With respect to fasting, premeal, or postprandial targets, there is little support for any particular level of glycemic control in the management of type 2 diabetes insofar as no large-scale outcome study has targeted particular levels of glucose with home glucose monitoring. The ADA target of fasting and premeal plasma glucose levels of 70 to 130 mg/dL (3.9 to 7.2 mM) is based on an estimate of the range of average glucose values that would be associated with a low risk of hypoglycemia and an A1C less than 7%. The American College of Endocrinology target of less than 110 mg/dL (6 mM) is an effort to achieve normal levels of glycemia. However, it should be recognized that consistent fasting and premeal glucose levels less than 110 mg/dL would be expected to be associated with an HbA1c of approximately 5.5% or lower.19
There are limited studies in which even safety, much less a clinical outcome, is documented for targeting a particular level of postprandial glucose. There are effective A1C-lowering agents that primarily target postprandial glucose levels. Monitoring postprandial glucose levels may allow more effective dose adjustment of these agents, though even this has not been demonstrated in clinical trials. Certainly there are patients with diabetes who have average fasting glucose levels within targets but whose A1C remains elevated. Monitoring and specifically treating postprandial elevations in these patients may provide improvements in A1C, perhaps with a lower risk of hypoglycemia and weight gain than further lowering fasting and premeal glucose levels. The ACE guidelines recommend targeting a 2-hour postprandial glucose level less than 140 mg/dL (7.8 mM) in an effort to achieve near-normal glycemia. Consistent postprandial glucose values less than 140 mg/dL would be associated with average A1C levels of approximately 5% or lower; 2-hour postprandial glucose levels of less than 180 mg/dL (10 mM) would generally be associated with A1C levels of 6% to 7% and is the recommended postprandial target of the ADA.1,19
Lifestyle Intervention
The components of lifestyle intervention include comprehensive diabetes education aimed at enabling patients to self-manage their diabetes, medical nutrition counseling, and exercise recommendations. The appropriate paradigm of care in diabetes is patient focused, since patients are responsible for almost every diabetes-related decision and behavior. Providers at their best can provide advice and help recognize and suggest techniques to overcome obstacles to achieving treatment goals.
EDUCATION OF PATIENTS
Arguably, over the last 10 years, nothing has changed more fundamentally in diabetes care than the emphasis on lifestyle intervention. For decades, physicians and patients paid lip service to the notion that lifestyle intervention is important. Now we have significant clinical trial evidence that each component of lifestyle intervention, when appropriately administered, can contribute to improved outcomes.20,21 Furthermore, since the Balanced Budget Act of 1997 and the passage of complementary legislation by most state governments, lifestyle intervention has become a covered benefit for most patients with diabetes in the United States.
Diabetes is a lifelong disease, and health care providers have almost no control over the extent to which patients adhere to the day-to-day treatment regimen. The appropriate role of the health care provider is to serve as a coach to the patient, who has primary responsibility for the delivery of daily care. Thus, it is essential that health care professionals understand the context in which patients are taking care of their disease. Using a prescriptive approach in which patients are told what to do can work on occasion but fails more often than not because of unrecognized barriers to the execution of a particular plan.
As defined by the ADA,22 diabetes self-management education is the process of providing the person with diabetes the knowledge and skills needed to perform self-care, manage crises, and make lifestyle changes. As a result of this process, the patient must become a knowledgeable and active participant in the management of his or her disease. To achieve this rather daunting goal, patients and providers work together in a long-term, ongoing process. Comprehensive diabetes education should be individualized, with emphasis on the issues highlighted in Table 48-2. There are many more specialized topics relevant to almost all patients, such as how to adjust therapy when eating out or during travel, as well as how to access available local health care resources and negotiate the complexity of health care financing in the United States. Although only limited studies are published to date, as a body of work, they do provide support for the concept that diabetes education can be cost effective and can improve outcomes.21–23
Table 48-2. Curricular Areas Which Should Be Addressed in Diabetes Self-Management Education
Adapted from American Diabetes Association: Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications, Diabetes Care 25:202–212, 2002.
A team of providers is generally required to optimally implement the process of diabetes self-management education, because the amount of information that needs to be exchanged is large and the range of expertise required is broad. It is generally impossible to cover the recommended content fully in the context of several or even many brief encounters with a physician in an office setting. Potential providers in a team care approach could include nurses, dietitians, exercise specialists, behavioral therapists, pharmacists, and other medical specialists including diabetologists or endocrinologists, podiatrists, medical subspecialists, obstetrician-gynecologists, psychiatrists, and surgeons. In the diabetes self-care process, the potential role of the community where the patient lives and works is enormous. At a minimum, this community includes family, friends, employers, health care systems, and health care insurers. Each member of the team has a role to play in the process, and it is useful to review these roles frequently (Table 48-3). The primary role of the providers in this process is to provide guidance in goal setting to manage the risk of complications, suggest strategies to achieve goals and techniques to overcome barriers, provide training in skills, and screen for complications. For this process to be a success, the patient must commit to the principles of self-care, participate fully in the development of a treatment plan, make ongoing decisions regarding self-care from day to day, and communicate honestly and with sufficient frequency with the team.
Table 48-3. Team Care: Roles of the Players
To be a source of accurate information and to refer to and coordinate with other sources of information as necessary |
Fortunately, barriers to providing team care are becoming less daunting, in large measure because of the rapidly expanding number of diabetes education programs and improved insurance coverage for services. The American Association of Diabetes Educators (800-TEAM-UP4; www.diabeteseducator.org) and the ADA (800-DIABETES; www.diabetes.org) can provide information regarding diabetes educators and education programs nationwide.
For diabetes care to be effective, communication and mutual respect among the patient-centered team is critical. Unfortunately, in many communities, the full benefit of the consultation and ongoing care with diabetes educators, nurses, dietitians, pharmacists, medical consultants, and primary care providers is not achieved because of overly hierarchical approaches to care. Non–health care professionals, such as lay or peer supporters, can provide benefit to patients in developing effective diabetes self-care behaviors. Key functions of such efforts have been identified and include assistance in managing and living with diabetes in daily life, social and emotional support, and linkage to clinical care.23
Perhaps some of the most overlooked contributors to ineffective care in the setting of type 2 diabetes are the relatively common barriers created by psychiatric, neurocognitive function, and adjustment disorders, which are largely responsive to psychosocial therapies.24
NUTRITION
The ADA has published technical reviews that exhaustively document the literature regarding the effect of medical nutrition therapy and specific advice on diabetes-related outcomes such as A1C and weight, as well as a position statement.25–27 These are summarized in Table 48-4. An individually negotiated nutrition program in which each patient’s circumstances, preferences, cultural background, and the overall treatment program are considered is most likely to result in optimal outcomes. Ideally, a registered dietitian with specific skill and experience in implementing nutrition therapy in diabetes management should work collaboratively with the patient and other health care team members in providing medical nutrition therapy. For optimum outcomes, this counseling should be performed over a series of visits initially, with intermittent follow-up thereafter. Analogously, physicians and other members of the health care team need to support the nutritional plan developed collaboratively.
Table 48-4. ADA Nutritional Principles and Recommendations
Adapted from Klein S, Sheard NF, Pi-Sunyer X, et al: Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition, Diabetes Care 27:2067–2073, 2004; Gillespie SJ, Kulkarni KD, Daly AE: Using carbohydrate counting in diabetes clinical practice, J Am Diet Assoc 98:897–905, 1998; and Egede LE, Ye K, Zhang D, et al: The prevalence and pattern of complementary and alternative medicine use in individuals with diabetes, Diabetes Care 25:324–329, 2002.
Individualized dietary advice can be developed by a physician from a brief diet history obtained by asking: “What do you eat for breakfast? … lunch? … supper? Do you have snacks between breakfast and lunch? … lunch and supper? … supper and bedtime? What do you drink during the day?” Ideally, this information should be obtained at each visit, with specific suggestions for change that both patient and provider agree are important in the context of the overall treatment plan as well as both achievable and sustainable. Easy issues to address include caloric beverages, which tend to elevate glucose levels dramatically and can generally be replaced relatively easily with artificially sweetened alternatives. Juices are generally perceived as healthy but can significantly affect glycemic control and total caloric intake. Portion control and recipe modification are excellent dietary techniques, particularly for meats and fried foods. Substituting low-fat products for higher-fat foods is often useful but needs to be done with the recognition that they are generally higher in carbohydrates. It is important that patients recognize that “fat-free” and “sugar-free” foods are not “free” and that attention to both total carbohydrate and calorie content are critical.
Eating approximately every 4 hours while awake is the most practical dietary plan for most overweight people. Frequent small meals have been shown to be of benefit when used in a controlled inpatient setting, but in general when overweight patients are encouraged to eat more frequently they often overeat more frequently. At a minimum, avoiding high-calorie snacks is reasonable advice for most people with diabetes. If all health care providers repeatedly obtain a diet history every few weeks to months, effective assessment of whether previously agreed-to changes were enacted, reinforcement of the importance of dietary efforts, and the patient’s gradual evolution to a more healthful diet through further dietary modification can be accomplished.
In general, the critical nutrient for glycemic control is carbohydrate. Essentially all carbohydrates consumed are converted to glucose in the gut and require the action of insulin to be cleared from the circulation. A dietary technique called carbohydrate counting can be used in patients with type 2 diabetes to facilitate consistent carbohydrate intake or to allow insulin dose adjustment in response to changes in carbohydrates consumed.28 Although this technique requires less insulin than fixed meal dosing and may help curb weight gain, it has not been shown to improve glycemic control or reduce the rate of hypoglycemia in patients with type 2 diabetes.29 Whereas the β cell in type 2 diabetes has generally lost its responsiveness to glucose, the second phase of insulin secretion is largely spared in type 2 diabetes and is in part driven by amino acids and fatty acids. Therefore, including some protein and fat in each meal and snack may be useful.
Dietary fat is the nutrient most closely associated in epidemiologic studies with the risk of developing type 2 diabetes. Although dietary fat clearly has a major impact on total caloric intake, as well as on circulating lipids, it has a minimal acute impact on glycemia. It is recommended that people with diabetes, if they are overweight, consume a diet modestly restricted in calories, with less than 10% of total calories as saturated fat, less than 10% as polyunsaturated fat, and total avoidance of trans fats. Some advocate substituting foods high in monounsaturated fatty acids—seeds, nuts, avocado, olives, olive oil, and canola oil—for carbohydrate, but most patients do not find adequate variety in the monounsaturated fatty acid category and often overeat these high-caloric-density foods.
Dietary protein similarly has a minimal impact on glucose levels, although as mentioned, amino acids do promote insulin secretion. Metabolism of protein results in the formation of acids and nitrogenous waste that may result in bone demineralization and glomerular hyperfiltration. At least 0.8 g of high-quality protein per kilogram is generally recommended. Protein restriction in the setting of kidney disease has been recommended and is more fully discussed in Chapter 54. There is no evidence that protein intake materially effects the risk of developing kidney disease in patients with diabetes.
The role of vitamins, trace minerals, and nutritional supplements in the treatment of diabetes is poorly understood. There are some patients and providers who are absolutely convinced of the utility of soluble fiber, magnesium, chromium, zinc, folic acid, pyridoxine, cyanocobalamin, vitamin A, vitamin C, vitamin E, vanadium, selenium, garlic, and others. Clinical trial data to support their safety and efficacy are inconclusive. Many patients are convinced that nutritional supplementation is healthful, and it is often counterproductive to engage in scholarly discussion of the nature of the evidence base for their decision. At a minimum, discussion should include the documented efficacy of lifestyle and pharmacologic interventions and the idea that these efforts should not be left by the wayside when budgetary constraints affect potentially more effective interventions.30,31 A multivitamin/mineral preparation may be reasonable for most patients with diabetes. A recent randomized control trial in patients with diabetes demonstrated fewer self-reported infections and related absenteeism.32 Studies demonstrating the benefits of B-vitamin supplementation on restenosis after angioplasty33 have recently been called into question; the possibility has been raised that such therapy could increase rates of restenosis after stent placement.34
Although there are proponents of a wide range of dietary composition, there are few data to support these recommendations from long-term outcome studies of prescribed diets. Mixed meals containing 10% to 20% of calories from protein, no more than 10% of calories from saturated fat, no more than 10% from polyunsaturated fats, and the remainder largely from monounsaturated fats (seeds, nuts, avocados, olives, olive oil, canola oil) and carbohydrates, particularly whole grains, fruit, vegetables, and low-fat milk, are probably most reasonable. High-carbohydrate, low-fat diets, although historically recommended by many health organizations, have been shown to increase postprandial blood glucose and triglyceride levels, elevate fasting triglyceride levels, and decrease high-density lipoprotein (HDL) cholesterol levels in insulin-resistant people, including those with type 2 diabetes. Several studies have demonstrated improved lipid levels and blood glucose control in both short- and intermediate-term studies in which total fat intake approaches 45% of calories and carbohydrate intake is as low as 40% of calories. Reducing fat or carbohydrate intake in obese individuals will not necessarily lead to reduced calories. Since weight loss will occur only in the setting of caloric restriction, arguably the most appropriate approach is to limit intake of both fat and highly processed, easily digestible carbohydrates. The treatment of obesity is discussed in Chapter 28; the principles discussed are appropriate when type 2 diabetes is complicated by obesity. To date, short-term studies of medical nutrition therapy, physical activity, and comprehensive lifestyle approaches have been shown to improve the control of classic CVD risk factors, as well as intermediate markers of CVD risk such as C-reactive protein; no long-term, large-scale study of intentional weight loss has been powered to examine CVD endpoints. Look AHEAD (Action for Health in Diabetes) will examine CVD events for up to 11.5 years in a study in which patients with type 2 diabetes 45 to 74 years of age with a body mass index = 25 kg/m2 will be recruited. Patients will be randomized to a 4-year intensive weight loss program (calorie restriction and physical activity) or to diabetes support and education. With planned recruitment of 5000 patients at 16 centers over 2.5 years, the study is designed to provide a 0.90 probability of detecting an 18% difference in major CVD event rates between arms.35
EXERCISE
There is a substantial body of literature supporting exercise as a modality of treatment in type 2 diabetes, including a recent technical review by the ADA.36,37 The recommendations of this technical review are summarized in Table 48-5. Exercise is perhaps the single most important lifestyle intervention in diabetes, because it is associated with improved glycemic control, insulin sensitivity, cardiovascular fitness, and cardiac remodeling. Aerobic exercise and resistance (strength) training both have a positive impact on glucose control. Improvements in glycemic control are generally apparent immediately, become maximal after a few weeks of consistent exercise, but only persist for 3 to 6 days after the cessation of training. To maintain effects on glycemia, a minimum of three exercise sessions a week is suggested, with no more than 2 days rest between sessions.
Table 48-5. ADA Recommendations Regarding Physical Activity in People with Type 2 Diabetes
Indications for Graded Exercise Test With ECG Monitoring In the absence of contraindications, a graded exercise test with ECG monitoring should be seriously considered before undertaking aerobic physical activity with an intensity exceeding the demands of everyday living (more intense than brisk walking) in previously sedentary diabetic individuals whose 10-year risk of a coronary event is ≥10%. This risk could be estimated directly using the UKPDS Risk Engine (www.dtu.ox.ac.uk/riskengine/download.htm) and would correspond approximately to meeting any of the following criteria: These criteria should not be construed as a recommendation against stress testing for individuals without the above risk factors or for those who are planning less intense exercise. [Note: The most recent ADA recommendations 1 do not advocate routine stress testing in this setting.] The amount and intensity recommended for aerobic exercise vary according to goals. • To improve glycemic control, assist with weight maintenance, and reduce risk of CVD, recommend at least 150 min/wk of moderate-intensity aerobic physical activity (40% to 60% of VO2max or 50% to 70% of maximum heart rate) and/or at least 90 min/wk of vigorous aerobic exercise (>60% of VO2max or >70% of maximum heart rate). The physical activity should be distributed over at least 3 days/wk and with no more than 2 consecutive days without physical activity. • Performing ≥4 hr/wk of moderate to vigorous aerobic and/or resistance exercise is associated with greater CVD risk reduction compared with lower volumes of activity. • In the absence of contraindications, people with type 2 diabetes should be encouraged to perform resistance exercise three times a week, including all major muscle groups, progressing to three sets of 8-10 repetitions at a weight that cannot be lifted greater than 8-10 times. |
CAD, Coronary artery disease; CVD, cardiovascular disease; ECG, electrocardiograph.
Adapted from Sigal RJ, Kenny GP, Wasserman DH, et al: Physical activity/exercise and type 2 diabetes, Diabetes Care 27:2518–2539, 2004.
The recommended approach to promoting an increase in physical activity is analogous to that discussed for diet. Goals, methods, intensity, and frequency have to be negotiated with patients, with great sensitivity to recognizing barriers and helping patients discover solutions. The role of educators, exercise specialists, physical therapists, and social supports in this process is critical. The major role for the physician is to screen for complications (neuropathy, nephropathy, retinopathy, vascular disease) and discover ways for patients to be able to exercise safely. Exercise in the presence of uncontrolled diabetes, hypertension, retinopathy, nephropathy, neuropathy, and cardiovascular disease can occasionally result in devastating problems. Vigorous exercise should be avoided in the setting of proliferative or severe nonproliferative diabetic retinopathy; waiting 3 or more months after successful laser photocoagulation is probably prudent. In the setting of severe peripheral neuropathy, non-weight-bearing activities such as bicycling are probably appropriate. In the setting of known ischemic heart disease, an initial period of exercise under monitoring as provided in cardiac rehabilitation programs is appropriate. These issues can all be addressed creatively and should never provide an insurmountable barrier to increasing physical activity.
Recommendations regarding stress testing before initiating an exercise program in previously sedentary people are provided in some detail in Table 48-5. The utility of such treadmill tests in potentially low-risk populations is limited by their poor sensitivity and specificity.38,39 It should also be noted that in an older asymptomatic population with normal electrocardiograms, adenosine technetium-99m sestamibi single-photon emission-computed tomography myocardial perfusion imaging revealed that over 20% of patients studied had silent ischemia. However, in 5-year follow-up, there was a spontaneous remission of abnormalities in many, generally very low event rates, and no difference in outcomes based on whether stress testing was performed or not.40 If the planned exercise program does not involve more strenuous (both intensity and duration) activity than the patient has engaged in recently but merely involves more frequent activity, screening cardiovascular stress testing is unlikely to be particularly useful.
Even if the stress test does not demonstrate characteristics of ischemia, it is appropriate to counsel patients to avoid overexertion and seek medical follow-up for exertional symptoms such as chest, jaw, or arm discomfort or for worsening dyspnea. Improving exercise tolerance should be viewed as a measure of improving cardiorespiratory function.
For the average patient with type 2 diabetes starting an exercise program, these levels of moderate exertion will generally be reached with quite low-level activity initially, such as walking at a pace of 2 miles an hour. Initially, it may even be necessary to negotiate once-weekly or shorter-duration exercise sessions and proceed from there, picking up the pace as tolerated and increasing the duration and frequency of exercise sessions slowly to avoid overuse injuries.
SELF-MONITORING OF BLOOD GLUCOSE
Self-monitoring of blood glucose (SMBG) has not been demonstrated in clinical trials to substantially change outcomes in non-insulin-treated type 2 diabetes when evaluated in isolation and may be associated with decreased quality of life.41,42 However, many diabetes self-management programs have been associated with improved glycemic control. In all of these, SMBG was integral to the overall process, suggesting that SMBG is at least a component of effective therapy. The frequency and type of glucose monitoring should be determined in collaboration with the patient, taking into account the overall treatment plan and goals, the patient’s abilities, and the stage of diabetes. SMBG can theoretically improve the safety of treatment with insulin or sulfonylureas, since it allows the identification of minimal or asymptomatic episodes of hypoglycemia. Coupled with appropriate education, dose adjustments, or modest changes in the lifestyle plan, SMBG can minimize risk of severe hypoglycemia or weight gain. Although severe hypoglycemia is relatively rare in type 2 diabetes, it is more common in the elderly and can have devastating consequences, such as physical injury as a result of trauma or a change in the perceived ability of a patient to continue to live independently as a result of confusion or loss of consciousness. In general, it is optimal to have patients use glucose monitoring to assess the nature of any hypoglycemic symptoms they experience. Many patients are fearful of hypoglycemia and routinely consume extra calories in response to a variety of life’s circumstances—such as when they are hungry, sweaty, nervous, or upset—without documenting hypoglycemia. Most “hypoglycemic” symptoms in patients with type 2 diabetes are not related to hypoglycemia and do not need to be treated with calorie consumption. Counseling patients to carry commercially available glucose tablets and glucose monitoring equipment at all times and to take a 15-g dose of carbohydrate for documented mild to moderate hypoglycemia helps patients avoid excessive calorie intake and recreational consumption of sweets for treatment of hypoglycemia.
Optimal timing of SMBG will vary depending on individual characteristics and treatment. It is important to advise patients to vary the time of the day at which blood glucose levels are checked. For some patients, the highest blood glucose of the day is the morning glucose, whereas for others the highest is before bed. Particularly in early diabetes, gestational diabetes, and well-controlled diabetes, monitoring 1 to 2 hours after meals allows patients to assess the effect of the combined lifestyle and pharmacologic efforts in controlling postprandial glucose levels, which often are the only glycemic abnormality present.
When glucose control is poor, having patients concentrate on premeal glucose levels is perhaps most productive. Once the premeal glucose levels reach the low 100s, many advocate that patients switch to checking 1- to 2-hour postprandial glucose levels, because it amplifies the observed effect of diet on glycemic control and enables patients to see that moderate changes in meal plan, activity, and medications have a significant impact on glucose levels. Even after substantial inappropriate changes in food intake, activity, or timing or dose of medication, blood sugar values often return to near-normal levels overnight or by the time of the next meal.
The frequency of glucose monitoring needs to be matched to individual patient needs and treatment. Many clinicians ask patients to monitor at least once a day, varying among breakfast, lunch, supper, bedtime, and mid-sleep, as well as with symptoms. Others ask patients to monitor with intensity similar to that described for patients with type 1 diabetes (four times per day before meals and bedtime, with occasional postprandial and mid-sleep checks). Some ask for sets of glycemic readings more infrequently (e.g., fasting and 1 hour after the biggest meal). In the subset of patients who achieve stable blood glucose levels without significant hypoglycemia, it is generally appropriate to decrease the frequency of SMBG to a few times a week. The important characteristic of the timing of SMBG is that it be frequent enough to optimally inform the treatment plan and that both patient and provider have a good understanding of both the adequacy and the stability of glycemic control.
It has been assumed that the benefits of SMBG stem from facilitating self-management. Theoretically, if patients are aware of the glycemic targets associated with the outcomes they seek to achieve, SMBG enables them to evaluate their response to therapy and make adjustments or seek help as needed to achieve goals. It is useful for patients to keep a daily diary of their SMBG results so they can assess their results periodically and share them with the health care team. Unfortunately, many patients faithfully perform daily or more frequent SMBG, record the results as instructed, and discuss them with their health care team only at quarterly visits. Unless SMBG results are within the agreed-to targets, they should be communicated and reviewed regularly with a member of the health care team by telephone, fax, mail, or e-mail or at an interim visit to trigger changes in therapy as the need arises. Unfortunately, such services are generally not reimbursed, which places an unsustainable burden on many health care teams.
Finally, one of the most difficult areas for health care providers to remain current is in the area of available equipment and supplies, particularly for glucose monitoring. Diabetes educators often have demonstration models and a robust understanding of patient characteristics that match well or poorly with particular devices; they can be exceptionally helpful to patients in selecting appropriate equipment. A useful resource in this regard is the annual Resource Guide, which comes out as the January issue of Diabetes Forecast, a magazine for lay people with diabetes and their families. It is available online at http://forecast.diabetes.org/magazine/archive to find the most recent January issue.
Pharmacotherapy of Type 2 Diabetes
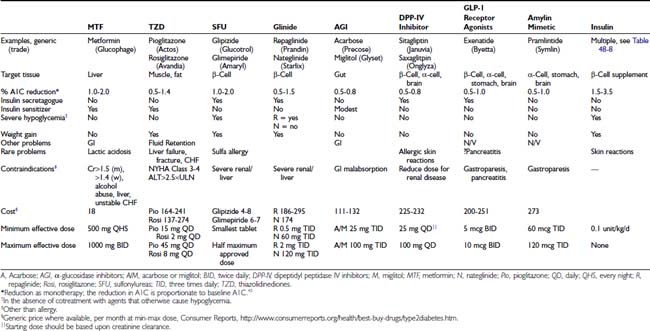
The revolution in the treatment of type 2 diabetes since 1995 in the United States has been driven by the release of multiple new classes of drugs that independently address different pathophysiologic mechanisms that contribute to the development of diabetes. The relative benefits of lifestyle intervention and the eight classes of drugs available for the management of type 2 diabetes are found in Table 48-6. This area has been the subject of extensive reviews and recent guidelines from professional groups.43–44 Because of limitations of space, basic principles are summarized with recent reviews, along with limited additional references derived from intensive management in the setting of randomized clinical trials and clinical practice.
INSULIN SENSITIZERS WITH PREDOMINANT ACTION IN THE LIVER: BIGUANIDES
Metformin is the only biguanide available in the United States and the subject of a recent review.45 Phenformin was removed from the United States market in the 1970s because of deaths associated with lactic acidosis. Metformin activates adenosine monophosphate–activated protein kinase (AMPK), a critical molecule in insulin signaling with effects on metabolism and energy balance. Metformin reduces hepatic insulin resistance, gluconeogenesis, and glucose release.46
Metformin has a half-life of 2 to 6 hours and reaches peak concentrations in approximately 1 hour when taken with meals. It is generally administered at least twice daily, although sustained-release formulations, which are particularly effective when administered with the evening meal, are now available. The glucose-lowering efficacy and the prevalence of adverse gastrointestinal effects increase proportionately in the dose range 500 to 2000 mg/d. The maximal dose of 2550 mg does not generally provide additional benefit beyond that seen at 2000 mg daily. Since metformin does not increase insulin levels, it is not associated with a significant risk of hypoglycemia. The most common adverse events are gastrointestinal: nausea, diarrhea, crampy abdominal pain, and dysgeusia. About a third of patients have some gastrointestinal distress, particularly early in their course of treatment. These adverse effects can be minimized by starting with a low dose (500 mg) once daily with a meal and titrating upward slowly (over weeks) to more effective doses (500 to 1000 mg twice daily). Sustained-release metformin is associated with less frequent and less severe upper gastrointestinal symptoms but can increase the frequency of diarrhea, a much less common adverse effect overall. The majority of patients have no treatment emergent complaints during metformin initiation, and at least 90% tolerate it adequately with long-term use. Perhaps as a result of clinical or subclinical gastrointestinal effects, metformin is associated with less weight gain than other available oral antihyperglycemic agents and in some studies has even been shown to produce modest mean weight loss.
The issue of greatest concern regarding metformin therapy is lactic acidosis, which is quite rare and occurs almost exclusively in patients who are otherwise at high risk of developing lactic acidosis.47 When used prudently, the risk of lactic acidosis is virtually zero. Patients at increased risk of developing lactic acidosis due to baseline medical conditions should avoid taking metformin.48 The package insert suggests that metformin is absolutely contraindicated in patients with renal insufficiency, because the drug is cleared renally.49 The drug should not be used in males with a serum creatinine greater than or equal to 1.5 mg/dL or in females at 1.4 mg/dL. There have been recent suggestions that metformin dosing should be based on estimated glomerular filtration rate (eGFR), with contraindication to use in stage 4 to 5 chronic kidney disease (eGFR < 30 mL/min/1.73 m2) and caution with eGFR less than 50 mL/min/1.73 m2.50
Metformin was originally contraindicated in patients with congestive heart failure requiring treatment. However, recent reports suggest improved morbidity and mortality in patients with stable compensated heart failure.51 This prompted the U.S. Food and Drug Administration (FDA) to recommend a change in labeling in 2005 to allow its use in patients with stable compensated heart failure without other contraindications to its use.52
Metformin is also contraindicated in patients with hepatic insufficiency, acidosis, hypoxia, and in those with a history of binge drinking or heavy alcohol use. Caution is required in elderly people, and specific recommendations to measure creatinine clearance with a timed urine collection prior to initiating therapy in patients over the age of 80 are provided in the package insert. Patients with acute illness, poorly controlled chronic illness, surgical indications, dehydration, or simultaneous treatment with nephrotoxic drugs (e.g., iodinated contrast dye) should have metformin withheld until the clinical course is stabilized and adequate renal function is confirmed.
Metformin has the strongest record of achievement of antidiabetic agents in outcome studies in patients with type 2 diabetes. In the UKPDS, among overweight subjects, those randomly assigned to metformin not only had improvements in microvascular complications similar to those of subjects randomly assigned to insulin and sulfonylurea but also exhibited a reduction in diabetes-related deaths and myocardial infarction.10 The validity of this observation has been challenged because of unusual responses in a subsequent subrandomization. Beneficial effects of metformin on macrovascular complications through glucose-independent mechanisms are plausible and suggested by consistent observations, such as metformin-associated reductions in low-density lipoprotein (LDL) and procoagulant factors.53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree