Management of young children with diabetes, especially those younger than 5 years old, must balance opposing risks of hypoglycemia (see section on Hypoglycemia below) and future vascular complications. The relative contribution of the prepubertal years to the development of microvascular complications has been uncertain; however, recent evidence indicates that longer prepubertal duration of diabetes increases the risk for retinopathy and, possibly, microalbuminuria in adolescence and young adulthood, but at a slower rate than in the postpubertal years.23
The risk for microalbuminuria increases steeply with HbA1c >8%.24,25 Based on these considerations, an HbA1c of ≤8.0% is a reasonable general goal for children with diabetes; however, biochemical goals should be individualized, taking into account both medical and psychosocial considerations. Less stringent treatment goals are appropriate for preschool-age children, those with developmental handicaps, psychosocial challenges, and lack of appropriate family support, for children who have experienced severe hypoglycemia or have hypoglycemia unawareness.
INSULIN THERAPY
Within days to months of diagnosis, most children with T1DM are severely insulin deficient and depend on insulin replacement for survival. The aim of insulin replacement therapy is to simulate as closely as possible patterns of plasma insulin levels that occur in nondiabetic individuals; however, truly physiologic replacement of insulin remains an elusive goal. Insulin pump therapy and multiple daily insulin injections are the two methods that most closely mimic insulin secretion. The first step in choosing an insulin regimen is to establish glycemic goals. For most patients, this means that more than one half of plasma glucose values should fall within the following ranges: preprandial 90 to 130 mg/dL (5 to 7.2 mmol/L), bedtime 100 to 140 mg/dL (5.6 to 7.8 mmol/L), and 1 to 2 hours postprandial <180 mg/dL (10 mmol/L) (see Table 49-1).
In children with severe insulin deficiency, practical considerations, including socioeconomic circumstances, age, supervision of care, ability and willingness to self-administer insulin several times each day, and difficulty maintaining long-term adherence, make physiologic replacement of insulin challenging. No universal insulin regimen can be successfully used for all children with T1DM. The diabetes team has to design an insulin regimen that meets the needs of the individual patient and is acceptable to the patient and/or family members(s) responsible for administering insulin to the child or supervising its administration.
The initial route of insulin administration is determined by the severity of the child’s condition at presentation. Insulin is preferably given intravenously as treatment for DKA. Children who are metabolically stable without vomiting or significant ketosis may be started with subcutaneous (SC) insulin administration. SC insulin treatment in the newly diagnosed child should, ideally, be started with at least three injections per day or a basal-bolus regimen (Table 49-2). Some clinicians have recently started insulin pump therapy at the time of diagnosis, regardless of the severity of presentation or the age of the child.
In addition to severity of metabolic decompensation, the child’s age, weight, and pubertal status guide the initial insulin dose selection. When diabetes has been diagnosed early, before significant metabolic decompensation, 0.25 to 0.5 unit/kg/day usually is an adequate starting dose. When metabolic decompensation is more severe (e.g., ketosis without acidosis or dehydration) the initial dose typically is at least 0.5 unit/kg/day. After recovery from DKA, prepubertal children usually require at least 0.75 unit/kg/day, whereas adolescents require at least 1 unit/kg/day. In the first few days of insulin therapy, while the focus of care is on diabetes education and emotional support, it is reasonable to aim for pre-meal blood glucose levels in the range of 80 to 200 mg/dL and to supplement, if necessary, with 0.05 to 0.1 unit/kg of rapid-acting insulin SC at 3 to 4 hour intervals.
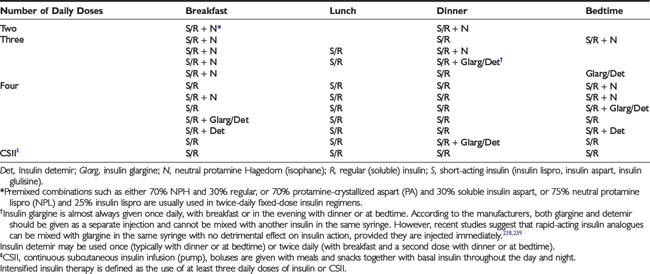
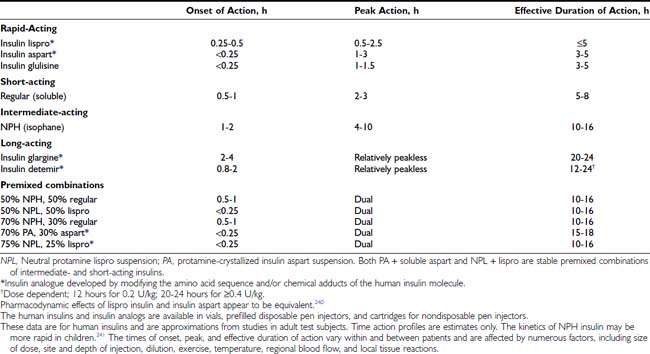
Three major categories of insulin preparations, classified according to their time course of action, are available (Table 49-3). Various insulin replacement regimens consisting of a mixture of short- or rapid-acting insulin and an intermediate- or long-acting insulin are used in children and adolescents (see Table 49-2) and typically are given two to four (or more) times daily. Clear superiority of any one regimen in children and adolescents, in terms of metabolic outcomes, has not been demonstrated.26,27 All insulin regimens have the same general goal: to provide basal insulin throughout the day and night and additional (prandial) insulin to cover meals and snacks.
When a two-dose regimen is used, the total daily dose is typically divided so that about two thirds is given before breakfast and one third is given in the evening. With a three-dose regimen, short- or rapid-acting insulin is administered before the evening meal, and the second dose of intermediate- or a long-acting insulin is given at bedtime rather than before the evening meal. The initial ratio of rapid- to intermediate-acting insulin at both times is approximately 1:2. Toddlers and young children typically require a smaller fraction of short- or rapid-acting insulin (10% to 20% of the total dose) and proportionately more intermediate- or long-acting insulin. Regular insulin is given at least 30 minutes before eating; rapid-acting insulin (lispro, aspart, glulisine) is given 5 to 15 minutes before eating (depending on the pre-meal blood glucose value). In toddlers and young children with unpredictable eating habits, rapid-acting insulin may be given immediately after the meal (dose based on estimated actual carbohydrate consumed) to prevent hypoglycemia from incorrect insulin dosing owing to the child’s not eating the entire meal.28,29
The optimal ratio of rapid- or short-acting to intermediate- or long-acting insulin for each patient is determined empirically, guided by the results of frequent blood glucose measurements. At least five daily measurements are required initially to determine the effects of each component of the insulin regimen. The blood glucose concentration is measured before each meal, before the bedtime snack, and once between midnight and 4 am. Parents are taught to look for patterns of hyperglycemia or hypoglycemia that indicate the need for an adjustment in the dose. Adjustments are made to individual components of the insulin regimen, usually in 5% to 10% increments or decrements, in response to patterns of consistently elevated (above the target range for several consecutive days) or unexplained low blood glucose levels, respectively. This is referred to as pattern adjustment. The insulin dose is adjusted until satisfactory blood glucose control is achieved with at least 50% of blood glucose values in or close to the individual child’s target range.
At the time of diagnosis, most children have some residual β cell function and within several days to a few weeks often enter a period of partial remission (“honeymoon”), during which normal or nearly normal glycemia is relatively easily achieved with a low dose of insulin. At this stage, the dose of insulin should be reduced to prevent hypoglycemia but should not be discontinued. As destruction of remaining β cells occurs, the insulin dose increases (“intensification phase”), eventually reaching a full replacement dose. The average daily insulin dose in prepubertal children with long-standing diabetes is approximately 0.8 unit/kg/day, and in adolescents 1 to 1.5 units/kg/day.
Insulin Therapy in Young Children: Technical Considerations
Caring for young children with diabetes is challenging for many reasons, one of which is the need to accurately and reproducibly measure and inject tiny doses of insulin that is supplied in a concentration of 100 units/mL (U 100 insulin). To administer a dose of 1 unit requires the ability to accurately measure 10 µL (1/100 mL) of insulin. When the dose is less than 2 U of U 100 insulin, neither parents of diabetic children nor skilled pediatric nurses are able to measure the dose accurately.30 Furthermore, a dose change of 0.25 U translates into a volume difference of 2.5 µL in a 300 µL (3/10 cc or 30 unit) syringe. When parents attempt to measure insulin doses in increments of 0.25 U of insulin (e.g., 3.0, 3.25, 3.5 U) using a standard commercial 30 unit (300 µL) syringe, they consistently measure more than the prescribed amount.31 Therefore, to enhance the accuracy and reproducibility of small doses, insulin should be diluted to U 10 (10 units/mL) with the specific diluent available from the insulin manufacturers. When U 10 insulin is used, each line (“unit”) on a syringe is actually 0.1 U of insulin.
To avoid intramuscular insulin injections in infants and young children with little subcutaneous fat, syringes with 8 mm needles or insulin pens with 31 gauge 5 mm needles should be used. Short needles (5 or 8 mm) are also desirable for use in older thin children.
Intensified Insulin Therapy in Children
Little evidence is available to guide clinical decisions concerning the risk-benefit ratio of strict control in the preadolescent patient. Clinical trials comparable to the DCCT have not been conducted in prepubertal children; nevertheless, it is reasonable to extrapolate that prepubertal children will also benefit from strict control of their diabetes.
Beyond the remission period, it generally is not possible to achieve near-normal glycemia with two injections per day without incurring a greater risk for hypoglycemia, especially during the overnight period. An important limitation of a two-dose “split-and-mixed” regimen is that the peak effect of the pre-dinner intermediate-acting insulin tends to occur at the time of lowest insulin requirement (midnight to 4 am), increasing the risk for nocturnal hypoglycemia. Thereafter, insulin action declines from 4 am to 8 am, when basal insulin requirements normally increase. Consequently, the tendency for blood glucose levels to rise before breakfast (dawn phenomenon) may be aggravated by waning insulin effect in the period before breakfast and/or by counterregulatory hormones secreted in response to a fall in blood glucose levels during sleep, resulting in post-hypoglycemic hyperglycemia (Somogyi phenomenon).
A three-dose insulin regimen with mixed short- or rapid- and intermediate-acting insulins before breakfast, only short- or rapid-acting insulin before dinner, and intermediate- or long-acting insulin at bedtime may significantly ameliorate these problems.32,33 Intensive insulin regimens that employ intermediate-acting insulin demand consistency in the daily meal schedule, amounts of food consumed at each meal, and the timing of insulin injections.
Basal-Bolus Regimens and Continuous Subcutaneous Insulin Infusion
Insulin therapy with at least three injections each day or with continuous subcutaneous insulin infusion (CSII) using an insulin pump can more closely simulate normal diurnal insulin profiles, overcome many of the limitations inherent in a two-dose regimen, and permit greater flexibility with respect to timing and content of meals. Doses of rapid-acting insulin are adjusted meal-to-meal based on preprandial glucose values, anticipated carbohydrate intake, and physical activity. A peakless long-acting insulin, such as insulin glargine or detemir, can be used to provide basal insulin (typically 40% to 60% of the total daily dose) and is used together with short- or rapid-acting insulin injected before each meal (basal-bolus regimen). Insulin glargine is an insulin analogue, produced by recombinant DNA technology, whose duration of action is approximately 24 hours. It has little peak activity and is administered once daily, either before breakfast or in the evening with dinner or at bedtime. It should be injected at about the same time each day, whereas short- or rapid-acting insulin is injected separately before each meal, whenever it is eaten. Insulin glargine has been used safely in children and adolescents,34 and because it does not have the peak of activity characteristic of NPH, Lente, and Ultralente insulins,35 it can reduce nocturnal hypoglycemic episodes without jeopardizing glycemic control.33,36 More recently, insulin detemir has become available as an alternative long-acting, peakless basal insulin.37 Detemir has effects similar to those of glargine during the first 12 hours after administration; thereafter its effects wane; accordingly, it usually has to be administered twice daily in patients with severe insulin deficiency.38
In 1996, less than 5% of patients starting pump therapy were <20 years of age. Over the past several years, a worldwide marked increase has occurred in the number of children and adolescents using CSII (pump) therapy39; a current estimate is that more than 80,000 children and adolescents worldwide are using a pump to deliver insulin. An insulin pump has one unique advantage over insulin injections—the ability to program changes in basal dosage to meet an anticipated increase or decrease in need (Fig. 49-1C). This feature can be advantageous in combating the dawn phenomenon (especially in adolescents) or preventing hypoglycemia during or after strenuous exercise. In addition to programming various basal rates, the use of dual-wave and square-wave bolus delivery significantly lowers 4-hour postprandial blood glucose levels.40 Also, the infusion set typically has to be replaced only every 2 to 3 days, sparing the child the discomfort of repeated injections. A meta-analysis of randomized controlled clinical trials concluded that CSII resulted in a small (≈0.5%) improvement in HbA1c.41
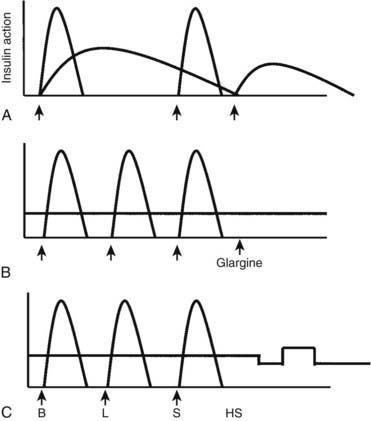
FIGURE 49-1. Insulin regimens. A, Schematic representation of idealized insulin action provided by a regimen consisting of a mixture of rapid-acting insulin (lispro or aspart) and intermediate-acting insulin (NPH or Lente) before breakfast, rapid-acting insulin (lispro or aspart) before supper, and intermediate-acting insulin (NPH or Lente) at bedtime. B, Schematic representation of idealized insulin action provided by an insulin regimen consisting of four daily injections: rapid-acting insulin (lispro or aspart) before each meal (B, L, S) and a separate injection of insulin glargine, at bedtime (as shown here) or at dinner or breakfast. C, Schematic representation of idealized insulin effect provided by continuous subcutaneous insulin infusion via an insulin pump with insulin aspart or lispro. In this figure, alternative basal rates are illustrated; insulin delivery is shown to decrease from midnight to 3 am and to increase before breakfast. B, Breakfast; HS, bedtime; L, lunch; S, supper. Arrows indicate times of insulin injection or bolus before meals.
Although an insulin pump is a complex and sophisticated medical device that requires extensive training in its proper use, with appropriate education and training and with support from parents and a school nurse, many children can manage the added responsibility of using an insulin pump and can benefit from its advantages.39,42 Only short- or rapid-acting insulin is used with CSII; therefore, any interruption in the delivery of insulin rapidly leads to metabolic decompensation. To reduce this risk, meticulous care must be devoted to the infusion system, and blood glucose levels must be measured at least four times daily. Increased lifestyle flexibility, reduced blood glucose variability, improved glycemic control, and reduced frequency of severe hypoglycemia are all documented advantages of CSII.39 Success requires motivation to achieve normal blood glucose levels, frequent blood glucose monitoring, record-keeping, carbohydrate counting, and frequent contact with the diabetes team. Patients must understand that to be successful, CSII therapy requires more time, effort, and active involvement in diabetes care by patients and parents, as well as considerable education and support from the diabetes team. The individual who is unable to master a multiple-dose injection regimen is not likely to be successful with CSII. Despite concerns that it might have adverse psychosocial consequences owing to the added burden of treatment, especially in adolescents, the opposite effect has been observed. Short-term studies have shown that more aggressive and successful management of their diabetes by teenagers can be accompanied by enhanced psychosocial well-being.43 In teenagers, CSII offers a treatment option that can lead to improved control and can lower the risk for severe hypoglycemia.44
Owing to physiologic peripheral insulin resistance of puberty,45 adolescents require large doses of rapid- or short-acting insulin to control postprandial blood glucose excursions. However, a large increase in the dose of regular insulin delays its peak effect (to 3 to 4 hours) and prolongs its total duration of action to 6 to 8 hours. Puberty does not cause hepatic insulin resistance; therefore, hyperinsulinemia suppresses hepatic glucose production for several hours and increases the risk for postprandial hypoglycemia, especially at night between 10 pm and 2 am.46 This is an important reason to recommend use of rapid-acting insulin analogs (lispro, aspart, or glulisine) in preference to regular (soluble) insulin in treating adolescents, especially before the evening meal, to reduce the risk for nocturnal hypoglycemia.
Technological innovations have provided patients with insulin preparations whose pharmacokinetic properties make it possible to crudely simulate physiologic insulin kinetics. It is now possible for children to safely achieve unprecedented levels of glycemic control without excessive severe hypoglycemia. The diabetes care provider should frankly discuss treatment options with parents and child and should explain the advantages and disadvantages of each in attempting to meet the overall goals of treatment. The most suitable regimen for a given child and family should be determined by mutual consent.
MEDICAL NUTRITION THERAPY
Nutritional management is one of the cornerstones of the management of all types of diabetes mellitus, and nutrition education is an essential component of a comprehensive program of diabetes education for patients and their families.47 There is no “diabetic diet” per se. Nutrition therapy should be individualized, with consideration given to the patient’s usual eating habits and other lifestyle factors. Monitoring clinical and metabolic parameters, including height and weight, blood pressure, blood glucose, HbA1c, and lipids, as well as quality of life, is crucial to ensure successful outcomes. Diabetes management that combines frequent self-monitoring of blood glucose with intensive insulin therapy and mastery of carbohydrate counting enables children and adolescents to enjoy dietary flexibility while maintaining glycemic control in the target range.
Patients with both T1DM and T2DM have the same goals: namely, to achieve and maintain target blood glucose and HbA1c levels (Table 49-4). The initial focus of medical nutrition therapy (MNT), however, differs between the two major types of diabetes. Children with T2DM typically are obese at presentation, and great emphasis is placed on weight loss, limiting caloric intake, and distributing meals evenly throughout the day. In T2DM, even modest weight reduction alone increases sensitivity to insulin and improves fasting and postprandial plasma glucose levels. Similarly, moderate caloric reduction decreases plasma glucose levels. In adults, structured, intensive lifestyle programs involving participant education, individualized counseling, reduced energy and fat intake (30% of total energy), regular physical activity, and frequent participant contact are necessary to produce long-term weight loss of 5% to 7% of starting weight.48 Accordingly, lifestyle changes that lead to weight loss are the cornerstone of therapy in patients with T2DM. In contrast, in the child with T1DM, the primary goal is to match insulin delivery with carbohydrate consumption to achieve blood glucose levels in the age-specific target range (see Table 49-1).
Table 49-4. Goals of Medical Nutrition Therapy for Children and Adolescents With Diabetes Mellitus
No evidence indicates that the nutritional needs of children with diabetes differ from those of otherwise healthy children. Therefore, nutrient recommendations are based on the requirements of healthy children and adolescents. The total intake of energy must be sufficient to balance the daily expenditure of energy and has to be adjusted periodically to achieve an ideal body weight and to maintain a normal rate of physical growth and maturation.
Carbohydrate
Approximately 60% to 70% of total energy should be obtained from carbohydrate and monounsaturated fat.49 Dietary dogma had been to avoid simple sugars and replace them with complex carbohydrates. This belief was based on the assumption that simple sugars are more rapidly digested and absorbed than starches and would aggravate hyperglycemia to a greater degree. The glycemic index (GI), proposed in 1981 as an alternative system for classifying carbohydrate-containing foods, measures the glycemic response after ingestion of carbohydrate. GI is defined as the incremental area under the plasma glucose response curve after consumption of a standard amount of carbohydrate from a test food relative to that of a control food, either white bread or glucose. Glycemic and hormonal responses to a large number of carbohydrates have been systematically examined and their GIs defined. There is a wide spectrum of biological responses to different complex and simple carbohydrates with so much overlap that they cannot be simply classified into two distinct groups. Even a single food produces a substantially different glycemic response when prepared in different ways. The physical structure and form of a carbohydrate-containing food, in addition to its chemical composition, influence postprandial glycemia by altering its rate of digestion and absorption. Fruits and milk cause a lower glycemic response than most starches, and sucrose causes a glycemic response similar to that of bread, rice, and potatoes. In general, most refined starchy foods have a high GI, whereas nonstarchy vegetables, fruits, and legumes tend to have a low GI.
The usefulness of low-GI diets in individuals with T1DM continues to be controversial, and data are sparse in children. A meta-analysis of randomized controlled clinical trials, some of which have included children, shows that low-GI diets have modest long-term beneficial effects on blood glucose and lipid concentrations.50
The glycemic load of meals and snacks is more important than the source or type of carbohydrate. The glycemic load, defined as the weighted average of the GI of individual foods multiplied by the percentage of dietary energy as carbohydrate, has been proposed as a method to characterize the impact of foods and dietary patterns with different macronutrient composition on glycemic responses. For example, a carrot has a high GI but a low glycemic load, whereas a potato has both a high GI and a high glycemic load. Although the use of low-GI foods may reduce postprandial glycemic excursions and may have long-term benefit on HbA1c levels, emphasis should be on the total amount of carbohydrate consumed, and its source should be a secondary consideration.51
Sucrose
Sucrose as part of the meal plan does not adversely affect blood glucose control in individuals with T1DM or T2DM. Sucrose and sucrose-containing foods may be substituted (gram-for-gram) for other carbohydrates. The nutrient content of sucrose-containing foods, as well as the presence of other nutrients frequently ingested with sucrose, such as fat, must be taken into consideration.
Fructose
Fructose is present as the free monosaccharide in many fruits, vegetables, and honey. About one third of dietary fructose comes from fruits, vegetables, and other natural sources in the diet, and about two thirds comes from food and beverages to which fructose has been added. Fructose is absorbed more slowly from the intestinal tract than is glucose, sucrose, or maltose, and it is converted to glucose and glycogen in the liver. Postprandial plasma glucose levels are reduced when an isocaloric amount of fructose replaces sucrose or starch in the diets of people with diabetes. Fructose has been used in children in amounts up to 0.5 g/kg/day; however, its potential benefit is tempered by concern that fructose may have adverse effects on serum lipids, especially low-density lipoprotein (LDL) cholesterol. Consumption of large amounts of fructose (15% to 20% of daily energy intake [90th percentile of usual intake]) increases fasting total and LDL cholesterol in subjects with diabetes and fasting total and LDL cholesterol and triglycerides in nondiabetic subjects. Because of the potential adverse effects of large amounts of fructose on serum lipids, fructose may offer no overall advantage over other nutritive sweeteners. There is no reason to avoid naturally occurring sources of fructose.
Carbohydrate Counting and Exchange Lists
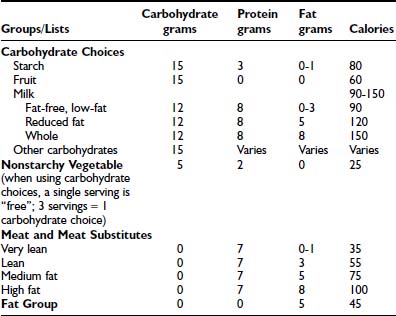
Carbohydrate counting is a meal planning method that entails counting the amount of carbohydrate or the number of carbohydrate servings eaten at each meal and snack. Carbohydrate is the main nutrient in starches, fruits, milk, and sugar-containing foods and has the greatest effect on blood glucose levels. Therefore, it is the most important macronutrient to control in order to maintain optimal glycemic control. With the use of exchange lists, one starch choice is considered to be equivalent to one fruit or milk choice; each contains approximately 15 grams of carbohydrate and is equal to one “carbohydrate choice” (Table 49-5). The “nutrition facts” on food labels list the portion size and total amount of carbohydrate measured in grams per serving. Carbohydrate counting allows flexibility in food choices and minimizes “cheating,” as all foods can be included in the meal plan. Table 49-6 shows an example of a patient’s daily meal plan, incorporating both exchange servings and grams of carbohydrate.
Individuals who use intensive insulin therapy usually select their pre-meal insulin doses based on the carbohydrate content of their meals, whereas individuals who receive fixed daily insulin dosages must attempt to maintain day-to-day consistency with respect to the carbohydrate content of their meals and snacks.
Fiber, which refers to the indigestible portion of a plant, influences the digestion, absorption, and metabolism of many nutrients. Inclusion of plant fiber in the diet may benefit patients with diabetes by diminishing postprandial glycemia. Certain soluble plant fibers significantly reduce serum cholesterol concentrations and decrease fasting serum triglyceride levels in patients with diabetes who have hypertriglyceridemia. Dietary fiber guidelines for children with diabetes are the same as for nondiabetic children and can be readily achieved by increasing the consumption of minimally processed foods, such as grains, legumes, fruits, and vegetables. Among diabetic adolescents using intensive insulin treatment methods, optimal blood glucose control is more common in those who have a higher intake of fiber, fruits, and vegetables.52
Protein
Protein requirements are not increased when diabetes is well controlled with insulin, and children with diabetes should follow the recommended daily allowance guidelines. Physiologic requirements are determined by the amount of protein necessary to sustain normal growth, which is based on ideal weight-for-height and varies with age, being highest in infancy and early childhood. Protein intake should be 0.9 to 2.2 g/kg body weight/day and constitutes 15% to 20% of the total daily intake of energy—the same as for nondiabetic children and adolescents.
Fat
A carbohydrate-containing meal that also has a high content of saturated fat significantly increases and prolongs the glycemic effect of the meal and requires anticipatory adjustment of the dose of insulin to combat the effect. Excessive saturated fat, cholesterol, and total energy lead to increased blood levels of cholesterol and triglycerides. Because hyperlipidemia is a major determinant of atherosclerosis, and patients with T1DM eventually develop atherosclerosis and its sequelae, the meal plan should attempt to mitigate this risk factor. The consumption of saturated fat can be reduced by eating less red meat, whole milk, and high-fat dairy foods and by eating more poultry, fish, and vegetable proteins, and by drinking more low-fat milk. Children and adolescents with well-controlled T1DM are not at high risk for dyslipidemia, but they should be screened and monitored according to recommended guidelines (see Chronic Complications section below). If the child or adolescent is growing and developing normally and has normal plasma lipid levels, less than 10% of energy should come from saturated fat, the daily intake of cholesterol should be less than 300 mg/day, and consumption of transunsaturated fatty acids should be minimized. Total dietary fat should be reduced in the obese child to reduce total energy consumption. The National Cholesterol Education Program (NCEP) Step II diet guidelines should be implemented in the patient with elevated LDL cholesterol (>2.6 mmol/L [100 mg/dL]). Total fat should constitute ≤30% of total calories, with <7% of calories from saturated fat, and dietary cholesterol should be limited to 200 mg/day.53
MNT Education and Formulation of the Meal Plan
Newly diagnosed children with T1DM usually present with weight loss; therefore, the initial meal plan includes an estimation of energy requirements to restore and then maintain an appropriate body weight and allow for normal growth and development. Energy requirements vary with age, height, weight, stage of puberty, and level of physical activity. Because the energy needs of growing children continuously change, the meal plan should be reevaluated at least every 6 months in young children and annually in adolescents.
MNT begins with an assessment by a registered dietitian, heeding the ethnic, religious, and economic factors pertaining to the individual patient and family. The meal plan must take account of the child’s school schedule, early or late lunches, physical education classes, after-school physical activity, and differences in a child’s activities on weekdays compared with weekends and holidays. Young children typically have three meals and two or three snacks daily, depending on the interval between meals, the age of the child, and the level of physical activity. Although their daily energy intake is relatively constant over time, young children adjust their energy intake at successive meals.54 The highly variable food consumption from meal to meal typical of normal young children is especially challenging when the child has T1DM. Rapid-acting insulin may be administered after the meal, based on estimation of the actual amount of carbohydrate consumed, and this diminishes parental anxiety.28,29 The purpose of snacks is to prevent hypoglycemia and hunger between meals. If the basal insulin component is adjusted appropriately, patients who use a basal-bolus insulin regimen or insulin pump therapy may not require snacks. Data from preprandial and postprandial blood glucose monitoring and individualized insulin-to-carbohydrate ratios are used to select insulin doses to match anticipated carbohydrate intake.
The dietitian’s role is to evaluate the patient’s and family’s knowledge and understanding of nutrition and to formulate an individualized meal plan. Even intensive insulin replacement regimens are not successful without careful attention to meal planning.55 Nutrition education, like all aspects of diabetes education, has to be an ongoing process with periodic review and revision of the meal plan and assessment of the child’s and parents’ levels of comprehension, ability to analyze and solve problems, and adherence to the nutrition goals. The patient with newly diagnosed diabetes and his or her parents should consult with a dietitian several times during the first few days after diagnosis. Within a few weeks of the child resuming his or her usual schedule and activities, the patient and family should review the meal plan with a dietitian, who also should be available to patients for telephone consultation. If the patient’s glycemic control is poor, if growth is failing, if weight gain is excessive, or if other problems related to MNT should arise, the dietitian should be re-consulted.
The Meal Plan
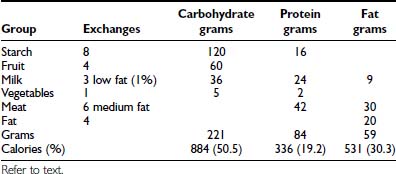
The individualized meal plan must be simple, practical, and easy to modify, and should offer foods that are interesting, tasty, and affordable. Dietary strategies principally are determined by the patient’s insulin replacement regimen (Table 49-7). We advocate meal planning adapted to the ethnic, religious, and economic circumstances of each family and based on a combination of carbohydrate counting and the exchange system. Each list in the exchange system for meal planning indicates the appropriate size or volume of each food exchange. Each portion of food within a group is exchangeable because it contains approximately the same nutritional value in terms of calories, carbohydrates, protein, and fat. By prescribing the meal plan in terms of a number of exchanges for each meal, the consistency of total calories and the proportions of nutrients can be maintained, while allowing the patient to choose among numerous foods. Accurate measurement of portion sizes has to be learned, and weighing and measuring of foods helps to achieve familiarity with the sizes of food portions specified in the exchange list. Weighing and measuring food should be viewed as an educational exercise to train the eye and need not be continued indefinitely; however, if blood glucose control appears inexplicably to deteriorate, it is useful to resume weighing and measuring of food portions to ensure that amounts are accurate. The exchange system should not be used in isolation; rather, it should be one component of a nutritional program directed by a trained dietitian. An example of how this system is applied to a hypothetical patient is illustrated below. An 11-year-old girl’s height is 144 cm (50th percentile on the Centers for Disease Control and Prevention growth chart) and her weight is 37.4 kg (50th percentile). Her daily energy requirement to support growth in the 50th percentile is 1756 calories. An appropriate distribution of macronutrients consists of 50% of total calories from carbohydrate, 20% as protein, and 30% as fat (see Table 49-6).
Table 49-7. General Approaches to Meal Management
Age-related issues: Children’s activities often differ on weekdays compared with weekends and holidays, and appropriate allowance must be made for these differences. The meal plan must take into account the child’s school schedule, early or late lunches, gym classes, and after-school physical activity.
Exercise
Children with diabetes are encouraged to participate in sports and to include regular exercise in their lives. Participation in physical exercise normalizes the child’s life, enhances self-esteem, improves physical fitness, helps to control weight, and may improve glycemic control. Regular exercise increases insulin sensitivity, cardiovascular fitness, and lean body mass, improves blood lipid profiles, and lowers blood pressure.
Although physical exercise is complicated for the child with T1DM, especially because of the need to prevent hypoglycemia, with proper guidance and planning, exercise can be a safe and enjoyable experience.
Exercise acutely lowers the blood glucose concentration by increasing utilization of glucose to a variable degree that depends on the intensity and duration of physical activity and the concurrent level of insulin in the blood. In T1DM, increased levels of epinephrine and glucagon in response to acute strenuous anaerobic exercise may cause transient hyperglycemia for 30 to 60 minutes.
Hypoglycemia usually can be prevented by a combination of anticipatory reduction in pre-exercise insulin dose or temporary interruption or reduction of basal insulin infusion (with CSII) and/or supplemental snacks before, during, and after activity, depending on the intensity and duration of the physical activity and its timing. Nearly all forms of activity lasting longer than 30 minutes require some adjustment to food and/or insulin. Continuous moderate-intensity exercise tends to cause a lesser decline in blood glucose levels than is produced by intermittent high-intensity exercise of short duration.56 The optimal strategy depends on the timing of the exercise relative to the child’s meal plan and on the insulin regimen. When the content and size of the snack are selected, consideration is given to several factors, including the current blood glucose level, the action of insulin most active during and after the period of anticipated exercise, the interval since the last meal, and the duration and intensity of physical activity. The appropriate amount is learned by trial and error; however, a useful initial guide is to provide up to 1 gram of carbohydrate per kg of body mass per hour of strenuous exercise. Prolonged and strenuous exercise in the afternoon or evening should be followed by a 10% to 30% reduction in pre-supper or bedtime dose of intermediate-acting insulin or long-acting insulin or an equivalent reduction in overnight basal insulin delivery in patients using CSII. In addition, to reduce the risk for nocturnal or early-morning hypoglycemia caused by the lag effect of exercise, the bedtime snack should be larger than usual and should contain carbohydrate, protein, and fat. Parents should be encouraged to monitor the blood glucose concentration in the middle of the night until they are experienced in modifying the evening dose of insulin after exercise.
Blood glucose monitoring is essential for the active child with diabetes because it allows identification of trends in glycemic responses. Records should include blood glucose levels and information on the timing, duration, and intensity of exercise, as well strategies used to maintain glucose concentrations in the target range. Blood glucose levels should be measured before, during, and after exercise and, to prevent nocturnal hypoglycemia, before bedtime (Table 49-8).
Table 49-8. Practical Guidelines for Exercise
Adapted from Riddell and Iscoe.244
Exercising the limb into which insulin has been injected accelerates the rate of insulin absorption. If possible, the insulin injection that precedes exercise should be given in a site least likely to be affected by exercise. Because physical training increases tissue sensitivity to insulin, children who participate in organized sports are advised to reduce the dose of the insulin preparation that is predominantly active during the period of sustained physical activity. The size of such reductions is determined by measuring blood glucose levels before and after exercise and is generally on the order of 10% to 30% of the usual dose.
In the child with poorly controlled diabetes, vigorous exercise can aggravate hyperglycemia and ketoacid production; accordingly, a child with ketonuria should not exercise until satisfactory biochemical control has been restored (see Table 49-8).
Type 2 Diabetes Mellitus in Children and Adolescents
Until recently, most children with diabetes had T1DM; however, as early as 1916, a phenotypically distinct form of diabetes, now classified as type 2 diabetes mellitus (T2DM), was recognized in childhood.57 Over the past 10 to 20 years, an alarming increase in the prevalence of pediatric T2DM has been reported from pediatric diabetes centers in North America58 and elsewhere in the world,59,60 and T2DM now accounts for up to 33% of new cases of diabetes in adolescents at centers that serve large numbers of minority youth.61,62 At least 90% of patients with newly diagnosed T2DM are obese,58 and the increased prevalence of pediatric T2DM temporally coincides with the increase in obesity noted in children in the United States; it has more than doubled in the past 20 years. In 2003-2004, 17.1% of U.S. children aged 2 to 19 years were overweight, defined as body mass index ≥95th percentile.63 As in adults, obesity in childhood is associated with insulin resistance, hyperinsulinism, and decreased insulin-stimulated glucose metabolism compared with nonobese children64,65 (Table 49-9). Factors that explain the increased prevalence of pediatric T2DM and strategies for primary prevention have been reviewed recently.66 The pathophysiology of T2DM is discussed in Chapter 41.
Table 49-9. Risk Factors for Type 2 Diabetes in Youth
TREATMENT
The general goals of treatment for T2DM are the same as those outlined above for T1DM: to normalize fasting and postprandial blood glucose concentrations. However, in addition to blood glucose control, from the outset treatment must include management of comorbidities such as obesity, dyslipidemia, hypertension, and microalbuminuria. The goals of treatment and the recommended standards of care for pediatric patients with T2DM are described in Tables 49-10 and 49-11. The UKPDS showed that intensive glycemic control in T2DM decreased the risk for microvascular complications by up to 25% for each 1% reduction in HbA1c.18 A multifactorial approach that addresses associated risk factors has been shown in adults to be essential to prevent or reduce complications, including cardiovascular disease (CVD).67 Evidence suggests that T2DM in children and adolescents may have a more rapid clinical course; therefore, optimal management is required to prevent diabetes-associated complications.68
Table 49-10. Goals of Treatment for Type 2 Diabetes Mellitus75
• Prevent or treat diabetes-associated comorbidities, including dyslipidemia, hypertension, and microalbuminuria |
BMI, Body mass index; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides.
* Blood pressure goals must be adjusted for age, height, and gender.
Table 49-11. Recommended Standards of Care for Patients With Type 2 Diabetes Mellitus47
Currently, no evidence-based guidelines are available for the management of T2DM in children and adolescents; however, as for T1DM, a multidisciplinary diabetes team that consists of a physician, a diabetes nurse educator, a registered dietitian, an exercise physiologist, and a behavioral specialist or social worker is essential. Results of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study on the treatment of T2DM in children and adolescents are eagerly anticipated. This study is expected to provide much needed information on the natural history of T2DM and the efficacy of various treatments for youth T2DM (Table 49-11).69
Nonpharmacologic Therapy
Weight Control and Physical Activity
Although weight loss and increased physical activity are the first-line therapies for prevention and management of T2DM and its comorbidities, the optimal strategy is still controversial. A recent review by the Cochrane Collaboration found few to no high-quality long-term data available on the optimal dietary treatment of T2DM in adults.70
Nutrition and lifestyle approaches to diabetes prevention and treatment should be given at least as much importance as drug therapy. A family-centered rather than patient-oriented approach usually is more successful. Patients and their families must acknowledge that lifestyle modifications such as eating a balanced diet, maintaining a healthy weight, and exercising regularly are essential.71 Nutrition recommendations should be culturally appropriate and sensitive to family resources. As a general rule, patients should be advised to restrict starches and refined carbohydrates, including sugary drinks and “fast foods.” Intake of salad, nonstarchy vegetables, fruits, and whole grains should be encouraged. Less than 30% of the daily caloric requirement should come from fat.
Increasing evidence suggests that a low-GI diet may have beneficial effects on metabolic control.50 A meta-analysis of randomized controlled trials comparing low-GI versus conventional or high-GI diets found a mean reduction in HbA1c in favor of low-GI diets. A low-GI diet may reduce insulin secretion and improve insulin sensitivity, and by reducing insulin secretion, may downregulate malonyl-CoA carboxylase activity, thereby decreasing formation of fatty acids and triglycerides. The amount of dietary fiber should also be increased as it reduces insulin levels, promotes weight loss, improves lipid profiles, and lowers cardiovascular risk. A recent study in adults comparing the effects of three different diets—low-GI, high-GI, and low-carbohydrate diets—showed no differences in HbA1c levels; however, a reduction in C-reactive protein (CRP) and a decrease in postprandial glucose concentrations was seen with the low-GI diet.72
Regular physical activity facilitates weight loss, increases high-density lipoprotein (HDL) cholesterol levels, lowers blood pressure, and improves metabolic control. Fasting serum insulin concentrations decrease and insulin sensitivity improves in obese children who exercise regularly.73,74 Youth with T2DM should participate in regular aerobic exercise with a gradual increase in the frequency, intensity, and duration, aiming for at least 30 minutes daily of moderate/intense physical activity. Exercise tolerance is reduced in obese children; therefore, advice to increase physical activity should be realistic and individualized. To increase children’s physical activity, the amount of time devoted to sedentary activities (screen time) must be strictly limited.
Pharmacologic Therapy
Oral Agents
Symptomatic patients with severe hyperglycemia, weight loss, and ketosis or ketoacidosis require a period of insulin therapy (similar to the treatment of T1DM) until fasting and postprandial glycemia have been restored to normal or near normal. Similarly, when the type of diabetes has not been defined, the patient initially should be treated with insulin (see Fig. 49-1).75
Less than 10% of children and adolescents with T2DM achieve adequate glycemic control with lifestyle changes alone. Most require pharmacologic therapy76; however, data on the efficacy and safety of oral antihyperglycemic agents in the pediatric population are sparse. Metformin monotherapy is recommended as the first choice for asymptomatic or mildly symptomatic patients. Some clinicians initiate pharmacologic therapy upon diagnosis, whereas others prescribe medication only after a 2 to 3 month trial of behavior modification and lifestyle intervention has failed, as evidenced by persistent or worsening hyperglycemia.
Metformin
Metformin is currently the only oral hypoglycemic agent specifically approved for pediatric use by the U.S. Food and Drug Administration (FDA) in children over 10 years of age, when given alone or in combination with insulin. Metformin is safe and efficacious in pediatric patients with T2DM.77,78 It suppresses basal hepatic glucose production and increases insulin-mediated glucose uptake in skeletal muscle, but it does not affect insulin secretion or cause hypoglycemia. Metformin causes a mild reduction in triglyceride and LDL concentrations. Its anorectic effect may contribute to modest weight loss.
Its most common side effects are nausea, vomiting, abdominal pain, and diarrhea. Lactic acidosis is a rare, potentially fatal side effect. Provided that it is not administered to patients with renal insufficiency (metformin is excreted unchanged in the urine) or poor tissue perfusion, the risk of lactic acidosis is not greater than that of other antihyperglycemic agents.79 Metformin must be discontinued before radiographic studies with contrast agents or surgery under general anesthesia is performed; in patients with renal, liver, or heart disease; and whenever tissue perfusion is poor. Because the absorption of vitamin B12 and/or folic acid may be compromised, patients are advised to take a daily multivitamin.
Metformin is available as 500-, 850-, and 1000-mg tablet strengths and as 500- and 750-mg extended-release tablets. A liquid formulation (500 mg/5 mL) is also available. For children 10 to 16 years of age, the recommended starting dose is 500 mg once daily. The dose may be increased to 500 mg twice daily, and further increases may be made at weekly intervals in 500 mg increments to a maximum daily dose of 2000 mg. The acute, reversible gastrointestinal adverse effects of metformin may be minimized by administration with or after food, and by use of lower dosages, which are increased slowly, as necessary. The extended-release preparation should be initiated at a dose of 500 mg once daily, given with the evening meal. The maximum recommended dose of the extended-release product is 2000 mg per day.
When metformin is prescribed for overweight females with polycystic ovary syndrome, a condition that is often associated with T2DM, menstrual cycles and fertility may be restored to normal. Sexually active females should be counseled regarding the need for birth control.
Insulin Secretagogues (Sulfonylureas and Meglitinides)
Although sulfonylureas have been used in adults for longer than half a century, only limited evidence of their efficacy in children has been found. A recent 24-week, randomized, single-blind comparative study in T2DM pediatric patients, showed that glimepiride was as safe and effective as metformin in terms of reduction of HbA1c and incidence of hypoglycemia. The glimepiride-treated group, however, showed greater weight gain compared with patients treated with metformin.80
A non-sulfonylurea insulin secretagogue such as repaglinide enhances insulin release within 10 to 30 minutes of its administration and has a shorter duration of effect (2 to 4 hours) than the sulfonylureas. It is taken before meals to improve postprandial blood glucose control.
Thiazolidinediones (TZDs)
TZDs are insulin sensitizers that act on the nuclear receptor peroxisome proliferator–activated receptor-gamma (PPAR-γ) and increase insulin sensitivity in muscle and adipose tissue. TZDs have favorable effects on lipid metabolism. Side effects include weight gain and fluid retention, which contraindicates their use in patients with heart failure. At the present time, TZDs are not approved for use in children, but clinical trials in pediatric T2DM are currently in progress.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree