FIGURE 63-1. Bone biopsy photomicrographs. A, Local osteolytic hypercalcemia secondary to leukemia. Note the presence of leukemic cells in the marrow space and numerous osteoclasts lining the trabecular surface. B, Humoral hypercalcemia of malignancy caused by squamous carcinoma. Note the absence of tumor in the marrow space but the presence of numerous active osteoclasts on the trabecular surface. Also note the absence of osteoblasts and osteoid. C, Hyperparathyroidism. Note the abundant osteoclasts, osteoblasts, and osteoid. Osteoblasts are indicated by small arrows, and osteoblasts by large arrows.
(B and C from Stewart AF, Vignery A, Silverate A, et al: Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity, J Clin Endocrinol Metab 55:219–227, 1982.)
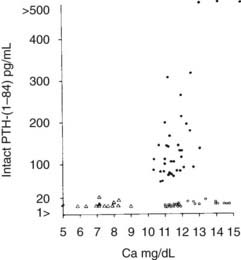
FIGURE 63-2. Immunoreactive parathyroid hormone in the serum of patients with primary hyperparathyroidism (solid circles), hypoparathyroidism (triangles), and hypercalcemia of malignancy (open circles) as measured with a two-site immunoradiometric assay for parathyroid hormone (1-84). Note the clear separation of patients with hyperparathyroidism from those with malignancy-associated hypercalcemia.
(Data from Nussbaum SR, Zahradnik RJ, Lavigne JR, et al: Highly sensitive two-site immunoradiometric assay of parathyrin and its clinical utility in evaluating patients with hypercalcemia, Clin Chem 33:1364–1366, 1987.)
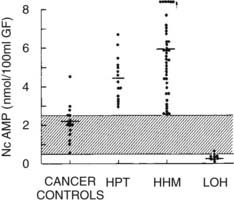
FIGURE 63-3. Nephrogenous cyclic adenosine monophosphate excretion in patients with normocalcemia and cancer (cancer controls), primary hyperparathyroidism (HPT), humoral hypercalcemia of malignancy (HHM), and local osteolytic hypercalcemia (LOH).
(Data from Stewart AF, Horst R, Deftos LJ, et al: Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and non-humoral groups, N Engl J Med 303:1377–1383, 1980.)
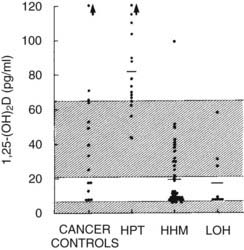
FIGURE 63-4. Plasma 1,25-dihydroxyvitamin D concentration in the four groups described in Figure 63-3.
(Data from Stewart AF, Horst R, Deftos LJ, et al: Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and non-humoral groups, N Engl J Med 303:1377–1383, 1980.)
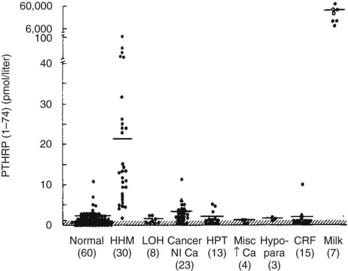
FIGURE 63-5. Immunoreactive parathyroid hormone–related protein (PTHrP) in plasma from patients with the clinical syndromes listed below the x-axis. Note that patients with humoral hypercalcemia of malignancy (HHM) have elevated circulating PTHrP concentrations.
(Data from Burtis WJ, Brady TG, Orloff JJ, et al: Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of malignancy, N Engl J Med 322:1106–1112, 1990.)
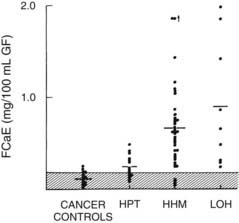
FIGURE 63-6. Fasting calcium excretion in the four groups described in Figure 63-3. Note that on average, patients with humoral hypercalcemia of malignancy (HHM) and local osteolytic hypercalcemia (LOH) appear to be more calciuric than do patients with primary hyperparathyroidism (HPT). Compare these findings with those in Figure 63-8.
(Data from Stewart AF, Horst R, Deftos LJ, et al: Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and non-humoral groups, N Engl J Med 303:1377–1383, 1980.)
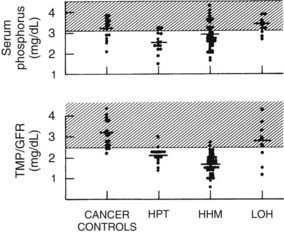
FIGURE 63-7. Serum phosphorus and renal phosphorus threshold in the four groups described in Figure 63-3.
(Data from Stewart AF, Horst R, Deftos LJ, et al: Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and non-humoral groups, N Engl J Med 303:1377–1383, 1980.)
Humoral Hypercalcemia of Malignancy
Humoral hypercalcemia of malignancy (HHM) accounts for approximately 80% of patients in unselected series of individuals with MAHC.2–12,17–22 Approximately 50% of patients with HHM have an underlying squamous carcinoma of the lung, cervix, esophagus, larynx, oropharynx, vulva, skin, or other site2–12,17–22 (see Table 63-1). Carcinomas of the kidney, ovary, and bladder also are very common.2–14,19–22 It is interesting to note that breast cancer not only may cause MAHC through LOH and skeletal metastases but also may cause hypercalcemia in the absence of skeletal metastases in a classic HHM scenario43,44 (see Table 63-1). Human T cell lymphoma/leukemia virus-I lymphomas, 90% of which are associated with hypercalcemia, also operate through this mechanism.45,46 Finally, hypercalcemia resulting from endocrine tumors such as pheochromocytomas47,48 and islet cell carcinomas49,50 may cause hypercalcemia through this mechanism. As is the case with LOH, virtually every tumor type has been reported on occasion to cause HHM. It is interesting to note that not every tumor that causes hypercalcemia is malignant. Examples of systemic secretion of PTHrP by benign neoplasms (mammary hypertrophy and uterine leiomyomas are examples) have been reported to cause “humoral hypercalcemia of benignancy.”51
Since the advent of Albright’s humoral theory of hypercalcemia of malignancy in the 1940s,3 several substances have been proposed as candidates for the humoral mediator responsible for HHM. In the 1960s, Gordan and coworkers52 suggested that elevated circulating levels of four phytosterols (plant-derived vitamin D analogues) were present in patients with breast carcinoma. Subsequent studies showed, however, that these same phytosterols were present in equivalent concentrations in normal and lactating women, and that the potency of these analogues was inadequate to cause hypercalcemia.53 The phytosterol theory thus lost support.
With the discovery in the 1970s that prostaglandin E2 (PGE2) was a potent stimulator of bone resorption both in tissue culture54 and in experimental animals in vivo,55 the possibility arose that the hypercalcemia associated with HHM was due to systemic PGE2 secretion by tumors. Seyberth and colleagues56 and others reported that urinary metabolites of PGE2 were elevated in patients with MAHC, and that therapy with prostaglandin synthesis inhibitors (aspirin, indomethacin) reversed the hypercalcemia in several patients. However, subsequent, more extensive studies have not shown frequent responses to indomethacin.57 It is the current view of most investigators that PGE2 does not act as a systemic mediator of bone resorption in most cases of HHM, and that therapy with prostaglandin synthesis inhibitors is usually ineffective. It should be clear, however, that these observations do not exclude a role for PGE2 or other arachidonate metabolites in HHM at the local level within the skeleton.
As was noted earlier, Albright3 initially had suggested that PTH was the responsible humoral factor. This concept subsequently gained wide acceptance, as evidenced by the entrance into common usage in the 1960s and 1970s of the terms “ectopic hyperparathyroidism” and “pseudohyperparathyroidism.”6 Evidence in support of the “ectopic PTH” thesis included (1) the humoral nature of the syndrome4,5,10; (2) the hypophosphatemia and renal phosphate–wasting characteristic of the syndrome; and (3) the apparent failure of suppression of PTH observed in the early generations of PTH radioimmunoassays.58,59 It is now clear, as is described later, that although bona fide ectopic hyperparathyroidism does indeed exist (see later), it is extremely rare and fails to account for most instances of HHM.
Today, it is widely accepted that the vast majority of cases of HHM are due to the secretion of PTHrP by tumors. Evidence for this statement can be summarized as follows: (1) tumors associated with HHM produce and secrete PTHrP, which leads to elevated circulating concentrations of PTHrP19–22 (see Fig. 63-5); (2) PTHrP infusion into laboratory animals and humans reproduces the key features of the HHM syndrome in vivo60–65; and (3) infusion of neutralizing antisera against PTHrP reverses the HHM syndrome in laboratory animal models.66,67
The pathophysiology of HHM is now quite clear. Under normal circumstances, PTHrP is widely expressed among essentially all tissues and serves as a local paracrine or autocrine factor, but it does not enter the systemic circulation.68,69 However, in malignant and occasionally benign tumors, PTHrP gene expression may be significantly upregulated, and PTHrP now enters the circulation. Because of the structural homology between PTHrP and PTH, PTHrP is able to bind to and activate the common PTH/PTHrP receptor in bone and kidney, with the result that patients with HHM mimic many of the cardinal features of primary hyperparathyroidism. As is summarized later, hypercalcemia in HHM results from a combination of accelerated osteoclastic bone resorption and reduced ability of the kidney to clear calcium. Biochemically, patients with HHM display increases in circulating concentrations of PTHrP19–22 (see Fig. 63-5), and bone biopsies display a marked increase in osteoclastic resorption, accompanied by a decrease in osteoblastic bone formation13 (see Fig. 63-1). These histologic results have been corroborated more recently by the use of biochemical markers of bone turnover such as bone-specific alkaline phosphatase, osteocalcin, N-telopeptide of collagen, and deoxypyridinoline cross-links.42 This quantitatively striking uncoupling of bone resorption from formation leads to a large calcium flux from the skeleton into the extracellular fluid and accounts primarily for the hypercalcemia observed in HHM. The cellular basis for this dramatic uncoupling is not known. Circulating PTH concentrations are reduced in patients with HHM19–2270 (see Fig. 63-2), but nephrogenous cyclic adenosine monophosphate (NcAMP) excretion is increased (see Fig. 63-3),11,12 a reflection of the increases in circulating PTHrP. Although the increase in NcAMP excretion is of little diagnostic importance today, the elevation in NcAMP and the ability of PTHrP to stimulate adenylyl cyclase in the kidney in vitro led to the identification and purification of PTHrP.69
HHM is associated with suppression of plasma 1,25(OH)2D11,12 (see Fig. 63-4). The mechanisms responsible for the reduction in 1,25(OH)2D in HHM as compared with primary hyperparathyroidism have recently begun to be elucidated. First, Horwitz et al.64 have demonstrated that chronic infusion of PTHrP in humans serves as a poor agonist of 1,25(OH)2D production, in contrast to infusion of PTH, which leads to robust, dose-related increases in 1,25(OH)2D. Second, Dean et al71 have recently demonstrated that whereas PTH and PTHrP may associate with the common human PTH-PTHrP receptor equivalently, their dissociation rates differ markedly, with PTH remaining bound to the receptor and activating signal transduction, while PTHrP dissociates rapidly, inactivating downstream signaling. Because of the low 1,25(OH)2D concentrations in patients with HHM, one might predict that intestinal calcium absorption does not contribute to their hypercalcemia, in contrast to those with primary hyperparathyroidism, in whom dietary calcium intake increases serum calcium. This inability to absorb dietary calcium in humans with malignancy-associated hypercalcemia has been directly documented using intestinal 45Ca absorption.72
Fractional calcium excretion by the kidney has been reported to be elevated10,11 (see Fig. 63-5) or reduced73,74 in patients with HHM. These conflicting reports result from the fact that accurate measurements of the GFR, on which calcium excretion measurement is based, are not possible in patients with advanced cancer, rapidly declining renal function, and markedly reduced muscle mass (and therefore creatinine release) who are undergoing aggressive hydration and diuretic therapy. To address this issue more directly, Horwitz and others62–64 recently demonstrated in normal healthy volunteers infused with PTH or PTHrP that both peptides have potent and equivalent effects in stimulating renal tubular (presumably distal) reabsorption of calcium (Fig. 63-8). Thus, in addition to the bone-resorbing effects of PTHrP, the anticalciuric effects of PTHrP contribute to hypercalcemia in HHM.
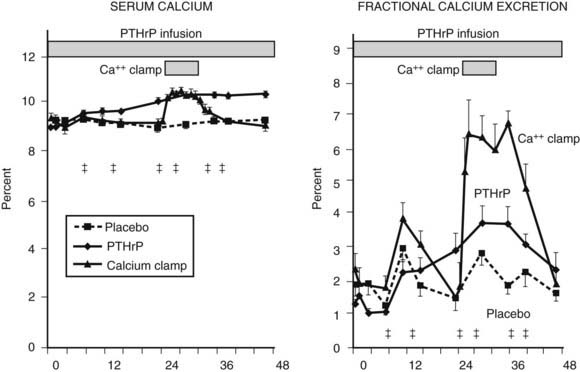
FIGURE 63-8. Fractional calcium excretion in normal subjects infused with calcium chloride or parathyroid hormone–related protein (PTHrP). Left, Serum calcium concentrations are shown in three groups of normal healthy volunteers infused with vehicle alone, with PTHrP to achieve a serum calcium of 10.3 mg/dL, or with calcium chloride (calcium clamp) to achieve an identical serum calcium of 10.3 mg/dL. Right, Response in fractional calcium excretion in the three groups. In the control (vehicle) group, fractional calcium excretion remains stable at approximately 2%. In the calcium clamp group, the fractional calcium excretion increases dramatically to approximately 6.5%, reflecting a combination of the increase in the filtered load of calcium from the calcium infusion and the suppression of endogenous PTH secretion. In the group receiving PTHrP, despite identical serum calcium concentration (and therefore an identical filtered load of calcium) to the calcium clamp group, fractional calcium excretion remains low, indicating that PTHrP is a potent stimulator of distal tubular calcium reabsorption. Studies comparing PTH with PTHrP indicate that the two peptides have equivalent effects in human renal calcium handling.62–64
(Data from Syed MA, Horwitz MJ, Tedesco MB, et al: Parathyroid hormone-related protein [1-36] stimulates renal tubular calcium reabsorption in normal human volunteers: implications for the pathogenesis of humoral hypercalcemia of malignancy, J Clin Endocrinol Metab 86:1525–1531, 2001.)
Finally, similar to PTH, PTHrP inhibits proximal tubular phosphorus reabsorption.60–64 Thus, in HHM, the serum phosphorus concentration is characteristically reduced11,12 (see Fig. 63-7) as long as renal function remains normal, a phenomenon that is reflected in a reduction in the TmP/GFR11,12 (see Fig. 63-7).
Bone radionuclide scans characteristically display a complete absence of skeletal metastases or the presence of only a few skeletal metastases,2–12,17–22 which is a reflection of the primarily humoral nature of the syndrome. It is important to note that the syndrome reverses with successful eradication of the tumor in question, thus underscoring the humoral nature of the syndrome.2–12,17,18 Unfortunately, this outcome is not common.
Authentic Ectopic Hyperparathyroidism
As noted earlier, from the 1940s through the 1970s, ectopic hyperparathyroidism was believed to be a common cause of paraneoplastic hypercalcemia. By the 1980s, the term had fallen into disuse, and the existence of authentic ectopic hyperparathyroidism was in doubt; most authors believed that all cases of what had previously been considered to be ectopic hyperparathyroidism were in fact cases of HHM. With the advent of sensitive and specific immunoassays and molecular probes for PTH and PTHrP, the situation has changed. Now, approximately 15 case reports describe patients with diverse types of cancer (e.g., small cell carcinomas of the lung, squamous carcinoma of the lung, hepatoma, thymoma, neuroendocrine tumors, clear cell adenocarcinoma of the ovary, thyroid papillary carcinoma) who also displayed elevations in immunoreactive PTH in plasma with the use of modern two-site PTH immunoassays or expressed PTH mRNA in their tumor, or both.75–82
In one thoroughly studied case, reported by Nussbaum and associates,75 immunoreactive PTH values were found to be elevated in plasma by a sensitive and specific two-site PTH immunoradiometric assay. At surgery, a fivefold gradient of PTH was demonstrated across the ovarian tumor. PTH values decreased precipitately after tumor resection, and the serum calcium concentration normalized (Fig. 63-9). Neck exploration before the ovarian surgery had revealed four normal parathyroid glands, and resection of three-and-a-half parathyroid glands had no effect on the serum calcium concentration. PTH mRNA was abundantly present in the tumor, whereas PTHrP mRNA was undetectable, as was PTHrP in plasma.
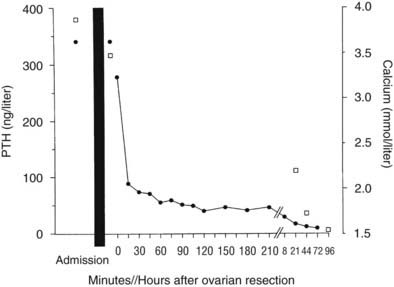
FIGURE 63-9. Serum calcium (open squares) and immunoreactive parathyroid hormone (PTH) (solid circles) in a patient with an ovarian carcinoma before and after oophorectomy, indicated by the black bar. The PTH immunoassay is that shown in Figure 63-2. Prior parathyroidectomy of 3.5 glands failed to influence her hypercalcemia or her elevated PTH concentration.
(Data from Nussbaum SR, Gaz RD, Arnold A: Hypercalcemia and ectopic secretion of PTH by an ovarian carcinoma with rearrangement of the gene for PTH, N Engl J Med 323:1324–1328, 1990.)
The basis of PTH gene overexpression in this tumor was found to be twofold. First, a clonal rearrangement in the upstream region of one copy of the PTH gene in the ovarian carcinoma apparently served to abolish a silencer in this region of the gene or included a promoter region of a normal ovarian gene. Second, the PTH gene was amplified in the tumor. In contrast, in the report by Yoshimoto and colleagues76 describing ectopic hyperparathyroidism caused by a pulmonary small cell carcinoma, no such gene rearrangement or amplification events were identified, and the cause of the PTH expression was unexplained. In a recent report by VanHouten et al., ectopic production of PTH was documented from a pancreatic neuroendocrine tumor both in vivo and in vitro, and this was shown to be due not to a gene rearrangement or amplification, but to transcriptional activation of the PTH gene within the malignant neuroendocrine cells.82
These case reports demonstrate that authentic ectopic hyperparathyroidism, although rare, can occur. From a clinical standpoint, because of elevations in immunoreactive PTH, these patients may be misdiagnosed as having primary hyperparathyroidism. Unless the offending malignant neoplasm is obvious at the initial evaluation, such confusion may lead to unsuccessful parathyroidectomy.
Unusual Causes of Malignancy-Associated Hypercalcemia
The vast majority of patients with HHM are striking in their homogeneity. As was indicated earlier, the histologic findings (squamous, renal, bladder, and ovarian carcinomas) and biochemical findings (elevated NcAMP, reduced renal phosphorus thresholds, reduced 1,25[OH]2D levels, suppressed immunoreactive PTH levels, elevated immunoreactive PTHrP concentrations) are so uniform that it seems inescapable that most cases of HHM are due to secretion of PTHrP.
It should be clear, however, that other humoral mediators undoubtedly exist in unusual patients. For example, a small number of patients with elevated PGE2 levels or clear responsiveness to prostaglandin synthesis inhibitors or both have been described,56,83 which suggests that in rare instances, PGE2 may act as a humoral, systemic agent.
In addition, more than 40 patients with a variety of types of lymphoma have been described in whom hypercalcemia occurred in the absence of bone metastases and with reduced urinary cAMP or NcAMP excretion but elevated circulating levels of 1,25(OH)2D84–86 (Fig. 63-10). These observations suggest that tumor-derived 1,25(OH)2D may have induced intestinal hyperabsorption of calcium or may have led to osteoclastic stimulation, or both. More recently, in patients harboring ovarian dysgerminomas, several groups have reported production and systemic elevations of 1,25(OH)2D leading to hypercalcemia.87,88
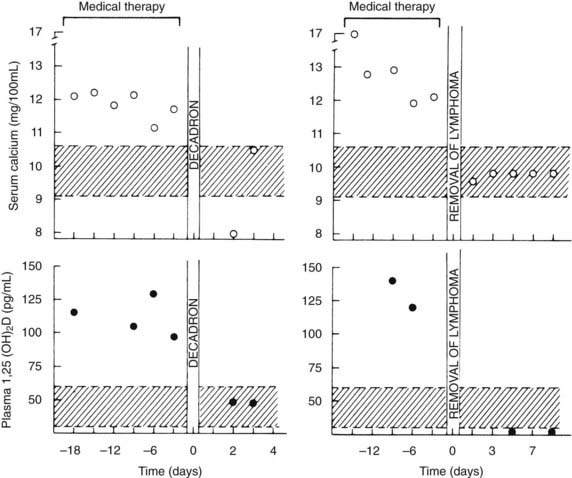
FIGURE 63-10. Serum calcium and plasma 1,25-dihydroxyvitamin (1,25[OH]2D) concentrations in patients with the 1,25(OH)2D-induced lymphoma syndrome before and after therapy. The patient shown in the two left panels was treated with dexamethasone (decadron) for systemic lymphoma. The patient in the two right panels had a splenectomy for a solitary splenic lymphoma. Note the decrease in plasma 1,25(OH)2D concentration after therapy in both patients and the normalization of serum calcium. Also compare the plasma 1,25(OH)2D concentrations in these patients versus those in patients with humoral hypercalcemia of malignancy and local osteolytic hypercalcemia shown in Figure 63-4.
(Data from Rosenthal ND, Insogna KL, Godsall JW, et al: Elevations in circulating 1,25-dihydroxyvitamin D in three patients with lymphoma-associated hypercalcemia, J Clin Endocrinol Metab 60:29–33, 1985.)
Further, a patient with an ovarian dysgerminoma, no skeletal involvement, hypercalcemia in the presence of an elevated renal phosphorus threshold, and reduced levels of NcAMP and 1,25(OH)2D has been described.89 All these abnormalities promptly normalized after eradication of the patient’s tumor, which suggests that the tumor produced a humoral agent distinct from PTH, PTHrP, or 1,25(OH)2D.
Finally, Bringhurst and others90,91 described patients with hypercalcemia in the setting of metastatic malignant melanoma and bladder carcinoma. Excision of the tumor reversed the patients’ hypercalcemia, thus indicating a humoral mechanism. In culture, the tumors were shown to produce a bone-resorbing protein devoid of adenylate cyclase–stimulating activity or PTH-like bioactivity. In the aggregate, these examples collectively indicate that humoral forms of MAHC can occur through the production of factors other than 1,25(OH)2D, PTHrP, or PTH; however, these examples appear to be rare.
Treatment of Hypercalcemia
OVERVIEW
This section summarizes treatment of hypercalcemia of any cause but with particular reference to that due to cancer. The treatment of hypercalcemia caused by cancer has received complete attention in several recent reviews.92–94 As was noted earlier, the mean life expectancy in patients following a diagnosis of MAHC is 30 days.14 The most effective long-term therapy for MAHC is successful antitumor therapy. This point should be borne in mind and acted on early in the management of any given patient. Therapies aimed at treatment of hypercalcemia in patients with MAHC per se are rarely effective over the long term. Hence, long-term control of hypercalcemia depends on tumor eradication or debulking through surgery, chemotherapy, or radiotherapy. It is therefore essential and urgent that a plan for chemotherapy, surgery, or radiotherapy be developed and acted upon very early on in the course of treatment for MAHC. Said another way, therapy aimed at correcting hypercalcemia is largely short term and palliative: it should be used only to temporize while a more concrete and effective long-term treatment plan is formulated.
Another important concept is that therapy should be aimed not at the serum calcium concentration, but at a given patient’s overall status. For example, a patient with a calcium level of 11.5 mg/dL who has been stable and asymptomatic may need no treatment at all, whereas aggressive therapy for a calcium level of 11.0 may be appropriate in an elderly patient with symptoms of hypercalcemia, or when a rapid subsequent increase seems likely. Conversely, no antihypercalcemic therapy may be most appropriate in a patient with advanced cancer who has failed all attempts to control the malignancy.
It is useful to conceptualize short-term antihypercalcemic therapy in terms of the three organ systems that regulate calcium homeostasis and whose aggregate homeostatic failure leads to hypercalcemia: the intestine, the kidney, and the skeleton. Therapy should be tailored to a given patient’s needs and with the factors in mind that may have precipitated development of the hypercalcemia. The development of hypercalcemia in a patient with MAHC may be primarily skeletal: it may be traced to tumor progression with progressive skeletal metastases, to increasing PTHrP levels in the circulation, or to recent immobilization. Therapy aimed at reducing tumor burden and/or reversing immobilization may be all that is necessary to correct such a patient’s hypercalcemia. A previously normocalcemic patient with cancer may develop hypercalcemia as a result of dehydration due to anorexia or diarrhea. Simple rehydration may be all that is required. As described previously, a patient with lymphoma or dysgerminoma may develop hypercalcemia owing to excessive intestinal absorption of calcium as a result of increased 1,25(OH)2D concentrations (or with vitamin D intoxication). Reducing dietary calcium intake and vitamin D may be the best therapeutic option.
Finally, most often, combinations of the agents and measures described below are used. For example, a regimen designed to increase hydration and increase physical mobility, combined with a loop diuretic and a potent intravenous bisphosphonate, would be appropriate in most patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








