FIGURE 142-1. Pooled summary of contraceptive efficacy from two World Health Organization male contraceptive efficacy studies,4,5 where contraceptive failure rate (pregnancy rate) is plotted against the current sperm concentration in the ejaculate. This illustrates a summation of all data pooled from both studies. Data comprise monthly observations of the mean sperm concentration (averaging monthly sperm counts) and whether a pregnancy occurred in that month or not. Pregnancy rate (per 100 person years, Pearl Index) on the Y-axis is plotted against the cumulative sperm density (in million sperm/mL), indicating that contraceptive failure rates are proportional to sperm output. The inset is the same data replotted in discrete sperm concentration bands rather than cumulatively. For comparison, the average contraceptive failure rates in the first year of use10,12 of modern reliable contraceptive methods are indicated by diamond symbols.
The reversibility of hormonal male contraceptive regimens is clearly established by an integrated reanalysis that pooled primary data from over 90% of all hormonal male contraceptive studies reported; it showed that all regimens show full reversibility within a predictable time course6 (Fig. 142-2). This comprehensive review of the recovery of 1549 healthy eugonadal men, aged 18 to 51 years, who underwent 1283.5 man years of treatment and 705 man years of posttreatment recovery, showed the median times for recovery to sperm densities of 10 and 20 million/mL were 2.5 months (2.4 to 2.7) and 3 months (2.9 to 3.1), respectively. Covariables such as age, ethnicity, and hormonal or sperm output kinetics had significant but minor influence on the rate but not the extent of recovery. Acceptability of a hormonal male contraceptive is high across a wide range of countries and cultures. Willingness to use a hypothetical hormonal male contraceptive averaged 55% (range 29% to 71%) in an extensive population-representative survey of 9342 men aged 18 to 50 years from 9 countries (4 European, 3 South American, Indonesia, and the United States), with consistency across a wide range of socioeconomic and cultural settings.138,139 Similar findings are reported in a four-country study (United Kingdom, South Africa, Hong Kong, Shanghai) with 44% to 83% in each center140 and 75% in Australia141 willing to try a hormonal male contraceptive. Female partners from a variety of cultures also indicate high acceptability in a survey of 1894 women in four countries, among whom 40% to 78% would support and trust their male partners in stable relationships to use a hormonal male contraceptive.142 Corroborating the acceptability of hormonal male contraception are findings from experimental studies of prototype regimens for up to 12 months usage in which most participants confirm high levels of satisfaction and willingness to try a commercial product.143,144 Hence, prototype hormonal methods have proven reliability and reversibility and reasonable prospects for being well accepted and safe. Although they are the most likely opportunity in the foreseeable future to develop a practical contraceptive method for men, progress depends on pharmaceutical industry development, but the commitment of drug companies continues to languish.7
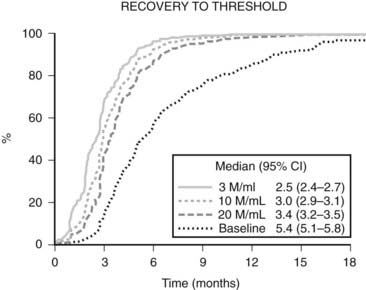
FIGURE 142-2. Recovery of spermatogenesis after cessation of treatment with hormonal male contraceptive regimens, modified after an integrated reanalysis of over 90% of all reported studies.6 Data are plotted as a Kaplan-Meier survival plot of the increasing proportion of men recovering to various thresholds over time since last treatment. The data of last treatment are defined as the time elapsed from the end of the last treatment cycle—that is, the latest date of the first missed treatment dose. The thresholds are a sperm concentration of 3, 10, or 20 million sperm/mL in the ejaculate or a return to their own pretreatment baseline sperm concentration. The median time to achieving each threshold is tabulated together with its 95% confidence interval.
STEROIDAL METHODS
Androgen Alone
Testosterone provides both gonadotropin suppression and androgen replacement, making it an obvious first choice as a single agent for a reversible hormonal male contraceptive. Although androgen-induced, reversible suppression of human spermatogenesis has long been known,145–148 systematic studies of androgens for male contraception began in the 1970s.149,150 Feasibility and dose-finding studies,151 mostly using testosterone enanthate (TE) in an oil vehicle as a prototype, showed that weekly IM injections of 100 to 200 mg TE induce azoospermia in most Caucasian men,152 but less frequent or lower doses fail to sustain suppression.153–156 The largest experience with an androgen-alone regimen arises from the two WHO studies in which over 670 men from 16 centers in 10 countries received weekly injections of 200 mg TE. In these studies approximately 60% of non-Chinese and more than 90% of Chinese men became azoospermic, and the remainder were severely oligozoospermic.4,5 The high efficacy among Chinese men has also been replicated using monthly TU injections.134 Effective gonadotropin suppression is a prerequisite for effective testosterone-induced spermatogenic suppression in human135,157–161 and nonhuman primates.162,163 However, the reasons for within- and between-population differences in susceptibility to hormonally-induced azoospermia remain largely unexplained.157 Possible factors include population differences in reproductive physiology of environmental,164,165 genetic,166,167 or uncertain168,169 origin that may lead to differences in suppressibility of circulating gonadotropins and/or depletion of intratesticular androgens. Limited invasive studies measuring intratesticular testosterone (and dihydrotestosterone [DHT]) suggest that the degree of depletion may not predict reliably complete suppression of sperm output,170–172 but other more widely applicable, noninvasive markers of endogenous Leydig-cell function such as circulating epitestosterone173 or 17-hydroxyprogesterone174 or nonsteroidal testicular products such as INSL3175 may be more analytically informative as to the relative roles of gonadotropin suppression and intratesticular androgen depletion. Exogenous testosterone causes suppression of sperm output, with an average of 13 weeks to reach severe oligozoospermia (<1 million/mL) or azoospermia and suppression maintained consistently during ongoing treatment.176 Following cessation of treatment, sperm reappear within 3 months to reach sperm densities of 10 and 20 million/mL at an average of 11.5 and 13.6 weeks, respectively,176 with ultimately full recovery6 (see Fig. 142-2). Apart from intolerance of weekly injections, there were few discontinuations due to acne, weight gain, polycythemia, or behavioral effects; these side effects were reversible, as were changes in hemoglobin, testis size, and plasma urea. There was no evidence of liver, prostate, or cardiovascular disorders.4,5,177
The pharmacokinetics of testosterone products are crucial for suppressing sperm output. Oral androgens have major first-pass hepatic effects, producing prominent route-dependent effects on hepatic protein secretion (e.g., sex hormone–binding globulin [SHBG], high-density lipoprotein [HDL] cholesterol) and inconsistent bioavailability. Short-acting testosterone products requiring daily or more frequent administration (oral, transdermal patches, or gels) that may be acceptable for androgen replacement therapy are not optimal for hormonal contraception. Weekly TE injections required for maximal suppression of spermatogenesis151 are far from ideal178 and cause supraphysiologic blood testosterone levels, risking both excessive androgenic side effects and preventing maximal depletion of intratesticular testosterone for optimal efficacy.179,180 Other currently available oil-based testosterone esters (cypionate, cyclohexane-carboxylate, propionate) are no improvement over the enanthate ester,181 and longer-acting depot preparations are needed. Subdermal testosterone pellets sustain physiologic testosterone levels for 4 to 6 months,182 and the newer injectable preparations, testosterone undecanoate,134 testosterone-loaded biodegradable microspheres,183 and testosterone buciclate,184 provide 2 to 3 months’ duration of action. Depot androgens suppress spermatogenesis faster at lower doses and with fewer metabolic side effects than TE injections, but azoospermia is still not achieved uniformly,185 although when combined with a depot progestin, this goal is achievable.135
Oral synthetic 17α-alkylated androgens such as methyltestosterone,186 fluoxymesterone,187 methandienone,188 and danazol189,190 suppress spermatogenesis, but azoospermia is rarely achieved and the inherent hepatotoxicity of the 17α-alkyl substitutent191 renders them unsuitable for long-term use in otherwise healthy men. Athletes self-administering supratherapeutic doses of androgens also exhibit suppression of spermatogenesis.188,192 Synthetic androgens lacking the 17α-alkyl substituent have been little studied, although injectable nandrolone esters produce azoospermia in 88% of European men,193,194 whereas oral mesterolone is ineffective.195 On the other hand, nandrolone hexyloxyphenylpropionate alone was unable to maintain spermatogenic suppression induced by a gonadotropin-releasing hormone (GnRH) antagonist196 in a prototype hybrid regime (where induction and maintenance treatment differ), whereas testosterone appears more promising.197 A 7-methyl derivative of nandrolone (7α-methyl-19-nortestosterone [MENT]), which is partly aromatizable but resistant to 5α reduction which amplifies androgenic potency, has been studied as a non-oral androgen for hormonal male contraceptive regimens.198 Although it is prostate sparing,199 dose titration to achieve essential androgen replacement at each relevant tissue is more complex than for testosterone and may be difficult to achieve.200,201 More potent synthetic androgens lacking 17α-alkyl groups,202,203 as well as the recent development of the first nonsteroidal androgens,204 remain to be fully evaluated in the context of male contraception, where aromatizability may be critical in exploiting testosterone’s feedback effects on pituitary gonadotropin secretion.
Antiandrogens have been used to selectively inhibit epididymal and testicular effects of testosterone without impeding systemic androgenic effects.205 Cyproterone acetate, a steroidal antiandrogen with progestational activity, suppresses gonadotropin secretion without achieving azoospermia but leads to androgen deficiency when used alone.206 In contrast, pure nonsteroidal antiandrogens lacking androgenic or gestagenic effects such as flutamide, nilutamide, and bicalutamide (Casodex) fail to suppress spermatogenesis when used alone.207,208 Two studies evaluating the hypothesis that incomplete suppression of spermatogenesis is due to persistence of testicular DHT have reported no additional suppression from administration of finasteride, a type II 5α-reductase inhibitor209,210; however, because testes express predominantly the type I isoforms,211 further studies are required to conclusively test this hypothesis, using an inhibitor of type I 5α-reductase.212
The long-term safety of androgen administration concerns mainly potential effects on cardiovascular and prostatic disease. The explanation for the higher male susceptibility to cardiovascular disease is not well understood, so the risks of exogenous androgens are not clear.213,214 In clinical trials, lipid changes are minimal with depot (non-oral) hormonal regimens.135,173,185,215 Changes in blood cholesterol fractions observed during high hepatic exposure to testosterone and/or progestins, due to either oral first-pass effects or high parenteral doses, have unknown clinical significance. In any case, maintenance of physiologic blood testosterone concentrations is the prudent and preferred objective. The real cardiovascular risks or benefits of hormonal male contraception will require long-term surveillance of cardiovascular outcomes.216
The long-term effects of exogenous androgens on the prostate also require monitoring, since prostatic diseases are both age- and androgen-dependent. Exposure to adult testosterone levels is required for prostate development and disease.217–219 The precise relationship of androgens to prostatic disease, and in particular any influence of exogenous androgens, remains poorly understood. Comprehensive analysis pooling 18 prospective studies shows no relationship between blood testosterone or androgen levels and the subsequent occurrence of prostate cancer.220 A genetic polymorphism, the CAG (polyglutamine) triplet repeat in exon 1 of the androgen receptor, is an important determinant of prostate sensitivity to circulating testosterone, with short repeat lengths leading to increased androgen sensitivity,221 but the relationship of the CAG triplet repeat length polymorphism to late-life prostate diseases remains unclear.222 Among androgen-deficient men, prostate size and PSA concentrations are reduced and returned towards normal by testosterone replacement, without exceeding age-matched eugonadal controls.221,223–225 Even self-administration of massive androgen overdosage does not increase total prostate volume or PSA in anabolic steroid abusers, although central prostate zone volume increases.226 In situ prostate cancer is common in all populations of older men, whereas rates of invasive prostate cancer differ considerably between populations, despite similar blood testosterone concentrations. This suggests that early and prolonged exposure to androgens may initiate in situ prostate cancer, but later, androgen-independent environmental factors promote the outbreak of invasive prostate cancer. Therefore, it is prudent to maintain physiologic androgen levels with exogenous testosterone, which then might be no more hazardous than exposure to endogenous testosterone. Prolonged surveillance comparable with that to quantify small increases in risk of cardiovascular and breast disease in users of female hormonal contraception would be equally essential to monitor both cardiovascular and prostate disease risk in men receiving exogenous androgens for hormonal contraception.
Extensive experience with testosterone in doses equivalent to replacement therapy in normal men indicates minimal effects on mood or behavior.4,5,151,227–229 A careful placebo-controlled crossover study showed that a 1000 mg TU injection in healthy young men produces minor mood changes without any detectable increase in self- or partner-reported aggressive, nonaggressive, or sexual behaviors.230 By contrast, extreme androgen doses used experimentally in healthy men can produce idiosyncratic hypomanic reactions in a minority.231 Aberrant behavior in observational studies of androgen-abusing athletes or prisoners are difficult to interpret, particularly to distinguish genuine androgen effects from the influence of self-selection for underlying psychological morbidity.232
Androgen Combination Regimens
Combination steroid regimens using nonandrogenic steroids (estrogens, progestins) to suppress gonadotropins, together with testosterone for androgen replacement, have shown the most promising efficacy, with enhanced rate and extent of spermatogenic suppression compared with androgen-alone regimens.173,233,234 Synergistic combinations reduce the effective dose of each steroid, and minimizing testosterone dosage could both enhance spermatogenic suppression (if high blood testosterone levels counteract the necessary maximal depletion of intratesticular testosterone235–237) and reduce androgenic side effects.
Progesterone is a key precursor and steroidogenic intermediate for all bioactive natural steroids, and the progesterone receptors A and B are structurally and evolutionarily the closest members of the nuclear receptor superfamily to the androgen receptor. Yet, although progesterone has crucial gestational and lactational roles in female reproductive physiology, it has no well-established role in male reproductive physiology apart from a possible role in sperm function,238 possibly via a nongenomic rather than a classically genomic mechanism.239 Nevertheless, functional nuclear progesterone receptors are expressed in male brain, smooth muscle, and reproductive (but not most nonreproductive) tissues.240 Synthetic progestins, steroidal structural agonistic analogs of progesterone, are potent inhibitors of pituitary gonadotropin secretion and used widely for female contraception and hormonal treatment of disorders such as endometriosis, uterine myoma, and mastalgia. Used alone, progestins suppress spermatogenesis but cause androgen deficiency, including impotence,241,242 so androgen replacement is necessary. Nonhuman primate studies indicate that this is mediated via a central hypothalamic-pituitary site of action rather than direct effects on the testis.243 Extensive feasibility studies concluded that progestin-androgen combination regimens had promise as hormonal male contraceptives if more potent and durable agents were developed.151,244 The most detailed information on androgen-progestin regimens derives from studies with medroxyprogesterone acetate (MPA) combined with testosterone. Monthly injections of both agents or daily oral progestin with dermal androgen gels produce azoospermia in about 60% of fertile men of European background, with the remainder having severe oligozoospermia and impaired sperm function.151,244,245 Nearly uniform azoospermia is produced in men treated with depot MPA and either of two injectable androgens in Indonesian men132,133 or testosterone depot implants in Caucasian men.173 Smaller studies with other oral progestins such as levonorgestrel233,246,247 and norethisterone248,249 combined with testosterone demonstrate similar efficacy to oral MPA, whereas cyproterone acetate with its additional antiandrogenic activity has higher efficacy in conjunction with TE234,250 but not oral testosterone undecanoate.251 Promising findings of highly effective suppression of spermatogenesis are reported with depot progestins in the form of non-biodegradable implants of norgestrel252–254 or etonorgestrel255,256 or depot injectable MPA135,173,257,258 or norethisterone enanthate259,260 coupled with testosterone. The pharmacokinetics of the testosterone preparation is critical to efficacy of spermatogenic suppression, with long-acting depots being most effective and transdermal delivery less effective than injectable testosterone.252 Progestin side effects are few, and sexual function is maintained by adequate androgen replacement dosage. Metabolic effects depend on the specific regimen, with oral administration and higher testosterone doses exhibiting more prominent hepatic effects such as lowering SHBG and HDL cholesterol. After treatment ceases, with depletion or withdrawal of hormonal depots, spermatogenesis recovers completely but gradually, consistent with the time-course of the spermatogenic cycle.6
Estradiol augments testosterone-induced suppression of primate spermatogenesis261 and fertility,262 but estrogenic side effects (gynecomastia) and modest efficacy at tolerable doses make estradiol-based combinations impractical for male contraception.263 The efficacy and tolerability of newer estrogen analogs in combination with testosterone remain to be evaluated.
Gonadotropin-Releasing Hormone Blockade
The pivotal role of GnRH in the hormonal control of testicular function makes it an attractive target for biochemical regulation of male fertility. Blockade of GnRH action by GnRH receptor blockade with synthetic analogs or GnRH immunoneutralization would eliminate luteinizing hormone (LH) and testosterone secretion, requiring testosterone replacement. Many superactive GnRH agonists are used to induce reversible medical castration for androgen-dependent prostate cancer. They cause a sustained paradoxical inhibition of gonadotropin and testosterone secretion and spermatogenesis through pituitary GnRH-receptor down-regulation. When combined with testosterone, GnRH agonists suppress spermatogenesis but rarely achieve azoospermia,235,236,264 making them less effective than androgen-progestin regimens. By contrast, pure GnRH antagonists create and sustain immediate competitive blockade of GnRH receptors265,266 and, in combination with testosterone, are highly effective at suppressing spermatogenesis. Early hydrophobic GnRH antagonists were difficult to formulate and irritating, causing injection-site mast cell histamine release. Newer more potent but less irritating GnRH antagonists produce rapid, reversible, and complete inhibition of spermatogenesis in monkeys267–269 and men270,271 when combined with testosterone. More immediate and effective inhibition of gonadotropin secretion, and thereby more effective depletion of intratesticular testosterone, may account for the striking superiority of GnRH antagonists over agonists. Owing to their highly specific site of action, GnRH analogs have few unexpected side effects. Depot GnRH antagonist plus testosterone formulations suitable for administration at up to 3-month intervals could be promising as a hormonal male contraceptive regimen. Whether GnRH antagonists are more cost effective than progestins as the second, nonandrogenic, component of combination male hormonal contraceptive regimens remains to be established.171,196,258,272 The drawback of high cost might be overcome by hybrid regimens using GnRH antagonists to initiate suppression, followed by a switch to more economical steroids for maintenance of spermatogenic suppression.197 A GnRH vaccine could intercept GnRH in the pituitary-portal bloodstream, preventing its reaching pituitary GnRH receptors. Gonadotropin-selective immunocastration would require androgen replacement in men,273 and pilot feasibility studies in advanced prostate cancer are underway,274 but the prospects for acceptably safe application for male contraception remain doubtful.275 By contrast, there are growing applications for antihormonal contraceptive vaccines in control of companion (pet), agricultural, zoo, feral, and wild animal populations.276,277
Follicle-Stimulating Hormone Blockade
Selective follicle-stimulating hormone (FSH) blockade theoretically offers the opportunity to reduce spermatogenesis without inhibiting endogenous testosterone secretion. FSH action could be abolished by selective inhibition of pituitary FSH secretion with inhibin278 or novel steroids,279 by FSH vaccine,280 or by FSH-receptor blockade with peptide antagonists.281 Although FSH was considered essential to human spermatogenesis, spermatogenesis and fertility persist in rodents282–284 and humans285 lacking FSH bioactivity. Even complete FSH blockade alone might produce insufficient reduction in sperm output and function required for adequate contraceptive efficacy.286 In addition to the usual safety concerns of contraceptive vaccines (e.g., autoimmune hypophysitis, orchitis, immune complex disease), an FSH vaccine might be overcome by reflex increases in pituitary FSH secretion. Hence, effective FSH suppression is a necessary but not sufficient requirement for a hormonal male contraceptive regimen.
1. Anderson RA, Baird DT. Male contraception. Endocr Rev. 2002;23:735-762.
2. Kamischke A, Nieschlag E. Progress towards hormonal male contraception. Trends Pharmacol Sci. 2004;25:49-57.
3. United Nations. Levels and Trends of Contraceptive Use as Assessed in 2002. New York: Department of International Economic and Social Affairs; 2006.
4. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet. 1990;336:955-959.
5. World Health Organization Task Force on Methods for the Regulation of Male Fertility. contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65:821-829.
6. Liu PY, Swerdloff RS, Christenson PD, et al. Rate, extent and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367(9520):1412-1420.
7. Handelsman DJ. Hormonal male contraception: Lessons from the East when the Western market fails. J Clin Endocrinol Metab. 2003;88:559-561.
8. World Health Organization Task Force on Methods for the Determination of the Fertile Period. A prospective multicentre trial of the ovulation method of natural family planning. III. Characteristics of the menstrual cycle and of the fertile phase. Fertil Steril. 1983;40:773-778.
9. Trussell J, Grummer-Strawn L. Contraceptive failure of the ovulation method of periodic abstinence. Fam Plann Perspect. 1990;22:65-75.
10. Trussell J. Contraceptive failure in the United States. Contraception. 2004;70:89-96.
11. Rogow D, Horowitz S. Withdrawal: a review of the literature and an agenda for research. Stud Fam Plann. 1995;26:140-153.
12. Kost K, Singh S, Vaughan B, et al. Estimates of contraceptive failure from the 2002 National Survey of Family Growth. Contraception. 2008;77:10-21.
13. Moreau C, Trussell J, Rodriguez G, et al. Contraceptive failure rates in France: results from a population-based survey. Hum Reprod. 2007;22:2422-2427.
14. Trussell J, Kost K. Contraceptive failure in the United States: a critical review of the literature. Stud Fam Plann. 1987;18:237-283.
15. Potts M, Diggory P. Textbook of Contraceptive Practice, ed 2. Cambridge: Cambridge University Press; 1983.
16. Steiner MJ, Cates WJr, Warner L. The real problem with male condoms is nonuse. Sex Transm Dis. 1999;26:459-462.
17. Grady WR, Klepinger DH, Nelson-Wally A. Contraceptive characteristics: the perceptions and priorities of men and women. Fam Plann Perspect. 1999;31:168-175.
18. Rosenberg MJ, Waugh MS, Solomon HM, et al. The male polyurethane condom: a review of current knowledge. Contraception. 1996;53:141-146.
19. Gallo MF, Grimes DA, Lopez LM, et al: Non-latex versus latex male condoms for contraception, Cochrane Database Syst Rev CD003550, 2006.
20. Macaluso M, Blackwell R, Jamieson DJ, et al. Efficacy of the male latex condom and of the female polyurethane condom as barriers to semen during intercourse: a randomized clinical trial. Am J Epidemiol. 2007;166:88-96.
21. Cates WJr, Steiner MJ. Dual protection against unintended pregnancy and sexually transmitted infections: what is the best contraceptive approach? Sex Transm Dis. 2002;29:168-174.
22. Carey RF, Lytle CD, Cyr WH. Implications of laboratory tests of condom integrity. Sex Transm Dis. 1999;26:216-220.
23. Walsh TL, Frezieres RG, Peacock K, et al. Use of prostate-specific antigen (PSA) to measure semen exposure resulting from male condom failures: implications for contraceptive efficacy and the prevention of sexually transmitted disease. Contraception. 2003;67:139-150.
24. United Nations. Levels and Trends of Contraceptive Use as Assessed in 1998. New York: Department of International Economic and Social Affairs; 2000.
25. Liskin L, Benoit E, Blackburn R. Vasectomy: New Opportunities. Baltimore: Population Information Program, Johns Hopkins University, Baltimore; 1992.
26. Schwingl PJ, Guess HA. Safety and effectiveness of vasectomy. Fertil Steril. 2000;73:923-936.
27. Awsare NS, Krishnan J, Boustead GB, et al. Complications of vasectomy. Ann R Coll Surg Engl. 2005;87:406-410.
28. Sokal D, Irsula B, Hays M, et al. Vasectomy by ligation and excision, with or without fascial interposition: a randomized controlled trial. BMC Med. 2004;2:6.
29. Li S, Goldstein M, Zhu J, et al. The no-scalpel vasectomy. J Urol. 1991;145:341-344.
30. Sokal D, McMullen S, Gates D, et al. A comparative study of the no scalpel and standard incision approaches to vasectomy in 5 countries. The Male Sterilization Investigator Team. J Urol. 1999;162:1621-1625.
31. Cook LA, Pun A, van Vliet H, et al: Scalpel versus no-scalpel incision for vasectomy, Cochrane Database Syst Rev CD004112, 2007.
32. Barone MA, Irsula B, Chen-Mok M, et al. Effectiveness of vasectomy using cautery. BMC Urol. 2004;4:10.
33. Sokal D, Irsula B, Chen-Mok M, et al. A comparison of vas occlusion techniques: cautery more effective than ligation and excision with fascial interposition. BMC Urol. 2004;4:12.
34. Shapiro EI, Silber SJ. Open-ended vasectomy, sperm granuloma, and postvasectomy orchialgia. Fertil Steril. 1979;32:546-550.
35. Errey BB, Edwards IS. Open-ended vasectomy: an assessment. Fertil Steril. 1986;45:843-846.
36. Moss WM. A comparison of open-end versus closed-end vasectomies: a report on 6220 cases. Contraception. 1992;46:521-525.
37. Berthelsen JG. Peroperative irrigation of the vas deferens during vasectomy. Scand J Urol Nephrol. 1976;10:100-102.
38. Leungwattanakij S, Lertsuwannaroj A, Ratana-Olarn K. Irrigation of the distal vas deferens during vasectomy: does it accelerate the post-vasectomy sperm-free rate? Int J Androl. 2001;24:241-245.
39. Mason RG, Dodds L, Swami SK. Sterile water irrigation of the distal vas deferens at vasectomy: does it accelerate clearance of sperm? a prospective randomized trial. Urology. 2002;59:424-427.
40. Pearce I, Adeyoju A, Bhatt RI, et al. The effect of perioperative distal vasal lavage on subsequent semen analysis after vasectomy: a prospective randomized controlled trial. BJU Int. 2002;90:282-285.
41. Eisner B, Schuster T, Rodgers P, et al. A randomized clinical trial of the effect of intraoperative saline perfusion on postvasectomy azoospermia. Ann Fam Med. 2004;2:221-223.
42. Albert PS, Seebode J. Nitrofurazone: vas irrigation as adjunct in vasectomy. Urology. 1977;10:450-451.
43. Edwards IS. Vasectomy: irrigation with euflavine. Med J Aust. 1977;1:847-849.
44. Gandrup P, Berthelsen JG, Nielsen OS. Irrigation during vasectomy: a comparison between sterile water and the spermicide euflavine. J Urol. 1982;127:60-61.
45. Henry Yu HY, Halim A, Evans PR. Chlorhexidine for irrigation of vas: a clinical trial and the study of viability of non-motile sperms in post-vasectomy patients with trypan blue uptake. Br J Urol. 1976;48:371-375.
46. Cook LA, Van Vliet H, Lopez LM, et al: Vasectomy occlusion techniques for male sterilization, Cochrane Database Syst Rev CD003991, 2007.
47. Wood BL, Doncel GF, Reddy PR, et al. Effect of diltiazem and methylene blue on human sperm motility, viability and cervical mucus penetration: potential use as vas irrigants at the time of vasectomy. Contraception. 2003;67:241-245.
48. Badrakumar C, Gogoi NK, Sundaram SK. Semen analysis after vasectomy: when and how many? BJU Int. 2000;86:479-481.
49. Griffin T, Tooher R, Nowakowski K, et al. How little is enough? The evidence for post-vasectomy testing. J Urol. 2005;174:29-36.
50. Labrecque M, Barone MA, Pile J, et al. Re: How little is enough? The evidence for post-vasectomy testing. J Urol. 2006;175:791-792.
51. Makhlouf AA, Niederberger CS. Ensuring vasectomy success: what is the standard? J Androl. 2006;27:637-640.
52. Barone MA, Nazerali H, Cortes M, et al. A prospective study of time and number of ejaculations to azoospermia after vasectomy by ligation and excision. J Urol. 2003;170:892-896.
53. Labrecque M, Hays M, Chen-Mok M, et al. Frequency and patterns of early recanalization after vasectomy. BMC Urol. 2006;6:25.
54. Deneux-Tharaux C, Kahn E, Nazerali H, et al. Pregnancy rates after vasectomy: a survey of US urologists. Contraception. 2004;69:401-406.
55. Philp T, Guillebaud J, Budd D. Complications of vasectomy: review of 16,000 patients. Br J Urol. 1984;56:745-748.
56. Rubin GL, Ory HW, Layde PM. The mortality risk of voluntary surgical contraception. Biomed Bull. 1982;3:1-5.
57. Peng XS, Li FD, Miao ZR, et al. Plasma reproductive hormones in normal and vasectomized Chinese males. Int J Androl. 1987;10:471-479.
58. Petitti DB. Epidemiologic studies of vasectomy. In: Zatuchni GI, Goldsmith A, Spieler JM, et al, editors. Male Contraception: Advances and Future Prospects. Philadelphia: Harper & Row; 1986:24-33.
59. Giovannucci E, Tosteson TD, Speizer FE, et al. A long-term study of mortality in men who have undergone vasectomy. N Engl J Med. 1992;326:1392-1398.
60. Nienhuis H, Goldacre M, Seagroatt V, et al. Incidence of disease after vasectomy: a record linkage retrospective cohort study. BMJ. 1992;304:743-746.
61. Moller H, Knudsen LB, Lynge E. Risk of testicular cancer after vasectomy: cohort study of over 73,000 men. BMJ. 1994;309:295-299.
62. Strader CH, Weiss NS, Daling JR. Vasectomy and the incidence of testicular cancer. Am J Epidemiol. 1988;128:56-63.
63. Giovannucci E, Tosteson TD, Speizer FE, et al. A retrospective cohort study of vasectomy and prostate cancer in US men. JAMA. 1993;269:878-882.
64. Giovannucci E, Ascherio A, Rimm EB, et al. A prospective cohort study of vasectomy and prostate cancer in US men. JAMA. 1993;269:873-877.
65. Borgmeier I, Holman CD. Does vasectomy reversal protect against prostate cancer? Ann Epidemiol. 2004;14:748-749.
66. Bernal-Delgado E, Latour-Perez J, Pradas-Arnal F, et al. The association between vasectomy and prostate cancer: a systematic review of the literature. Fertil Steril. 1998;70:191-200.
67. Dennis LK, Dawson DV, Resnick MI. Vasectomy and the risk of prostate cancer: a meta-analysis examining vasectomy status, age at vasectomy, and time since vasectomy. Prostate Cancer Prostatic Dis. 2002;5:193-203.
68. Cox B, Sneyd MJ, Paul C, et al. Vasectomy and risk of prostate cancer. JAMA. 2002;287:3110-3115.
69. Carbone DJJr, Shah A, Thomas AJJr, et al. Partial obstruction, not antisperm antibodies, causing infertility after vasovasostomy. J Urol. 1998;159:827-830.
70. Royle MG, Parslow JM, Kingscott MM, et al. Reversal of vasectomy: the effects of sperm antibodies on subsequent fertility. Br J Urol. 1981;53:654-659.
71. Holman CD, Wisniewski ZS, Semmens JB, et al. Population-based outcomes after 28,246 in-hospital vasectomies and 1,902 vasovasostomies in Western Australia. BJU Int. 2000;86:1043-1049.
72. Silber SJ, Grotjan HE. Microscopic vasectomy reversal 30 years later: a summary of 4010 cases by the same surgeon. J Androl. 2004;25:845-859.
73. Hsieh MH, Meng MV, Turek PJ. Markov modeling of vasectomy reversal and ART for infertility: how do obstructive interval and female partner age influence cost effectiveness? Fertil Steril. 2007;88:840-846.
74. Kuang W, Shin PR, Oder M, et al. Robotic-assisted vasovasostomy: a two-layer technique in an animal model. Urology. 2005;65:811-814.
75. Schiff J, Li PS, Goldstein M. Robotic microsurgical vasovasostomy and vasoepididymostomy in rats. Int J Med Robot. 2005;1:122-126.
76. Witt MA, Heron S, Lipshultz LI. The post-vasectomy length of the testicular vasal remnant: a predictor of surgical outcome in microscopic vasectomy reversal. J Urol. 1994;151:892-894.
77. Labrecque M, Hoang DQ, Turcot L. Association between the length of the vas deferens excised during vasectomy and the risk of postvasectomy recanalization. Fertil Steril. 2003;79:1003-1007.
78. Gerrard ERJr, Sandlow JI, Oster RA, et al. Effect of female partner age on pregnancy rates after vasectomy reversal. Fertil Steril. 2007;87:1340-1344.
79. Shiraishi K, Takihara H, Naito K. Quantitative analysis of testicular interstitial fibrosis after vasectomy in humans. Aktuelle Urol. 2003;34:262-264.
80. Raleigh D, O’Donnell L, Southwick GJ, et al. Stereological analysis of the human testis after vasectomy: impairment of spermatogenic efficiency with increasing obstructive interval. Fertil Steril. 2004;81:1595-1603.
81. Silber SJ. Epididymal extravasation following vasectomy as a cause for failure of vasectomy reversal. Fertil Steril. 1979;31:309-315.
82. Andonian S, Jarvi K, Zini A, et al. Ultrastructural features of the vas deferens from patients undergoing vasectomy and vasectomy reversal. J Androl. 2002;23:691-701.
83. Jarow JP, Budin RE, Dym M, et al. Quantitative pathologic changes in the human testis after vasectomy. A controlled study. N Engl J Med. 1985;313:1252-1256.
84. Shiraishi K, Takihara H, Naito K. Influence of interstitial fibrosis on spermatogenesis after vasectomy and vasovasostomy. Contraception. 2002;65:245-249.
85. Pavlovich CP, Schlegel PN. Fertility options after vasectomy: a cost-effectiveness analysis. Fertil Steril. 1997;67:133-141.
86. Heidenreich A, Altmann P, Engelmann UH. Microsurgical vasovasostomy versus microsurgical epididymal sperm aspiration/testicular extraction of sperm combined with intracytoplasmic sperm injection. A cost-benefit analysis. Eur Urol. 2000;37:609-614.
87. Zhang GY, Wang XH, Chen ZW, et al. Research on male contraception in China. In: Waites GMH, Frick J, Baker GWH, editors. Current Advances in Andrology. Bologna: Monduzzi Editore; 1997:233-239.
88. Zhao S-C. Vas deferens occlusion by percutaneous injection of polyurethane elastomer plugs: clinical experience and reversibility. Contraception. 1990;41:453-459.
89. Soebadi DM, Gardjito W, Mensink HJ. Intravasal injection of formed-in-place medical grade silicone rubber for vas occlusion. Int J Androl. 1995;18(Suppl 1):45-52.
90. Zambon JV, Barone MA, Pollack AE, et al. Efficacy of percutaneous vas occlusion compared with conventional vasectomy. BJU Int. 2000;86:699-705.
91. Song L, Gu Y, Lu W, et al. A phase II randomized controlled trial of a novel male contraception, an intra-vas device. Int J Androl. 2006;29:489-495.
92. Guha SK, Singh G, Ansari S, et al. Phase II clinical trial of a vas deferens injectable contraceptive for the male. Contraception. 1997;56:245-250.
93. Guha SK. Biophysical mechanism-mediated time-dependent effect on sperm of human and monkey vas implanted polyelectrolyte contraceptive. Asian J Androl. 2007;9:221-227.
94. Moore CR, Oslund R. Experiments on the sheep testis: Cryptorchidism, vasectomy and scrotal insulation. Am J Physiol. 1924;67:595-607.
95. Kandeel FR, Swerdloff RS. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril. 1988;49:1-23.
96. Thonneau P, Bujan L, Multigner L, et al. Occupational heat exposure and male fertility: a review. Hum Reprod. 1998;13:2122-2125.
97. Mieusset R, Bujan L. The potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Int J Androl. 1994;17:186-191.
98. Wang C, McDonald V, Leung A, et al. Effect of increased scrotal temperature on sperm production in normal men. Fertil Steril. 1997;68:334-339.
99. Setchell BP. The Parkes Lecture. Heat and the testis. J Reprod Fertil. 1998;114:179-194.
100. Baskin MJ. Temporary sterilization by injection of human spermatozoa: a preliminary report. Am J Obstet Gynecol. 1932;24:892-897.
101. Naz RK, Rajesh C. Passive immunization for immunocontraception: lessons learned from infectious diseases. Front Biosci. 2004;9:2457-2465.
102. McLaughlin EA, Holland MK, Aitken RJ. Contraceptive vaccines. Expert Opin Biol Ther. 2003;3:829-841.
103. Ford WCL, Waites GMH. Chlorinated sugars: a biochemical approach to the control of male fertility. Int J Androl Suppl. 1978;2:541-564.
104. van der Spoel AC, Jeyakumar M, Butters TD, et al. Reversible infertility in male mice after oral administration of alkylated imino sugars: a nonhormonal approach to male contraception. Proc Natl Acad Sci U S A. 2002;99:17173-17178.
105. Suganuma R, Walden CM, Butters TD, et al. Alkylated imino sugars, reversible male infertility-inducing agents, do not affect the genetic integrity of male mouse germ cells during short-term treatment despite induction of sperm deformities. Biol Reprod. 2005;72:805-813.
106. Walden CM, Butters TD, Dwek RA, et al. Long-term non-hormonal male contraception in mice using N-butyldeoxynojirimycin. Hum Reprod. 2006;21:1309-1315.
107. Bone W, Walden CM, Fritsch M, et al. The sensitivity of murine spermiogenesis to miglustat is a quantitative trait: a pharmacogenetic study. Reprod Biol Endocrinol. 2007;5:1.
108. Amory JK, Muller CH, Page ST, et al. Miglustat has no apparent effect on spermatogenesis in normal men. Hum Reprod. 2007;22:702-707.
109. Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41-s49.
110. Vickery BH, Grigg MB, Goodpasture JC, et al. Towards a same-day, orally administered male contraceptive. In: Zatuchni GI, Goldsmith A, Spieler JM, et al, editors. Male Contraception Advances and Future Prospects. Philadelphia: Harper & Row; 1986:271-292.
111. Giwercman A, Skakkebaek NE. The effect of salicylazosulphapyridine (sulphasalazine) on male fertility. A review. Int J Androl. 1986;9:38-52.
112. Kjaergaard N, Kjaergaard B, Lauritsen JG. Prazosin, an adrenergic blocking agent inadequate as male contraceptive pill. Contraception. 1988;37:621-629.
113. Wu D. An overview of the clinical pharmacology and therapeutic potential of gossypol as a male contraceptive agent and in gynaecological disease. Drugs. 1989;38:333-341.
114. Waites GMH, Wang C, Griffin PD. Gossypol: reasons for its failure to be accepted as a safe, reversible male antifertility agent. Int J Androl. 1998;21:8-12.
115. Huynh PN, Hikim AP, Wang C, et al. Long-term effects of triptolide on spermatogenesis, epididymal sperm function, and fertility in male rats. J Androl. 2000;21:689-699.
116. Breton S, Smith PJ, Lui B, et al. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med. 1996;2:470-472.
117. Ren D, Navarro B, Perez G, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603-609.
118. Liu J, Xia J, Cho KH, et al. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem. 2007;282:18945-18952.
119. Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci U S A. 2007;104:7688-7692.
120. Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737-740.
121. Carlson AE, Westenbroek RE, Quill T, et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A. 2003;100:14864-14868.
122. Quill TA, Ren D, Clapham DE, et al. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci U S A. 2001;98:12527-12531.
123. Qi H, Moran MM, Navarro B, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A. 2007;104:1219-1223.
124. Esposito G, Jaiswal BS, Xie F, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A. 2004;101:2993-2998.
125. Xie F, Garcia MA, Carlson AE, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353-362.
126. Sanchez G, Nguyen AN, Timmerberg B, et al. The Na,K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol Hum Reprod. 2006;12:565-576.
127. Mulryan K, Gitterman DP, Lewis CJ, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86-89.
128. O’Rand MG, Widgren EE, Wang Z, et al. Eppin: an epididymal protease inhibitor and a target for male contraception. Soc Reprod Fertil Suppl. 2007;63:445-453.
129. Wolgemuth DJ, Chung SS. Retinoid signaling during spermatogenesis as revealed by genetic and metabolic manipulations of retinoic acid receptor alpha. Soc Reprod Fertil Suppl. 2007;63:11-23.
130. Chung SS, Wang X, Wolgemuth DJ. Male sterility in mice lacking retinoic acid receptor alpha involves specific abnormalities in spermiogenesis. Differentiation. 2005;73:188-198.
131. Aaltonen P, Amory JK, Anderson RA, et al. 10th Summit Meeting consensus: recommendations for regulatory approval for hormonal male contraception. J Androl. 2007;28:362-363.
132. Pangkahila W. Reversible azoospermia induced by an androgen-progestagen combination regimen in Indonesian men. Int J Androl. 1991;44:248-256.
133. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Comparison of two androgens plus depot-medroxyprogesterone acetate for suppression to azoospermia in Indonesian men. Fertil Steril. 1993;60:1062-1068.
134. Gu YQ, Wang XH, Xu D, et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab. 2003;88:562-568.
135. Turner L, Conway AJ, Jimenez M, et al. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab. 2003;88:4659-4667.
136. Steiner MJ, Hertz-Picciotto I, Schulz KF, et al. Measuring true contraceptive efficacy. A randomized approach: Condom vs. spermicide vs. no method. Contraception. 1998;58:375-378.
137. Grimes DA, Lopez LM, Gallo MF, et al: Steroid hormones for contraception in men, Cochrane Database Syst Rev CD004316, 2007.
138. Heinemann K, Saad F, Wiesemes M, et al. Attitudes toward male fertility control: results of a multinational survey on four continents. Hum Reprod. 2005;20:549-556.
139. Heinemann K, Saad F, Wiesemes M, et al. Expectations toward a novel male fertility control method and potential user types: results of a multinational survey. J Androl. 2005;26:155-162.
140. Martin CW, Anderson RA, Cheng L, et al. Potential impact of hormonal male contraception: Cross-cultural implications for development of novel preparations. Hum Reprod. 2000;15:637-645.
141. Weston GC, Schlipalius ML, Bhuinneanin MN, et al. Will Australian men use male hormonal contraception? Med J Aust. 2002;176:208-210.
142. Glasier AF, Anakwe R, Everington D, et al. Would women trust their partners to use a male pill? Hum Reprod. 2000;15:646-649.
143. Meriggiola MC, Cerpolini S, Bremner WJ, et al. Acceptability of an injectable male contraceptive regimen of norethisterone enanthate and testosterone undecanoate for men. Hum Reprod. 2006;21:2033-2040.
144. Amory JK, Page ST, Anawalt BD, et al. Acceptability of a combination testosterone gel and depomedroxyprogesterone acetate male contraceptive regimen. Contraception. 2007;75:218-223.
145. Heckel NJ. Production of oligospermia in a man by the use of testosterone propionate. Proc Soc Exp Biol Med. 1939;40:658-659.
146. McCullagh EP, McGurl FJ. The effects of testosterone propionate on epiphyseal closure, sodium and chloride balance and on sperm counts. Endocrinology. 1940;26:377-384.
147. Heller CG, Nelson WO, Hill IB, et al. Improvement in spermatogenesis following depression of the human testis with testosterone. Fertil Steril. 1950;1:415-422.
148. Heckel NJ, Rosso WA, Kestel L. Spermatogenic rebound phenomenon after administration of testosterone propionate. J Clin Endocrinol Metab. 1951;11:235-245.
149. Reddy PRK, Rao JM. Reversible antifertility action of testosterone propionate in human males. Contraception. 1972;5:295-301.
150. Mauss J, Borsch G, Richter E, et al. Investigations on the use of testosterone enanthate as a male contraceptive agent. A preliminary report. Contraception. 1974;10:281-289.
151. Patanelli DJ, editor. Hormonal Control of Fertility. Washington, DC: U.S. Department of Health, Education and Welfare, 1977. (DHEW Pub. No. 78-1097)
152. Matsumoto AM. Effects of chronic testosterone administration in normal men: Safety and efficacy of high dosage testosterone and parallel dose-dependent suppression of luteinizing hormone, follicle-stimulating hormone and sperm production. J Clin Endocrinol Metab. 1990;70:282-287.
153. Cunningham GR, Silverman VE, Thornby J, et al. The potential for an androgen male contraceptive. J Clin Endocrinol Metab. 1979;49:520-526.
154. Steinberger E, Smith KD, Rodriguez-Rigau LJ. Suppression and recovery of sperm production in men treated with testosterone enanthate for one year. A study of a possible reversible male contraceptive. Int J Androl Suppl. 1978;2:748-760.
155. Swerdloff RS, Palacios A, McClure RD, et al. Male contraception: Clinical assessment of chronic administration of testosterone enanthate. Int J Androl Suppl. 1978;2:731-747.
156. Paulsen CA. Male contraceptive development: Re-examination of testosterone enanthate as an effective single entity agent. In: Patanelli DJ, editor. Hormonal Control of Male Fertility. Washington, DC: Department of Health Education and Welfare; 1978:17-40.
157. Handelsman DJ, Farley TMM, Peregoudov A, et al. Factors in nonuniform induction of azoospermia by testosterone enanthate in normal men. Fertil Steril. 1995;63:125-133.
158. McLachlan RI, O’Donnell L, Meachem SJ, et al. Hormonal regulation of spermatogenesis in primates and man: Insights for development of the male hormonal contraceptive. J Androl. 2002;23:149-162.
159. Nieschlag E, Zitzmann M, Kamischke A. Use of progestins in male contraception. Steroids. 2003;68:965-972.
160. McLachlan RI, Robertson DM, Pruysers E, et al. Relationship between serum gonadotropins and spermatogenic suppression in men undergoing steroidal contraceptive treatment. J Clin Endocrinol Metab. 2004;89:142-149.
161. Matthiesson KL, McLachlan RI, O’Donnell L, et al. The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J Clin Endocrinol Metab. 2006;91:3962-3969.
162. Weinbauer GF, Schlatt S, Walter V, et al. Testosterone-induced inhibition of spermatogenesis is more closely related to suppression of FSH than to testicular androgen levels in the cynomolgus monkey model (Macaca fascicularis). J Endocrinol. 2001;168:25-38.
163. Narula A, Gu YQ, O’Donnell L, et al. Variability in sperm suppression during testosterone administration to adult monkeys is related to follicle stimulating hormone suppression and not to intratesticular androgens. J Clin Endocrinol Metab. 2002;87:3399-3406.
164. Santner S, Albertson B, Zhang GY, et al. Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. J Clin Endocrinol Metab. 1998;83:2104-2109.
165. Jin B, Turner L, Zhou Z, et al. Ethnicity and migration as determinants of human prostate size. J Clin Endocrinol Metab. 1999;84:3613-3619.
166. Yu B, Handelsman DJ. Pharmacogenetic polymorphisms of the AR and metabolism and susceptibility to hormone-induced azoospermia. J Clin Endocrinol Metab. 2001;86:4406-4411.
167. Eckardstein SV, Schmidt A, Kamischke A, et al. CAG repeat length in the androgen receptor gene and gonadotrophin suppression influence the effectiveness of hormonal male contraception. Clin Endocrinol (Oxf). 2002;57:647-655.
168. Johnson L, Barnard JJ, Rodriguez L, et al. Ethnic differences in testicular structure and spermatogenic potential may predispose testes of Asian men to a heightened sensitivity to steroidal contraceptives. J Androl. 1998;19:348-357.
169. Veldhuis JD, Bae A, Swerdloff RS, et al. Experimentally induced androgen depletion accentuates ethnicity-related contrasts in luteinizing hormone secretion in Asian and Caucasian men. J Clin Endocrinol Metab. 2005;90:1632-1638.
170. Coviello AD, Bremner WJ, Matsumoto AM, et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl. 2004;25:931-938.
171. Matthiesson KL, Amory JK, Berger R, et al. Novel male hormonal contraceptive combinations: the hormonal and spermatogenic effects of testosterone and levonorgestrel combined with a 5alpha-reductase inhibitor or gonadotropin-releasing hormone antagonist. J Clin Endocrinol Metab. 2005;90:91-97.
172. Page ST, Kalhorn TF, Bremner WJ, et al. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J Androl. 2007;28:734-741.
173. Handelsman DJ, Conway AJ, Howe CJ, et al. Establishing the minimum effective dose and additive effects of depot progestin in suppression of human spermatogenesis by a testosterone depot. J Clin Endocrinol Metab. 1996;81:4113-4121.
174. Amory JK, Coviello AD, Page ST, et al. Serum 17-hydroxyprogesterone strongly correlates with intratesticular testosterone in gonadotropin-suppressed normal men receiving various dosages of human chorionic gonadotropin. Fertil Steril. 2008;89:380-386.
175. Amory JK, Page ST, Anawalt BD, et al. Elevated end-of-treatment serum INSL3 is associated with failure to completely suppress spermatogenesis in men receiving male hormonal contraception. J Androl. 2007;28:548-554.
176. Ly LP, Liu PY, Handelsman DJ. Rates of suppression and recovery of human sperm output in testosterone-based hormonal contraceptive regimens. Hum Reprod. 2005;20:1733-1740.
177. Wu FCW, Farley TMM, Peregoudov A, et al. Effects of testosterone enanthate in normal men: experience from a multicenter contraceptive efficacy study. Fertil Steril. 1996;65:626-636.
178. Mackey MA, Conway AJ, Handelsman DJ. Tolerability of intramuscular injections of testosterone ester in an oil vehicle. Hum Reprod. 1995;10:862-865.
179. McLachlan RI, O’Donnell L, Stanton PG, et al. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546-556.
180. Meriggiola MC, Costantino A, Bremner WJ, et al. Higher testosterone dose impairs sperm suppression induced by a combined androgen-progestin regimen. J Androl. 2002;23:684-690.
181. Behre HM, Nieschlag E. Comparative pharmacokinetics of testosterone esters. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. ed 2. Berlin: Springer; 1998:329-348.
182. Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab. 1990;71:216-222.
183. Amory JK, Anawalt BD, Blaskovich PD, et al. Testosterone release from a subcutaneous, biodegradable microcapsule formulation (Viatrel) in hypogonadal men. J Androl. 2002;23:84-91.
184. Behre HM, Baus S, Kliesch S, et al. Potential of testosterone buciclate for male contraception: endocrine differences between responders and nonresponders. J Clin Endocrinol Metab. 1995;80:2394-2403.
185. Handelsman DJ, Conway AJ, Boylan LM. Suppression of human spermatogenesis by testosterone implants in man. J Clin Endocrinol Metab. 1992;75:1326-1332.
186. McCullagh EP, Rossmiller HR: Methyl testosterone. I androgenic effects and production of gynecomastia and oligospermia, J Clin Endocrinol Metab 1:1941.
187. Jones TM, Fang VS, Landau RL, et al. The effects of fluoxymesterone administration on testicular function. J Clin Endocrinol Metab. 1977;44:121-129.
188. Holma PK. Effects of an anabolic steroid (metandienone) on spermatogenesis. Contraception. 1977;15:151-162.
189. Skoglund RD, Paulsen CA. Danazol-testosterone combination: a potentially effective means for reversible male contraception: A preliminary report. Contraception. 1973;7:357-365.
190. Sherins RJ, Gandy HM, Thorslund TW, et al. Pituitary and testicular function studies. I. Experience with a new gonadal inhibitor, 17a-pregn-4-en-20-yno-(2,3-d) isoxazol-17-ol (Danazol). J Clin Endocrinol Metab. 1971;32:522-531.
191. Ishak KG, Zimmerman HJ. Hepatotoxic effects of the anabolic-androgenic steroids. Semin Liver Dis. 1987;7:230-236.
192. Knuth UA, Maniera H, Nieschlag E. Anabolic steroids and semen parameters in bodybuilders. Fertil Steril. 1989;52:1041-1047.
193. Knuth UA, Behre H, Belkien L, et al. Clinical trial of 19-nortestosterone hexoxyphenylpropionate (Anadur) for male fertility regulation. Fertil Steril. 1985;44:814-821.
194. Schurmeyer T, Knuth UA, Belkein L, et al. Reversible azoospermia induced by the anabolic steroid 19-nortestosterone. Lancet. 1984;1:417-420.
195. Schellen TNCM, Beek JMJHA. The influence of high doses of mesterolone on the spermiogram. Fertil Steril. 1972;23:712-714.
196. Behre HM, Kliesch S, Lemcke B, et al. Suppression of spermatogenesis to azoospermia by combined administration of GnRH antagonist and 19-nortestosterone cannot be maintained by this non-aromatizable androgen alone. Hum Reprod. 2001;16:2570-2577.
197. Swerdloff RS, Bagatell CJ, Wang C, et al. Suppression of spermatogenesis in man induced by Nal-Glu gonadotropin releasing hormone antagonist and testosterone enanthate (TE) in maintained by TE alone. J Clin Endocrinol Metab. 1998;83:3527-3533.
198. Sundaram K, Kumar N. 7alpha-methyl-19-nortestosterone (MENT): the optimal androgen for male contraception and replacement therapy. Int J Androl. 2000;23(Suppl 2):13-15.
199. Cummings DE, Kumar N, Bardin CW, et al. Prostate-sparing effects in primates of the potent androgen 7alpha-methyl-19-nortestosterone: a potential alternative to testosterone for androgen replacement and male contraception. J Clin Endocrinol Metab. 1998;83:4212-4219.
200. Anderson RA, Wallace AM, Sattar N, et al. Evidence for tissue selectivity of the synthetic androgen 7 alpha-methyl-19-nortestosterone in hypogonadal men. J Clin Endocrinol Metab. 2003;88:2784-2793.
201. Walton MJ, Kumar N, Baird DT, et al. 7alpha-methyl-19-nortestosterone (MENT) vs testosterone in combination with etonogestrel implants for spermatogenic suppression in healthy men. J Androl. 2007;28:679-688.
202. Avery MA, Tanabe M, Crowe DF, et al. Synthesis and testing of 17ab-hydroxy-7a methyl-D-homoestra-4,16-dien-3-one: a highly potent orally active androgen. Steroids. 1990;55:59-64.
203. Grootenhuis AJ, de Gooyer ME, Louw J, et al. Synthesis and pharmacological profiling of new orally active steroidal androgens. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. ed 3. Berlin: Springer-Verlag; 2004:665-684.
204. Chen J, Hwang DJ, Bohl CE, et al. A selective androgen receptor modulator for hormonal male contraception. J Pharmacol Exp Ther. 2005;312:546-553.
205. Prasad MRN, Singh SP, Rajalakshmi M. Fertility control in male rats by continuous release of microquantities of cyproterone acetate from subcutaneous Silastic capsules. Contraception. 1970;2:165-178.
206. Wang C, Yeung KK. Use of low-dosage oral cyproterone acetate as a male contraceptive. Contraception. 1980;21:245-272.
207. Chandolia RK, Weinbauer GF, Simoni M, et al. Comparative effects of chronic administration of the non-steroidal antiandrogens flutamide and Casodex on the reproductive system of the male rat. Acta Endocrinol (Copenh). 1991;125:547-555.
208. Dhar JD, Sety BS. Effect of a nonsteroidal antiandrogen, anadron, on the reproductive system and fertility in male rats. Contraception. 1990;42:121-138.
209. McLachlan RI, McDonald J, Rushford D, et al. Efficacy and acceptability of testosterone implants, alone or in combination with a 5alpha-reductase inhibitor, for male hormonal contraception. Contraception. 2000;62:73-78.
210. Kinniburgh D, Anderson RA, Baird DT. Suppression of spermatogenesis with desogestrel and testosterone pellets is not enhanced by addition of finasteride. J Androl. 2001;22:88-95.
211. Killian J, Pratis K, Clifton RJ, et al. 5alpha-reductase isoenzymes 1 and 2 in the rat testis during postnatal development. Biol Reprod. 2003;68:1711-1718.
212. Amory JK, Kalhorn TF, Page ST. Pharmacokinetics and pharmacodynamics of oral testosterone enanthate plus dutasteride for four weeks in normal men: Implications for male hormonal contraception. J Androl. 2008;29:260-271.
213. Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183-217.
214. Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313-340.
215. Atkinson LE, Chang YL, Snyder PJ. Long-term experience with testosterone replacement through scrotal skin. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. ed 2. Berlin: Springer; 1998:364-388.
216. Handelsman DJ. The safety of androgens: prostate and cardiovascular disease. In: Wang C, editor. Male Reproductive Function. Boston: Kluwer; 1998:173-190.
217. Wu JP, Gu FL. The prostate 41–65 years post castration. Chin Med J (Engl). 1987;100:271-272.
218. Imperato-McGinley J, Gautier T, Zirinsky K, et al. Prostate visualization studies in males homozygous and heterozygous for 5α-reductase deficiency. J Clin Endocrinol Metab. 1992;75:1022-1026.
219. Quigley CA, DeBellis A, Marschke KB, et al. Androgen receptor defects: Historical, clinical and molecular perspectives. Endocr Rev. 1995;16:271-321.
220. Roddam AW, Allen NE, Appleby P, et al. Endogenous sex hormones and prostate cancer: A collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170-183.
221. Zitzmann M, Depenbusch M, Gromoll J, et al. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab. 2003;88:2049-2054.
222. Nelson KA, Witte JS. Androgen receptor CAG repeats and prostate cancer. Am J Epidemiol. 2002;155:883-890.
223. Behre HM, Bohmeyer J, Nieschlag E. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol (Oxf). 1994;40:341-349.
224. Meikle AW, Arver S, Dobs AS, et al. Prostate size in hypogonadal men treated with a nonscrotal permeation-enhanced testosterone transdermal system. Urology. 1997;49:191-196.
225. Jin B, Conway AJ, Handelsman DJ. Effects of androgen deficiency and replacement on prostate zonal volumes. Clin Endocrinol (Oxf). 2001;54:437-445.
226. Jin B, Turner L, Walters WAW, et al. Androgen or estrogen effects on the human prostate. J Clin Endocrinol Metab. 1996;81:4290-4295.
227. Archer J. The influence of testosterone on human aggression. Br J Psychiatry. 1991;82:1-28.
228. Anderson RA, Bancroft J, Wu FCW. The effects of exogenous testosterone on sexuality and mood of normal men. J Clin Endocrinol Metab. 1992;75:1503-1507.
229. Christiansen K. Behavioural correlates of testosterone. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. ed 2. Berlin: Springer; 1998:107-142.
230. O’Connor DB, Archer J, Wu FC. Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over study. J Clin Endocrinol Metab. 2004;89:2837-2845.
231. Pope HGJr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000;57:133-140.
232. Bahrke MS, Yesalis CE, Wright JE. Psychological and behavioural effects of endogenous testosterone levels and anabolic-androgenic steroids among male. A review. Sports Med. 1990;10:303-337.
233. Bebb RA, Anawalt BD, Christensen RB, et al. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab. 1996;81:757-762.
234. Meriggiola MC, Bremner WJ, Paulsen CA, et al. A combined regimen of cyproterone acetate and testosterone enanthate as a potentially highly effective male contraceptive. J Clin Endocrinol Metab. 1996;81:3018-3023.
235. Bouchard P, Garcia E. Influence of testosterone substitution on sperm suppression by LHRH agonists. Hormone Res. 1987;28:175-180.
236. Behre HM, Nashan D, Hubert W, et al. Depot gonadotropin-releasing hormone agonist blunts the androgen-induced suppression of spermatogenesis in a clinical trial of male contraception. J Clin Endocrinol Metab. 1992;74:84-90.
237. Handelsman DJ, Spaliviero JA, Simpson JM, et al. Spermatogenesis without gonadotropins: maintenance has a lower testosterone threshold than initiation. Endocrinology. 1999;140:3938-3946.
238. Garcia MA, Meizel S. Progesterone-mediated calcium influx and acrosome reaction of human spermatozoa: pharmacological investigation of T-type calcium channels. Biol Reprod. 1999;60:102-109.
239. Losel RM, Falkenstein E, Feuring M, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965-1016.
240. Luetjens CM, Didolkar A, Kliesch S, et al. Tissue expression of the nuclear progesterone receptor in male non-human primates and men. J Endocrinol. 2006;189:529-539.
241. Heller CG, Moore DJ, Paulsen CA, et al. Effects of progesterone and synthetic progestins on the reproductive physiology of normal men. Fed Proc. 1959;18:1057-1064.
242. Frick J, Danner C, Joos H, et al. Spermatogenesis in men treated with subcutaneous application of levonorgestrel and estrone rods. J Androl. 1981;2:331-338.
243. Junaidi A, Luetjens CM, Wistuba J, et al. Norethisterone enanthate has neither a direct effect on the testis nor on the epididymis: a study in adult male cynomolgus monkeys (Macaca fascicularis). Eur J Endocrinol. 2005;152:655-661.
244. Schearer SB, Alvarez-Sanchez F, Anselmo J, et al. Hormonal contraception for men. Int J Androl Suppl. 1978;2:680-712.
245. Wu FCW, Aitken RJ. Suppression of sperm function by depot medroxyprogesterone acetate and testosterone enanthate in steroid male contraception. Fertil Steril. 1989;51:691-698.
246. Foegh M. Evaluation of steroids as contraceptives in men. Acta Endocrinol Suppl (Copenh). 1983;260:3-48.
247. Anawalt BD, Bebb RA, Bremner WJ, et al. A lower dosage levonorgestrel and testosterone combination effectively suppresses spermatogenesis and circulating gonadotropin levels with fewer metabolic effects than higher dosage combinations. J Androl. 1999;20:407-414.
248. Lobel B, Olivo JF, Guille F, et al. Contraception in men: efficacy and immediate toxicity, a study of 18 cases. Acta Urol Belg. 1989;57:117-124.
249. Guerin JF, Rollet J. Inhibition of spermatogenesis in men using various combinations of oral progestagens and percutaneous or oral androgens. Int J Androl. 1988;11:187-199.
250. Meriggiola MC, Bremner WJ, Constantino A, et al. Low dose of cyproterone acetate and testosterone enanthate for contraception. Hum Reprod. 1998;13:1225-1229.
251. Meriggiola MC, Pavani A, Bremner WJ, et al. An oral regimen of cyproterone acetate and testosterone undecanoate for spermatogenic suppression in men. Fertil Steril. 1997;68:844-850.
252. Gonzalo IT, Swerdloff RS, Nelson AL, et al. Levonorgestrel implants (Norplant II) for male contraception clinical trials: combination with transdermal and injectable testosterone. J Clin Endocrinol Metab. 2002;87:3562-3572.
253. Gui YL, He CH, Amory JK, et al. Male hormonal contraception: suppression of spermatogenesis by injectable testosterone undecanoate alone or with levonorgestrel implants in Chinese men. J Androl. 2004;25:720-727.
254. Wang C, Wang XH, Nelson AL, et al. Levonorgestrel implants enhanced the suppression of spermatogenesis by testosterone implants: comparison between Chinese and non-Chinese men. J Clin Endocrinol Metab. 2006;91:460-470.
255. Anderson RA, Kinniburgh D, Baird DT. Suppression of spermatogenesis by etonogestrel implants with depot testosterone: Potential for long-acting male contraception. J Clin Endocrinol Metab. 2002;87:3640-3649.
256. Brady BM, Amory JK, Perheentupa A, et al. A multicentre study investigating subcutaneous etonogestrel implants with injectable testosterone decanoate as a potential long-acting male contraceptive. Hum Reprod. 2006;21:285-294.
257. Gu YQ, Tong JS, Ma DZ, et al. Male hormonal contraception: effects of injections of testosterone undecanoate and depot medroxyprogesterone acetate at eight-week intervals in Chinese men. J Clin Endocrinol Metab. 2004;89:2254-2262.
258. Page ST, Amory JK, Anawalt BD, et al. Testosterone gel combined with depomedroxyprogesterone acetate is an effective male hormonal contraceptive regimen and is not enhanced by the addition of a GnRH antagonist. J Clin Endocrinol Metab. 2006;91:4374-4380.
259. Kamischke A, Heuermann T, Kruger K, et al. An effective hormonal male contraceptive using testosterone undecanoate with oral or injectable norethisterone preparations. J Clin Endocrinol Metab. 2002;87:530-539.
260. Meriggiola MC, Costantino A, Saad F, et al. Norethisterone enanthate plus testosterone undecanoate for male contraception: Effects of various injection intervals on spermatogenesis, reproductive hormones, testis, and prostate. J Clin Endocrinol Metab. 2005;90:2005-2014.
261. Ewing LL, Cochran RC, Adams RJ, et al. Testis function in rhesus monkeys treated with a contraceptive steroid formulation. Contraception. 1983;27:347-362.
262. Lobl TJ, Kirton KT, Forbes AD, et al. Contraceptive efficacy of testosterone-estradiol implants in male rhesus monkeys. Contraception. 1983;27:383-389.
263. Handelsman DJ, Wishart S, Mackey MA, et al: Efficacy and safety of low-dose estradiol supplementation to augment depot testosterone-induced suppression of human spermatogenesis. Paper presented at the Annual Scientific Meeting of the Endocrine Society of Australia, 1998, Perth, Australia.
264. Lunn SF, Dixson AF, Sandow J, et al. Pituitary-testicular function is suppressed by an LHRH antagonist but not by an LHRH agonist in the marmoset monkey. J Endocrinol. 1990;125:233-239.
265. Marshall GF, Akhtar FB, Weinbauer GF, et al. Gonadotrophin-releasing hormone (GnRH) overcomes GnRH antagonist-induced suppression of LH secretion in primates. J Endocrinol. 1986;110:145-150.
266. Herbst KL, Coviello AD, Page S, et al. A single dose of the potent gonadotropin-releasing hormone antagonist acyline suppresses gonadotropins and testosterone for 2 weeks in healthy young men. J Clin Endocrinol Metab. 2004;89:5959-5965.
267. Weinbauer GF, Surmann FJ, Nieschlag E. Suppression of spermatogenesis in a non-human primate (Macaca fascicularis) by concomitant gonadotrophin-releasing hormone antagonist and testosterone treatment. Acta Endocr. 1987;114:138-146.
268. Weinbauer GF, Khurshid S, Findscheidt U, et al. Sustained inhibition of sperm production and inhibin secretion by a gonadotrophin-releasing hormone antagonist and delayed testosterone substitution in non-human primates (Macaca fascicularis). Acta Endocrinol (Copenh). 1989;123:303-310.
269. Bremner WJ, Bagatell CJ, Steiner RA. Gonadotropin-releasing hormone antagonist plus testosterone: a potential male contraceptive. J Clin Endocrinol Metab. 1991;73:465-469.
270. Tom L, Bhasin S, Salameh W, et al. Induction of azoospermia in normal men with combined Nal-Glu GnRH antagonist and testosterone enanthate. J Clin Endocrinol Metab. 1992;75:476-483.
271. Pavlou SN, Brewer K, Farley MG, et al. Combined administration of a gonadotropin-releasing hormone antagonist and testosterone in men induces reversible azoospermia without loss of libido. J Clin Endocrinol Metab. 1991;73:1360-1369.
272. Bagatell CJ, Matsumoto AM, Christensen RB, et al. Comparison of a gonadotropin-releasing hormone antagonist plus testosterone (T) versus T alone as a potential male contraceptive. J Clin Endocrinol Metab. 1993;77:427-432.
273. Simms MS, Scholfield DP, Jacobs E, et al. Anti-GnRH antibodies can induce castrate levels of testosterone in patients with advanced prostate cancer. Br J Cancer. 2000;83:443-446.
274. Talwar GP. Fertility regulating and immunotherapeutic vaccines reaching human trials stage. Hum Reprod Update. 1997;3:301-310.
275. Naz RK. Contraceptive vaccines. Drugs. 2005;65:593-603.
276. Naz RK, Gupta SK, Gupta JC, et al. Recent advances in contraceptive vaccine development: a mini-review. Hum Reprod. 2005;20:3271-3283.
277. Delves PJ, Roitt IM. Vaccines for the control of reproduction–status in mammals, and aspects of comparative interest. Dev Biol (Basel). 2005;121:265-273.
278. Burger HG. Inhibin. Reprod Med Rev. 1992;1:1-20.
279. Wiebe JP, Wood PH. Selective suppression of follicle-stimulating hormone by 3-alpha-hydroxy-4-pregnen-20-one, a steroid found in Sertoli cells. Endocrinology. 1987;120:2259-2264.
280. Moudgal NR, Jeyakumar M, Krishnamurthy HN, et al. Development of male contraceptive vaccine: A perspective. Hum Reprod Update. 1997;3:335-346.
281. Leng N, Grasso P, Reichert LE. D-amino acid substitution of residues 32 to 46 of the glycoprotein hormone common alpha-subunit: development of a synthetic glycoprotein hormone antagonist. Pept Res. 1996;9:188-194.
282. Singh J, O’Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136:5311-5321.
283. Kumar TR, Wang Y, Lu N, et al. FSH is required for ovarian follicle maturation but not for male fertility. Nat Genet. 1997;15:201-204.
284. Dierich A, Sairam MR, Monaco L, et al. Impairing follicle-stimulating hormone (FSH) signalling in-vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612-13617.
285. Tapanainen JS, Aittomaki K, Min J, et al. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205-206.
286. Nieschlag E. Reasons for abandoning immunization against FSH as an approach to male fertility regulation. In: Zatuchni GI, Goldsmith A, Spieler JM, et al, editors. Male Contraception: Advances and Future Prospects. Philadelphia: Harper & Row; 1986:395-400.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree