTIS and, because of low TRH, results in low TSH and thus low output of thyroid hormones by the thyroid.
There is other evidence of diminished hypothalamic function in patients with serious illness. Serum testosterone drops rapidly, as do follicle-stimulating hormone (FSH) and luteinizing hormone (LH).61,62 Typically serum cortisol is elevated as part of a stress response, but this is not always the case. Some patients develop hypotension in association with apparent transient central hypoadrenalism, have low or normal serum ACTH, and cortisol levels under 20 µg/dL. The patients respond dramatically to cortisol replacement and may manifest normal adrenal function at a later time if they recover.
Centrally mediated hyposomatotropism, hypothyroidism, and pronounced hypoandrogenism were observed in a study of patients in the catabolic state of critical illness. In these patients, pulsatile LH secretion and mean LH secretions are very low, even in the presence of extremely low circulating total testosterone and low estradiol. Pulsatile growth hormone (GH) and TSH secretion are also, as is known, suppressed. Interleukin 1 β (IL-1β) levels are normal, whereas IL-6 and tumor necrosis factor α (TNF-α) are elevated. Exogenous IV gonadotropin-releasing hormone (GnRH) partially return serum testosterone levels toward normal but do not completely overcome hypoandrogenism, suggesting that combined deficiency of GH, GnRH, and TSH secretagogues may be important in this low androgen syndrome.63
THYROID HORMONE TURNOVER
Kaptein et al.64,65 studied a group of patients who were critically ill, all of whom had total T4 below 4 µg/dL, low fT4 index, low normal free T4 by dialysis, and TSH which was normal or slightly elevated. In these patients, the mean T4 by dialysis was significantly below the normal mean. There was on average a 35% decrease in thyroxine disposal per day (Table 86-1). The T4 production rate in NTIS was significantly below the mean of 17 normal subjects (p < 0.005). In a similar study of T3 kinetics,65 free T3 was found to be 50% of normal serum values. The production rate of T3 was reduced by 83% (Table 86-2). These two studies document a dramatic reduction in provision of T4 and T3 to peripheral tissues, which would logically indicate that the effects of hormone lack (hypothyroidism) should be present. A third study reported dramatically reduced total T4 and T3 turnover, with normal thyroidal secretion of T3 in patients with NTIS due to uremia.66 However, this was a calculated rather than directly measured value, was highly variable, and does not negate the extreme reduction in T3 supply due to diminished T4>T3 conversion in peripheral organs.
T4 ENTRY INTO CELLS AND GENERATION OF T3
Using deiodination of T4 as an index of cellular transport of T4 into rat hepatocytes, Lim et al.67 and Vos et al.68 found that serum from patients with NTIS inhibited T4 uptake. Sera from critically ill NTIS patients caused reduced T4 uptake compared to control sera in one study, and the authors considered elevated nonesterified fatty acids (NEFA) and bilirubin and reduced albumin to play a role. Serum from patients with mild NTIS did not cause impaired deiodination of T4 and T3.69 Inhibition of uptake of T4 into hepatocytes caused by sera of patients with NTIS also was observed by Sarne and Refetoff.70 There is a diminution in the “reducing equivalents” available for the deiodination of T4 to T3 in liver, and presumably elsewhere, thus lowering transport and the function of the type 1 iodothyronine deiodinase.71 In animals, and probably in man, there is also a drop in the level of type 1 iodothyronine deiodinase enzyme, apparently due to hypothyroidism, since it can be reversed by giving T3. Recently a study was performed on blood, liver, and skeletal-muscle biopsies of patients immediately after death in intensive care unit settings. Liver T4 deiodinase 1 was found to be down-regulated, and deiodinase 3 was induced in liver and muscle, especially in situations associated with poor tissue perfusion. These changes contribute to the low generation of T3 and its increased metabolism in NTIS, thus lowering the intracellular T3 levels.35
In theory, reduced cellular uptake would cause tissue hypothyroidism, reduced T3 generation and serum T3 levels, and elevated serum T4, which is not observed. It is likely that reduced hormone supply in NTIS is caused by multiple factors, and that reduced cell uptake is one of the factors. T4 is converted to T3, although at a reduced rate. In addition, T4 is rapidly converted to rT3 by an intracellular process, suggesting that entry into cells is not seriously impaired, but the pathways of intracellular deiodination are abnormal.
THYROID HORMONE IN TISSUES
There are few significant data on thyroid hormone in tissues of patients with NTIS.72 In one study, there was of a dramatically reduced level of T3 in tissues (Table 86-3). While most samples had very low levels of T3 compared to normal tissues, some patients with NTIS showed sporadically and inexplicably high levels of T3 in certain tissues, especially skeletal muscle and heart.
Table 86-3. Tissue T3 Concentrations in Nonthyroidal Illness Syndrome (nmol of T3/kg of Wet Weight)72
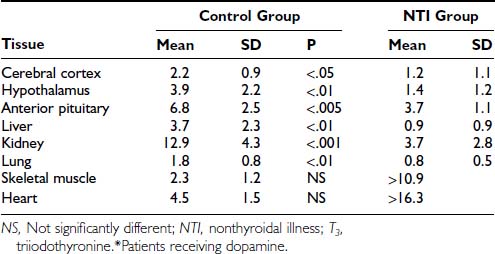
Peeters et al.73 investigated 79 patients who died after intensive care, some of whom received thyroid hormone treatment. Tissue iodothyronine levels were positively correlated with serum levels, indicating that the decrease in serum T3 during illness is associated with decreased levels of tissue T3. Higher serum T3 levels in patients who received thyroid hormone treatment were accompanied by higher levels of liver and muscle T3, with evidence for tissue-specific regulation. Tissue rT3 and the T3/rT3 ratio were correlated with tissue deiodinase activities. Monocarboxylate transporter 8 expression was not related to the ratio of the serum over tissue concentration of the different iodothyronines.73
Information on expression of TRs in human tissues during illness is limited. Increased expression of the messenger ribonucleic acid (mRNA) for thyroid hormone receptors α1, α2, and β1 has been reported in cardiac tissue of patients with dilated cardiomyopathy; α1 and α2 isoforms also had increased expression in ischemic heart disease.74 Rodriguez-Perez et al. studied subcutaneous fat and skeletal muscle in patients with septic shock.75 In muscle, mRNA for TRβ1 and RXR gamma was reduced, and mRNA for RXR alpha was increased, compared to normals. In adipose tissue, MCT8, TRβ1, TRα1, and RXR gamma mRNAs were lower. The authors conclude that in these patients, tissue responses were oriented toward decreased hormone levels and decreased hormone action. In animals, starvation and illness are associated with a reduction in thyroid hormone receptor levels. In experimental studies in mice, LPS induces NTIS, and this is associated with an early decrease in binding of the RXR/TR dimer to DNA due to limiting amounts of RXR, and later an up to 50% decrease in levels of RXR and TR protein.76–77
ARE PATIENTS WITH NTIS CLINICALLY HYPOTHYROID?
It is straightforward that the clinical parameters of severe hypothyroidism are absent in patients with NTIS. However, these patients usually present with a serious illness and are diagnostically challenging in view of their complicated state. Many are febrile, have extensive edema, have sepsis or pneumonia, may have hypermetabolism associated with burns, have severe cardiac or pulmonary disease, and in general have features that could easily mask evidence of hypothyroidism. Further, the common clinical picture of hypothyroidism does not develop within 2 to 3 weeks of complete thyroid hormone deprivation, but rather requires a much longer period for expression. General laboratory tests are also suspect. Thus starvation or disease-induced alterations in cholesterol, liver enzymes, TBG, creatine kinase, and even basal metabolic rate generally rule out the use of these associated markers for evidence of hypothyroidism. Angiotensin-converting enzyme levels are low,78 as seen in hypothyroidism, while high-affinity testosterone-binding globulin (TeBG) and osteocalcin levels are not altered.79 Antithrombin III levels are reduced in a septic rat model of NTIS. T3 supplementation returned the sepsis-induced decrease in antithrombin III levels toward normal.80
Mechanism of Thyroid Hormone Suppression in NTIS
It is probable that the cause of NTIS is multifactorial and may differ in different groups of patients. Specifically, the changes in liver disease and renal disease are probably somewhat different from those occurring in other forms of illness. Certainly one important cause of the drop in serum T3 is a decreased generation of T3 by type 1 iodothyronine deiodinase.81 If reduced entry of T4 into cells was a primary event and the major problem, then serum T4 levels would become elevated rather than suppressed. Some studies have suggested that individuals with NTIS may have selenium deficiency, and this may contribute to a malfunction of the selenium-dependent iodothyronine deiodinase.82 However, supplements of 500 μg of selenium given to patients in a surgical ICU during the first 5 days after serious injury caused only modest changes in thyroid hormones. The data did not suggest a major role for selenium deficiency in this condition.83
The overall degradation of thyroid hormone, both thyroxine and T3, is radically diminished in the NTIS syndrome in the presence of low hormone serum levels. The reduced degradation cannot produce the lowering of serum hormone levels; a primary reduction in degradation would increase serum hormone. The change in degradation must be secondary to the low hormone supply. Schussler and co-workers have observed a sharp drop in TBG levels during cardiac bypass surgery, which their studies indicate is due to some selective consumption of TBG. It is possible that this occurs because of activation of serine protease inhibitors (serpins) at sites of inflammation, which cleave the TBG into an inactive form.38
Considerable evidence suggests that an alteration in hypothalamic and pituitary function causes the low production of T4, which in turn causes the low production of T3. In rats, starvation reduces hypothalamic mRNA for TRH, reduces portal serum TRH, and lowers pituitary TSH content.84 A recent study documents low TRH mRNA in hypothalamic paraventricular nuclei85 in NTIS patients (Fig. 86-4). Responses to administered TRH vary in different reports, being suppressed or even augmented.57,58 Administration of TRH has been suggested as an effective means of restoring serum hormone levels to normal in individuals with NTIS. A recent report of great significance by Van den Berghe and co-workers proves that administration of TRH to patients with severe NTIS leads directly to increased TSH levels, increased T4 levels, and increased T3 levels.86 This data is strong support (albeit not proof) for the role of diminished hypothalamic function as an important factor causing NTIS.
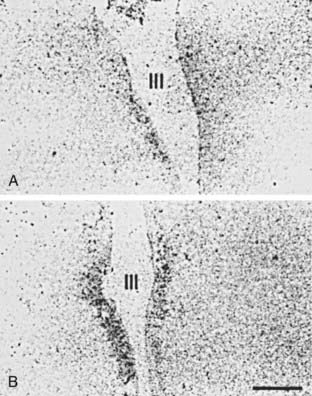
FIGURE 86-4. In situ hybridization study demonstrating mRNA for TRH in the periventricular nuclei of A, a subject who died with NTIS and B, a subject who died accidentally. In patients with NTIS, mRNA for TRH is significantly reduced.85 mRNA, Messenger ribonucleic acid; NTIS, nonthyroidal illness syndrome; TRH, thyrotropin-releasing hormone.
Quite possibly the production of TRH, and responses to TRH, are reduced by cytokines (to be discussed later) or by glucocorticoids.87 The diurnal variation in glucocorticoid levels at least in part controls the normal diurnal variation in TSH levels, perhaps by affecting pituitary responsiveness to TRH.88 High levels of glucocorticoids in Cushing’s disease suppress TSH and cause a modest reduction in serum hormone levels.89 High levels of glucocorticoids are known to suppress pituitary response to TRH in man.87 Stress-induced elevation of glucocorticoids in animals causes suppression of TSH and serum T4 and T3 hormone levels.90 Thus stress-induced glucocorticoid elevation may be one factor affecting TRH and TSH production.
Why should pituitary production of TSH be diminished in the presence of low serum thyroid hormone levels? A possibility is that there is augmented intrapituitary conversion of T4 to T3, thus allowing the pituitary to remain “euthyroid” while the rest of the body is actually hypothyroid. There is experimental support for this idea in a uremic rat model of NTIS.91 Another suggestion is that some other metabolite of thyroxine may be involved in control of pituitary responsiveness. For example, possibly triiodoacetic acid (triac) or tetraiodoacetic acid (tetrac) generated by metabolism of thyroxine could control pituitary responsiveness,92 but there is no experimental proof of this idea, and even if true, it would mean that the pituitary was normal but the rest of the body hypothyroid. As suggested earlier, elevated serum cortisol levels could play a role. The most obvious possibility is that low TSH stems from diminished TRH production, as previously described. It must also be remembered that the defect in pituitary function is not restricted to TSH, but that LH and FSH are also suppressed in seriously ill patients, and testosterone is reduced, in contrast to the generally augmented glucocorticoid response. Quite possibly these changes are the effect on the hypothalamus of neural integration of multiple factors including stress, starvation, glucocorticoids, and cytokines.
Van den Berghe has proposed that the changes in endocrine function seen during severe illness have a biphasic course. Possibly the initial suppression of T3 levels represents a genetically engineered adaptive response of the organism, allowing reduced metabolic rate and conservation of energy and protein stores for a longer period of time, while the animal or man goes through a period of starvation. However, the circumstances surrounding severe illness, and the resuscitative efforts applied in an intensive care unit over 1 or more weeks, presumably have not resulted in some genetically induced metabolic response, since survival under such extreme organ failure is a very recent phenomenon. This second phase of the syndrome, with associated suppression of thyroid hormone and other pituitary hormones and a variety of other changes, represents in this construction a maladaptive response. Patients in this situation tend to have elevated insulin levels, nitrogen wasting, retention of fats if calories are made available, and a variety of other metabolic abnormalities that include neuropathy and cardiomyopathy. These authors consider that provision of multiple hormonal support, including thyroid hormone, growth hormone, and androgens, may be beneficial.93–96
CYTOKINES IN NTIS
In a series of septic patients studied shortly after admission to an ICU, total T4, free T4, total T3, and TSH were depressed, and IL-1β, soluble interleukin-2 receptor (sIL-2R), IL-6, and TNF-α were elevated.97 The hypothalamo-pituitary-adrenal axis was activated as expected. The data suggest central suppression of TSH as the cause of the problem, but the relation to cytokines is unclear, as seen in the following reports. Hermus et al.98 showed that continuous infusion of IL-1 in rats causes suppression of TSH, T3, and free T4. Higher doses of IL-1 were accompanied by a febrile reaction and suppression of food intake, which presumably played some role in the altered thyroid hormone economy. IL-1 did not reproduce the diminution in hepatic 5′-deiodinase activity believed to be so characteristic of NTIS. IL-1 is also known to impair thyroid hormone synthesis by human thyrocytes and is enhanced in many diseases associated with NTIS.99 van der Poll et al.100 studied the effect of IL-1 receptor blockade in human volunteers to determine if it could alter the NTIS induced by endotoxin. Blockade of IL-1 activity was achieved by infusing recombinant human IL-1 receptor antagonist, but this did not prevent the drop in T4, free T4, T3, and TSH or the rise in rT3 caused by endotoxin. This is evidence against an important role for IL-1.
Interferon γ
Interferon-γ (IFN-γ) 100 μg/m2 administered subcutaneously to normal volunteers did not alter TNF-α levels, caused a small elevation of IL-6 levels, and thus did not support a role for IFN-γ in the pathogenesis of the euthyroid sick syndrome in humans.101
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree