FIGURE 76-1. Regulation of thyroid hormone production in the hypothalamic-pituitary axis. Thyroid hormone is produced in the thyroid gland under control of thyroid-stimulating hormone (TSH). Circulating hormones exert multiple effects on different tissues in the periphery and also feed back to inhibit hypothalamic signals that stimulate TSH release and pituitary production of TSH, thereby maintaining thyroid hormone levels in defined limits.
Thyroid hormone production is also subject to additional influences. For example, rates of thyroid hormone synthesis and release often become depressed in conditions of inflammation and illness.
Thyroid Hormone Action in the Periphery
The transport of thyroid hormones in the periphery is discussed in Chapter 92. Plasma thyroid hormone constitutes the reservoir for distribution to target tissues. Higher hormone levels are generally correlated with increased TH response. However, there are differences in availability of hormone in peripheral tissues, which are consequences of variations in transport processes and metabolic conversions that generate T3 and degrade T4 and T3 to inactive forms. The activity of these pathways means that the correlation between plasma hormone levels and response is not absolute.
Both major forms of thyroid hormone (T4 and T3) are transported in the circulation in complex with plasma proteins (Fig. 76-2). The ratio of total T4 to T3 in plasma is about 60:1. This is higher than the 20-fold ratio of T4 to T3 that is initially secreted by the thyroid gland because of greater plasma binding of T4 versus T3, resulting in greater clearance rates of T3. About 99.98% of total circulating T4 and 99.7% of T3 form noncovalent interactions with serum proteins, mostly to thyroxine-binding globulin but to a lesser extent to thyroxine-binding prealbumin, albumin and lipoproteins. The fact that less T3 circulates in complex with plasma proteins means that the ratio of free T4 to T3 is around three- to sixfold, with typical circulating free hormone levels around 20 picomolar (pM) T4 and 6 pM T3, respectively. The free fraction is biologically active and can enter target tissues. Thus, interactions with plasma binding proteins help to ensure even hormone delivery throughout the body.
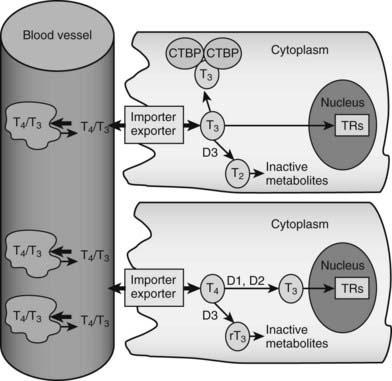
FIGURE 76-2. Peripheral actions of thyroid hormone. The figure summarizes possible fates of thyroid hormone in the periphery. Hormones are mostly transported as complexes with serum binding globulins with limited amounts of free T4 and T3. Hormones are actively taken up into cells by facilitated transport mechanisms, where they undergo several different fates. The upper cell represents possible intracellular fates of T3; it can be sequestered in complexes with CTBP dimers, undergo conversion to inactive metabolites, or enter the nucleus to interact with TRs. The lower cell represents fates of T4, which is either activated by metabolic modification to form T3 or converted to inactive metabolites. It should be noted that expression of D1 and D2 is under transcriptional control, making this step an important control point in thyroid hormone response.
IMPORT AND EXPORT
Thyroid hormone entry into cells is mediated by specific transporters (see Fig. 76-2).11–13 While early models suggested that lipophilic thyroid hormone molecules enter cells by diffusion across the plasma membrane, and this may occur to some extent, it is now clear that T4 and T3 entry mostly involves facilitated transport, a form of passive diffusion that requires membrane transport proteins.
Several proteins involved in thyroid hormone import and efflux across the plasma membrane of target cells have been identified. However, the quantitative contributions of these and possibly other transporters to overall thyroid hormone entry and export have not been established, and concentrations of various transporters in different tissues may vary. Potential transporters include members of several different multigene families: monocarboxylate transporters (MCTs), organic anion transporters (OATP), l-amino acid transporters, multidrug resistance–associated proteins, fatty acid translocase, and Na+/taurocholate-cotransporting polypeptide.
Presently, the best-characterized thyroid hormone transporter is the X-linked MCT8 isoform, which is important for thyroid hormone import into the brain. Expression of the MCT8 gene in cultured cells and frog oocytes enhances thyroid hormone transport into cells. Inactivating human MCT8 mutations are associated with severe neurologic defects in affected males, suggesting that MCT8-dependent thyroid hormone transport into neurons is essential for proper brain development. There are also changes in circulating thyroid hormones (low T4 and high T3). Analysis of MCT8 mutants in cultured cells reveals that the mutations abolish T3 transport, likely explaining elevated circulating T3 levels. Targeted deletion of the mouse MCT8 gene leads to alterations in thyroid hormone levels that are similar to those of affected humans, although mice are devoid of the neurologic defects. Here, liver thyroid hormone levels are normal, suggesting that other transporters are more important in this tissue.
Of other transporters, recent observations show that a closely related protein, MCT10, can also mediate T4 and T3 import into cells. MCT10 is a more effective transporter than MCT8 and displays a preference for T3. It is widely expressed and particularly abundant in the liver, intestine, kidney, and placenta. Finally, OATP1C1-expressing cells exhibit preferential import of T4 and rT3. The protein is distributed widely in the brain, with locations consistent with a specific role in thyroid hormone transport across the blood-brain barrier. It is not clear whether there are other high-affinity thyroid hormone transporters that are yet to be identified or whether apparently low-affinity transporters may play a physiologically important role in some tissues.
Less is known about mechanisms involved in thyroid hormone export from cells. It is clear that MCT8 and MCT10 enhance both thyroid hormone uptake and export, but it is not clear whether these proteins are physiologically relevant hormone exporters or whether other, undescribed, exporters may alsoplay a role.
In some tissues—brown fat in the mouse is an example—hormone response is dependent on T3 that is generated from intracellular T4. The implication here is that free T4 mostly penetrates these cells, where it constitutes a reservoir for T3 generation, and that tissue uptake of T3 is much lower. Regulated conversion of T4 to T3 provides an important control point; induction of the type 2 deiodinase via induction of second messenger pathways regulates T3 levels and therefore the extent of the thyroid hormone response (also discussed later).
ACCUMULATION OF THYROID HORMONE IN CELLS
While free thyroid hormones are present at low concentrations in the circulation, levels of hormone can be much higher in target tissues. Although the extent to which this pool of hormone is active is not known, this observation suggests that there are mechanisms which permit thyroid hormone accumulation in target cells.
T3 binds with nanomolar affinity to an intracellular protein that was originally called cytoplasmic T3-binding protein (CTBP).14 This protein is found at high abundance in the kidney and, to a lesser extent, in liver. Because CTBP was later found to be homologous to mu-crystallin, a protein abundant in the lens of the kangaroo eye, the gene is now often called CRYM. Overexpression of CRYM in stable cell lines increases maximal cellular T3 binding capacity and decreases T3 efflux rates. However, these effects are coupled to reduced transcriptional responses to T3, suggesting that CRYM sequesters T3 in an inactive cytoplasmic complex, away from nuclear TRs.
CRYM is important for thyroid hormone action in vivo. Mice with a targeted CRYM gene deletion appear normal but exhibit moderate decreases in circulating T4 (25%) and T3 (13%) levels and perhaps more importantly, also exhibit extremely rapid rates of T3 entry into and escape from target tissues, presumably because there is no CRYM to sequester cytoplasmic hormone from export mechanisms. Human patients with a natural CRYM mutation that blocks its ability to bind T3 (K314T) are deaf, a known consequence of defective thyroid hormone signaling during development. Interestingly, CRYM requires NADPH for dimerization and T3 binding (Fig. 76-3). As NADPH levels are reflective of levels of cellular reducing power and anabolic capacity, it is conceivable that CRYM may sequester or release T3 in response to alterations in cellular metabolic status. This notion is attractive but not proven.
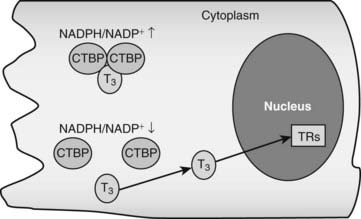
FIGURE 76-3. Thyroid hormone interactions with CTBP. Intracellular T3 can bind CTBP dimers and become sequestered in the cytoplasm. CTBP dimer formation is sensitive to intracellular NADPH/NADP+ concentrations such that free hormone will probably be released in response to reductions of NADPH levels.
There could be other T3-binding proteins in addition to CRYM and the nuclear TRs (which are described later). Radioisotope-labeled T3 interacts with proteins in the endoplasmic reticulum, mitochondrion, and nuclear envelope.2 These putative T3-binding proteins have not been identified, and the functional significance of these interactions is unknown.
DEIODINASES IN THYROID HORMONE ACTIVATION AND INACTIVATION
As discussed earlier and in Chapter 73, the thyroid gland produces mostly thyroxine. Limited amounts of T3 and rT3 are secreted by the gland, but more than 80% of both these forms of thyroid hormone are produced in the periphery via actions of specific deiodinases, D1, D2, and D3.15,16 D1 and D2 remove the iodine group from the 5′ position of the outer thyronine ring (Chapter 74). As T3 binds to TRs with higher affinity than T4, the actions of D1 and D2 deiodinases serve to increase thyroid hormone activity through generation of T3, and this represents a step-up in activity. Conversely, the action of D3 decreases hormone activity and represents a step down by removing the iodine group from the 5 position of the inner thyronine ring and converting T4 and T3 to rT3 and 3,3′-T2, respectively, neither of which interacts substantially with nuclear TRs at physiologic concentrations. Changes in deiodinase expression and activity are important control points in thyroid hormone signaling; they influence the production and availability of the biologically active form of thyroid hormone, T3.
D2 activity generates most plasma T3 in euthyroid (defined as levels of thyroid hormone within normal range) conditions via conversion of T4 to T3 in peripheral tissues. Comparisons of rates of T3 production by D2 and D1 indicate that D2 is probably more important for T4 to T3 conversion at physiologic T4 concentrations; it displays a higher Km value for substrate than D1 and is likely to be sensitive to alterations in T4 concentrations and more active than D1 at physiologic hormone concentrations. In addition, environmental stimuli regulate D2 activity catalyzing local T3 production from T4 in certain tissues. For example, signals such as cold exposure increase D2 expression in brown fat (discussed earlier), and the resulting increase in intracellular T3 promotes induction of uncoupling protein with consequent heat generation. These lines of evidence suggest that D2 is important in generating T3 that activates TRs. Accordingly, D2 is located in the perinuclear region where it can deliver T3 efficiently to TRs.
D1 acts in a similar way to D2, but its functions are not as clear as with D2, because it displays a relatively high Km value for T4 to T3 conversion (i.e., the enzyme binds the T4 substrate with low affinity). D1 is highly expressed in liver and kidney, and its expression is induced by thyroid hormone. While previous models suggested that D1 is important for regulation of circulating T3, more recent models suggest that D1 may only contribute substantially to plasma T3 in conditions of thyroid hormone excess. D1 may also be involved in 5′ deiodination of other forms of thyroid hormone, including rT3.
D3 is important for clearance of plasma T3.17 It is highly expressed in human placenta, where it is thought to be important for protection of the developing fetus from effects of maternal thyroid hormones. D3 is also highly expressed in the brain and skin. Increased D3 levels may account for reductions of circulating thyroid hormone levels that are often observed in critically ill patients and states of inflammation. Finally, D3 is often overexpressed in vascular tumors, and this in turn can result in marked reductions in circulating thyroid hormone levels.
ALTERNATE METABOLIC FATES OF THYROID HORMONE: INACTIVE AND ACTIVE DERIVATIVES
In addition to 5′ deiodination, T4 undergoes other modifications which are generally believed to inactivate the hormone.18 About 45% of circulating T4 is converted to rT3 and 35% to T3. T3 and rT3 undergo further deiodinations to create T4 derivatives with all possible combinations of iodinated and deiodinated inner and outer rings (3,3′-T2, 3,5-T2, 3′,5-T2, 3′-T1, 3-T1 and T0), none of which bind significantly to nuclear TRs. T4 and T3 can also be inactivated by glucuronidation in liver, followed by secretion into the bile or by sulfation in liver and kidney and excretion in the urine.
The fact that most secreted T4 is rapidly converted to apparently inactive forms of the hormone has led investigators to question whether some of these T4 derivatives have unappreciated functions in activation of alternate receptors or as metabolic precursors of different active forms of the hormone. As mentioned earlier, one of the most prominent, rT3, is a very weak thyroid hormone partial agonist that is unlikely to exert significant actions through the TRs. However, there are two cases in which alternate metabolic modification of T4 does produce biologically active forms of hormone:
Actions of Thyroid Hormone
Thyroid hormones exert profound influences on nearly all tissues (Fig. 76-4),2 as discussed in more detail in Chapter 79. Receptors for thyroid hormones (TRs) are expressed fairly ubiquitously, although well-defined target tissues such as liver and heart express higher levels of TRs than others, and there are differences in distributions of particular TR isoforms20 (described in more detail in a following discussion). Essentially, thyroid hormone is needed for proper growth and development of the fetus and children and exerts widespread influences on multiple aspects of metabolism in adults.
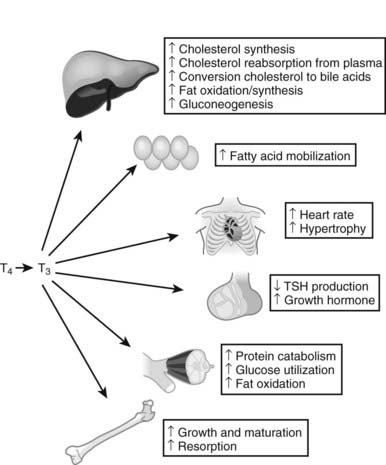
FIGURE 76-4. Tissue effects of thyroid hormones: summary of thyroid hormone effects on different tissues.
Analysis of gene expression patterns in mouse knockout models has confirmed that TRs mediate nearly all thyroid hormone responses, and that many of these effects involve changes in gene expression. While actual influences of thyroid hormone on gene expression are complex, there are important underlying principles.2 First, developmental defects that arise in cretinism and children born to hypothyroid mothers are not reversed by later hormone replacement, whereas adults with thyroid hormone imbalances exhibit metabolic disturbances that in most cases can be readily reversed by restoring TH levels to correct levels. This implies that hormone must trigger key developmental events in defined temporal windows, but thyroid hormone–regulated genes involved in metabolic regulation remain sensitive to alterations in hormone levels and continuously couple gene expression to thyroid status. Consideration of gene expression patterns, described in more detail later, reveals that some genes are induced in multiple tissues (D1 is an example), whereas other target genes are regulated in a manner that is highly tissue- and context-specific. Thus, thyroid hormone is a primary regulator of some genes, but hormone must cooperate with other factors to induce expression of other genes. Specific effects of thyroid hormone follow.
BASAL METABOLIC RATE
One of the most important effects of thyroid hormone involves changes in basal metabolic rate (BMR) in multiple tissues.21–24 Thyroid hormones stimulate oxygen consumption (indicative of enhanced metabolism) in multiple locations, including skeletal muscle, liver, kidney, and intestine. Increases in BMR are probably partly related to increased mitochondrial activity and number. Importantly, thyroid hormones induce expression of uncoupling proteins (UCPs), mitochondrial membrane proteins that dissipate the proton gradient in the absence of ATP synthesis, thereby converting potential energy to heat. This effect is probably a contributor to thyroid hormone–dependent increases in BMR.
Thyroid hormone does not always enhance BMR. For example, BMR is not enhanced by thyroid hormone in most regions of the brain, and the hormone actually suppresses metabolic activity in the pituitary. The receptors in these tissues are functional, suggesting that key mediators of thyroid hormone regulation of BMR are blocked or absent.
TISSUE EFFECTS
Thyroid hormone exerts specific effects on growth, development, and metabolism in a variety of tissues. Some of these effects are summarized briefly here:
Liver
Thyroid hormones exert multiple effects on the liver.2,25 They are potent mitogens in this tissue, especially in growing animals. Thyroid hormone also influences multiple metabolic processes. There is stimulation of fatty acid β-oxidation and gluconeogenesis, both key aspects of fasting response.26 However, thyroid hormone can also stimulate expression of enzymes involved in lipogenesis and generation of NADPH-reducing equivalents that are required for fat synthesis and protection against reactive oxygen species. Since it is thought that fat oxidation and synthesis do not occur simultaneously, these processes are probably separated spatially or temporally, and thyroid hormone must cooperate with other signaling mechanisms to regulate these effects.
Thyroid hormone plays a major role in regulation of cholesterol metabolism in liver in rodents, and studies of patients with thyroid excess and deficiency states suggest that some of these pathways are also thyroid hormone regulated in humans. The hormone induces expression of enzymes involved in cholesterol synthesis but also increases levels of the low-density-lipoprotein (LDL) cholesterol receptor, which promotes cholesterol uptake from the circulation. Likewise, thyroid hormone increases expression of apolipoprotein A1, the key protein component of high-density lipoprotein (HDL) and increases expression of an HDL receptor (SR-B1). Consequently, thyroid hormone excess may promote increased cholesterol flux from the plasma to the liver through both the LDL and HDL pathways. Additionally, thyroid hormone stimulates cholesterol efflux and cholesterol to bile acid conversion. Collectively these mechanisms account for the net reductions of serum cholesterol levels that are observed with thyroid hormone and a net reverse cholesterol transport reflected by an increased flow of bile acids into the gut. Although not observed with T3, some thyroid hormone analogues promote a lowering of plasma triglycerides that may be due to net suppression of triglyceride synthesis through poorly defined mechanisms.
Adipose Tissue27
Thyroid hormones promote differentiation of precursors into white fat and induce lipogenic enzymes in pre-adipocytes from young rats and cell lines, but they also increase lipolysis in animals and humans. However, the lipolytic effects must predominate, because there is a net loss of fat in hyperthyroid states and a gain in hypothyroidism.
Thyroid hormone stimulates adaptive thermogenesis in brown adipose tissues. In cold or in response to overeating, there is increased local production of T3 via transcriptional induction of D2. This T3 cooperates with norepinephrine outputs from the sympathetic nervous system to induce uncoupling proteins that promote dissipation of the mitochondrial protein gradient as heat rather than ATP storage. Whereas previous reports suggested that adaptive thermogenesis was only important for temperature regulation in human newborns and in small mammals, recent studies have suggested that brown fat may also be important in adult humans. There are currently no reports of how thyroid hormones regulate development of this potentially important tissue.
Heart and Blood Vessels28,29
Thyroid hormones have multiple effects on the cardiovascular system. One influence is to increase cardiac output. Excess thyroid hormone is associated with increased heart rate, atrial arrhythmias, and development of heart failure. Conversely, reduced thyroid hormone is associated with reduced heart rate, lower vascular resistance, and increased blood volume. Thyroid hormone regulates genes involved in cardiac contractility. There is induction of the sarcoplasmic reticulum Ca2+ ATPase 2 (SERCA2), involved in calcium reuptake during the diastolic phase, and α myosin heavy chain (MHC), a fast ATPase required for heart contractility that is expressed in adult heart. Conversely, thyroid hormone inhibits expression of βMHC, a slow ATPase expressed in embryonic heart and up-regulated in stress conditions that diminish cardiac function. Thus, changes in thyroid hormone response in heart may be an important component of heart failure. Thyroid hormones also inhibit injury at sites of stroke, attenuate cardiac remodeling, and improve hemodynamics.
Pituitary2
Thyroid hormones repress production of pituitary TSH and hypothalamic TRH. This feedback loop is critical for maintenance of normal thyroid homeostasis and can be deranged in diseases, as described earlier and in other chapters. Thyroid hormones also stimulate expression of factors such as growth hormone, which influence growth and metabolism of many other tissues.
Muscle2
Thyroid hormones promote muscle catabolism and increase skeletal muscle energy expenditure in adults. Thyroid hormone induces the insulin-sensitive glucose transporter in muscle and also promotes fat burning.30 These effects may help to sensitize muscle to insulin response. Accordingly, a human D2 gene polymorphism that reduces activity of the enzyme in muscle leads to a 20% reduction in insulin-dependent glucose disposal in humans and is correlated with insulin resistance in human populations that harbor this alteration.
Skeletal System31
Thyroid hormone is required for normal bone growth and maturation in children. Juvenile hypothyroidism causes delayed bone formation and short stature, whereas thyrotoxicosis leads to increased growth and advanced skeletal development. In adults, however, thyroid hormone excess promotes bone resorption. Thyroid hormone excess can lead to osteoporosis, especially in postmenopausal women.
Brain32,33
Thyroid hormone regulates brain development, and hypothyroidism results in mental retardation and multiple neurologic defects. Slow mentation and other CNS disturbances characterize hypothyroidism. In hyperthyroidism, there can be episodes of anxiety and even psychosis.
Intestine
Thyroid hormone is required for normal maturation of the small intestine. TRα1 is required for proliferation of intestinal epithelial progenitor cells via induction of the β-catenin proto-oncogene.34
Skin35
Thyroid hormone is important for skin function, and thyroid hormone imbalances are often first manifested in changes in appearance. Hypothyroidism leads to cold and dry, thickened skin. There is also increased hair loss. Conversely, hyperthyroidism leads to warm, moist, and smooth skin with fine soft hair. Hormone effects are a combination of inhibitory changes in keratin expression, sterol biosynthesis, diminished sebaceous gland secretion, and increased collagen breakdown.
SYSTEMATIC ANALYSIS OF THYROID HORMONE TARGET GENES
Many thyroid hormone–responsive genes involved in the responses described earlier are known.2 For example, in liver, thyroid hormone induces carnitine palmitoyl transferase 1a (that mediates the rate-limiting step in fatty acid oxidation); glucose-6-phosphatase and phosphoenolpyruvate carboxykinase (rate-limiting steps in gluconeogenesis); the NR coregulator PGC-1α, which is an important transcriptional coregulator that stimulates genes important for mitochondrial biogenesis, gluconeogenesis, and fat oxidation; CYP-7a1 (cholesterol-to–bile acid conversion); fatty acid synthetase; acetyl-CoA carboxylase; malic enzyme (increases fat synthesis); and spot 14, which is required for fat synthesis in some tissues and up-regulated in contexts in which carbohydrates are converted to fat. Many other hormone-responsive genes are probably still unknown.
The earliest systematic attempt to define thyroid hormone response was performed in the late 1970s. Use of two-dimensional (2D) gels to analyze extracts from radiolabeled methionine–pulsed cells revealed that about 1% of 1000 rat liver proteins change in response to altered thyroid hormone.36,37 There were inductions and repressions, and the studies began to define the “domain” of the thyroid hormone response. Recent advances in gene-profiling technology have permitted much more detailed descriptions of hormone-dependent alterations in gene profile. Presently there have been relatively few systematic genome-wide descriptions of thyroid hormone responses.25,26,38,39 An early study of thyroid hormone action in mouse liver revealed that about 1% of mouse genes change in response to altered thyroid hormone (hypothyroid versus hyperthyroid),25 confirming estimates from 2D gels. This study revealed target genes involved in carbohydrate, fat, and amino acid metabolism and unexpected thyroid hormone–regulated pathways; for example, genes involved in apoptosis are induced. The experiment also provided insights into patterns of thyroid hormone–responsive gene expression. Most regulated genes (>65%) in liver were repressed by thyroid hormone. This preponderance of negatively regulated genes is not seen in all tissues. Target genes are generally up-regulated by hormones in human muscle primary culture. Later studies of thyroid hormone patterns of gene expression in TR knockout mice provided insights into TR-specific effects and subtle differences in thyroid hormone–responsive gene expression and are discussed in more detail later in the chapter. Systematic screening of thyroid hormone–responsive genes should provide detailed descriptions of novel target genes and pathways in different tissues.
THYROID HORMONE–REGULATED MICRORNAs
MicroRNAs (miRs) are small (18 to 25 nucleotides), noncoding RNAs that hybridize with coding mRNAs to inhibit translation and, in some cases, also promote mRNA degradation.40 The enormous roles these miRs play in regulation is just beginning to be appreciated. MiRs are produced by cleavage and processing of large primary transcripts, which often code for proteins. Thus, many of the influences that regulate expression of parental primary transcripts also regulate expression of the associated miR. While effects of thyroid hormone on miR expression have not been studied extensively, one miR (miR-208) lies within a noncoding region of the thyroid hormone–responsive human αMHC transcript (and is therefore up-regulated by thyroid hormone itself). MiR-208 is implicated in up-regulation of stress-dependent cardiac responses. It is likely that other thyroid hormone–responsive miRs exist, and they may play important roles in hormone effects on protein translation.
History of the Mechanism of Action of Thyroid Hormones
As the physiologic effects of thyroid hormones became known, investigators focused on roles of these hormones on stimulating the basal metabolic rate and on the liver.1,2 It was originally proposed that the hormones directly uncouple oxidative phosphorylation and that the primary site of action of thyroid hormones was at the mitochondria; here, investigators related actions of thyroid hormones to those of dinitrophenol. Later studies revealed other influences of thyroid hormones, including cholesterol reduction, that were hard to relate to mitochondrial actions. The notion that thyroid hormones directly uncouple mitochondrial oxidative phosphorylation did not provide a coherent unifying hypothesis to explain all actions of thyroid hormone.
In the late 1960s, Tata and colleagues conducted a number of measurements of the effects of thyroid hormones on the liver.41 He found that thyroid hormone–dependent increases in metabolic rate were blocked by actinomycin D, an inhibitor of RNA and, secondarily, protein synthesis. Also, there was an increased uptake of radiolabeled uridine into trichloric acid (TCA) precipitable material following administration of TH to animals, as well as increased activity of RNA polymerase, suggesting that TH stimulates RNA synthesis. Similar studies were being conducted with NR ligands such as with glucocorticoids, estrogens, and progestins, and there was great controversy as to whether these ligands acted through (1) transcriptional control, (2) precursor uptake, or (3) posttranscriptional control. The experiments did not address high specificity of TH responses, and as a result, controversy remained as to which of the three mechanisms applied to thyroid hormone action. Nevertheless, this study represented the first fundamentally correct proposal for the mechanism of thyroid hormone action.
DISCOVERY OF THYROID HORMONE RECEPTORS AND THEIR MECHANISM OF ACTION
TRs were identified in the 1970s.42,43 Following the discovery of several nuclear receptors, including the estrogen and glucocorticoid receptors, investigators in the thyroid hormone field utilized similar approaches to look for TRs. Specific binding of radiolabeled T3 to liver cells was initially discovered by Oppenheimer and colleagues using intact cells and later characterized further by Samuels and colleagues, who found that the mechanism of TR actions differed mechanistically from the steroid receptors, which translocate into the nucleus on ligand binding. For TRs, hormone could bind directly to nuclei, and the location of receptors did not alter with T3.
In the late 1970s and early 1980s, multiple aspects of the receptors were revealed.36,44 Analysis of properties of TRs revealed a single class of high-affinity binding sites with Kd values for T3 in the 0.1 nM range. Specific hormone-binding sites were observed in extracts of several responsive tissues, including liver, anterior pituitary, brain, and heart. Estimates of the number of binding sites per cell suggested that the proteins are rare; highly responsive tissues only contained about 10,000 specific hormone-binding sites per cell. Evidence that the receptor preparations correspond to physiologic hormone targets came from observations that affinities for different thyroid hormones parallel their biological potencies (Triac>T3>T4>rT3). Partial TR purification and photo-affinity labeling revealed two major nuclear hormone-binding proteins with molecular weights of 46 and 57 kD, which were later found to correspond to products of distinct genes—the TR α and β isoforms—described later in the chapter. TRs were also found to be associated with chromatin, and preferentially with active chromatin, suggesting that they influence gene expression through interactions with DNA.45–48
DISCOVERY THAT THYROID HORMONES REGULATE SPECIFIC mRNAS AND GENE TRANSCRIPTION
As molecular biology techniques became available, it was possible to isolate specific mRNAs from tissues where they were abundant and to translate them. By the 1970s, regulation of specific mRNAs had been demonstrated for estrogens, progestins, and glucocorticoids. Growth hormone (GH) mRNA was abundant in rat pituitary cells, and Baxter and colleagues were able to demonstrate that thyroid hormones could specifically regulate GH mRNA, conclusively demonstrating for the first time that thyroid hormones alter the levels of specific mRNAs. The same group cloned rat GH gene sequences, and these permitted demonstrations that thyroid hormones increased mRNA hybridizable to a rat GH cDNA probe. The cloned GH sequences were also used in so-called “polymerase run-on experiments” that showed that hormones stimulate transcription rather than induce some posttranscriptional modification of the mRNA. These studies resolved the controversies prevalent at the time of experiments by Tata and colleagues, discussed previously. Thus the fundamental concept of thyroid hormone action whereby the receptor regulates the transcription of specific genes was established.
DISCOVERY THAT TRS BIND PREFERENTIALLY TO ACTIVE CHROMATIN AND SPECIFIC DNA SEQUENCES
Studies during the 1970s and 1980s also set the stage for understanding the nature of TR binding sites and their relationship to active chromatin. GH genomic DNA was used for interaction studies with partially purified TR preparations, demonstrating that TRs bound to specific DNA sequences. These experiments were forerunners of the now familiar concept that receptors act by binding to specific thyroid hormone response elements (TREs). That the receptors could affect DNA was further evidenced by the finding that they induce bending of rat or human TR DNA binding sites. Subcellular fractionation revealed that TRs were associated with chromatin, and preferentially with active chromatin, suggesting a relationship between the receptors and transcriptionally active chromatin.45–48 These studies set the stage for definition of thyroid hormone response elements (TREs).
CLONING OF TRS
TRs were cloned in 1986.49,50 They were identified as cellular homologs of a retroviral oncogene (cancer-causing gene), v-erbA,51 which causes erythroblastoma in chickens. Isolation of these cDNAs occurred after those for other members of the NR family, the glucocorticoid and estrogen receptors, which led to the realization that v-erbA was derived from an NR. Both of the v-erbA homologs bound thyroid hormone with high affinity and appropriate specificity. The Vennstrom group clone was most closely related to v-erbA and was originally designated as c-erbA, but it is now called thyroid hormone receptor α (TRα). As compared to TRα, v-erbA contains 17 different amino acid substitutions and a C-terminal truncation, which are now known to prevent the viral oncogene from binding hormone.49 The Evans group clone encoded a highly homologous but distinct 57-kD protein that was closely related to TRα and v-erbA but was the product of the THRB gene.50 The characterization of TR genes (THRA and THRB), which encode the two major TR isoforms (TRα1 and TRβ1) and several variants that arise from differential splicing (described later), helped to reveal that TRs belong to the NR family, which includes receptors for the steroid hormones, vitamins A and D, and other small lipophilic molecules.4
Identification of these receptor cDNAs paved the way for many studies of receptor structure and function. Although it was known at the time that NRs were composed of modular domains, the receptor structures provided the primary sequences in these domains and the abilities to express them and study their functions. In addition, the availability of TR cDNAs paved the way for functional studies, analog development, and improvements in understanding TR mechanisms. While these studies are not described in historical context, there have been rapid advancements in understanding of TR action, including the definition that unliganded TRs are transcriptionally active, leading to a new paradigm for thyroid hormone action; definition of TRE sequences; identification of retinoid X receptor (RXR) heterodimer partners and coregulators; x-ray structural analysis of DBDs and LBDs, which demonstrated a new and unexpected concept that the receptor folds around the ligands; improved understanding of TR function through generation of TR gene knockout animals; and development of new thyroid hormone analogs with selective actions.
Thyroid Hormone Receptors: Mechanism
TRs work by altering gene expression in response to changes in thyroid hormone concentrations (mostly T3).2,38 This alteration in gene transcription profile is believed to account for most of the observed physiologic effects of thyroid hormones, although there are also actions of thyroid hormones that do not involve transcription (discussed later). Unlike the case with steroid receptors, TRs are active in the absence of hormone.
HORMONE BINDING AND OCCUPANCY
Measurements of thyroid hormone affinity for its receptors reveal that T3 binds to purified and recombinant TRs with high affinity. T3 binds with about 10- to 15-fold greater affinity than T4 (Kd values are around 2 nM for T4 and 0.2 nM for T3). These values appear higher than measured concentrations of circulating free hormones (0.02 nmol for T4 and 0.06 nmol with T3), implying that receptor occupancy could be minor in normal conditions. However, based on direct estimates of occupancy of nuclear TRs in different tissues after radiolabeled T3 and T4 injection into hypothyroid rats, about 25% to 35% of liver and kidney TRs and 58% of pituitary TRs become occupied with thyroid hormones in euthyroid conditions. Thus, there appear to be mechanisms to bring the hormone into the correct range to bind and activate receptors. One possibility was described earlier: intracellular generation of T3 from T4 leads to increased T3 production in target cells. Facilitated transport alone would not be enough to concentrate T3, but combinations of transport and cytoplasmic sequestration by CTBP/CRYM or similar proteins could play a role. Finally, TR complexes with stabilizing coactivators could display higher affinities for target hormones than isolated TRs tested in vitro or partially purified from cell extracts. In any case, it is clear that thyroid hormone concentrations are in the correct range to achieve reasonable receptor occupancy in target tissues under euthyroid conditions.
TR ACTION
Unlike classic models of hormone action in which unliganded receptor is inactive and hormone binding triggers its activity, TRs are transcriptionally active in the absence and in the presence of hormone (Fig. 76-5).52 Rather than activating the receptor, hormone binding alters the spectrum of its actions.
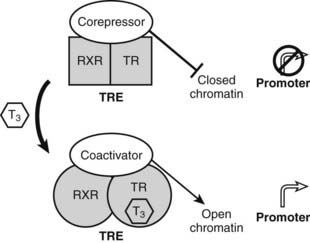
FIGURE 76-5. Thyroid hormone receptor action. Actions of unliganded and liganded TRs at positively regulated genes. The receptor binds DNA as a heterodimer with RXR in the absence and presence of the hormone. Unliganded TRs recruit corepressors, which repress gene transcription by condensing local chromatin structure and blocking coactivator binding. Hormone binding promotes a conformational change, which leads to exchange of corepressor for coactivators, which reverses effects of coactivators.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree