FIGURE 85-1. Individual and median values of thyroid function tests in various grades of primary hypothyroidism. Interrupted horizontal lines indicate upper (thyroid-stimulating hormone [TSH]) and lower (free thyroxine [FT4] and triiodothyronine [T3]) limits of the normal reference range. Progression from grade I to III can be observed in the transition from the euthyroid to the severely hypothyroid state and vice versa on treatment of hypothyroidism.
History
Hypothyroidism as a clinical syndrome was described in 1874 by Gull under the name of myxedema in view of the swollen skin (edema) and its excessive content of mucin (myx-). In 1883, Semon noted striking similarities between patients with myxedema and patients who had undergone total thyroidectomy. The Clinical Society of London nominated a committee to investigate this matter. In 1888, the committee reported in what has become a classic paper3 that cretinism, myxedema, and postthyroidectomy changes all were due to loss of thyroid function. In 1891, Murray reported cure of myxedema by hypodermic injections of sheep thyroid extract. Simply eating ground or dried animal thyroid tissue proved equally effective. The active principle of thyroid extract was isolated by Kendall on Christmas Day, 1914, and was named thyroxine. Harrington elucidated the precise constitution of thyroxine in 1926 and was able to synthesize it. Desiccated thyroid remained the usual treatment for hypothyroidism, however, because thyroxine was more expensive and less efficacious owing to poor absorption of the free acid. As of the 1960s, levothyroxine sodium surplanted gradually desiccated thyroid as the preferred treatment modality for hypothyroidism.
Epidemiology
Primary hypothyroidism is a common disease worldwide, especially in iodine-deficient areas (see Chapter 88). It also is a prevalent disease in iodine-replete regions. The most extensive epidemiologic data have been obtained from a population-based study of subjects 18 years old and older in Whickham County in northeast England (Table 85-1).4,5 The initial survey was done between 1972 and 1974, with a follow-up 20 years later. The data seem representative of other countries inasmuch as similar figures have been reported from Sweden, Japan, and the United States.6 Most striking are the high prevalence (especially of subclinical hypothyroidism), the marked female preponderance, and the increasing occurrence with advancing age. The mean age at diagnosis of hypothyroidism in women is 60 years. Most cases are due to chronic autoimmune thyroiditis (incidence of 3.5 per 1000 women per year), followed by destructive treatment for thyrotoxicosis (incidence of 0.6 per 1000 women per year). The probability of spontaneous hypothyroidism developing in women at a particular time increases with age: from 1.4 per 1000 per year at ages 20 to 25, to 14 per 1000 per year at 75 to 80 years. Risk factors for progression to overt hypothyroidism include the presence of thyroid autoantibodies and an already elevated TSH (Table 85-2). The risk correlates directly with the serum concentration of thyroid peroxidase autoantibodies and with the extent of the TSH increase. The probability that hypothyroidism will develop increases even at TSH levels in the high-normal range of 2 to 5 mU/L, independent of age or antibody status.5,7
Table 85-6. Characteristics and Treatment of Myxedema Coma
| Hypothyroxinemia | Large doses of intravenous levothyroxine |
| Hypothermia | Blankets, no active rewarming |
| Hypoventilation | Mechanical ventilation |
| Hypotension | Cautious volume expansion with crystalloid or whole blood |
| Hyponatremia | Mild fluid restoration |
| Hypoglycemia | Glucose administration |
| Hypocortisolemia | Glucocorticoid administration |
| Precipitating event | Identification and elimination by specific treatment |
Pathogenesis
The various causes of hypothyroidism can be classified according to their site of interference (in the hypothalamus-pituitary, in the thyroid gland, or in the peripheral target tissues) and their nature (organic lesions resulting in loss of functional tissue, or functional disturbances resulting in deficient hormone biosynthesis and release) (Table 85-3). Most cases of hypothyroidism are acquired and permanent; congenital hypothyroidism and transient forms of hypothyroidism are in the minority.
Table 85-2. Percentage of Women Acquiring Spontaneous Primary Hypothyroidism During 20 Years of Follow-up in the Whickham Survey
| Initial Serum TSH | Initial Thyroid Autoantibodies | |
|---|---|---|
| Negative | Positive | |
| Normal | 4% | 27% |
| Elevated | 33% | 55% |
TSH, Thyroid-stimulating hormone.
Data from Vanderpump et al5 and Wang and Crapo.6
CENTRAL HYPOTHYROIDISM
Reduced T4 secretion in central hypothyroidism is due to insufficient stimulation of the thyroid gland by TSH, which is caused by lesions in the pituitary (secondary hypothyroidism) or the hypothalamus (tertiary hypothyroidism resulting from deficient thyrotropin-releasing hormone [TRH] release). The term central hypothyroidism is preferred because lesions sometimes involve both sites, which prevents clear-cut distinction. Although an absent TSH response to exogenous TRH would suggest a pituitary cause, and a delayed response would suggest a hypothalamic cause,8 the TSH profiles after TRH are not well correlated to the anatomic site of the lesion. Basal serum TSH values in central hypothyroidism can be low, normal, or even slightly elevated (up to 10 mU/L).9,10 The apparent paradox of central hypothyroidism in the presence of a normal or increased serum TSH concentration is explained by the reduced biological activity of TSH in these patients related to abnormal sialylation of TSH. Central hypothyroidism also is associated with a decreased nocturnal TSH surge (because of loss of the usual nocturnal increase in TSH pulse amplitude, but not TSH pulse frequency), which might hamper further maintenance of normal thyroid function.11,12
The prevalence of central hypothyroidism in the general population is unknown; a rough estimate is 0.005%. The sex distribution is about equal, and central hypothyroidism occurs with peaks in childhood and in adults 30 to 60 years old.13 Congenital cases are due to pituitary hypoplasia, midline defects such as septo-optic dysplasia (TSH deficiency in 20%), Rathke’s pouch cysts, or rare loss-of-function mutations in genes encoding for TRH receptors, the TSH-β subunit, or pituitary transcription factors. Childhood cases are caused most often by craniopharyngioma (TSH deficiency in 53%) or cranial irradiation for brain tumors (TSH deficiency in 6%).14 Adult cases most frequently are due to pituitary macroadenomas (hypothyroidism in 10% to 25%) and pituitary surgery or irradiation. TSH deficiency caused by loss of functional tissue usually becomes manifest after the development of growth hormone and gonadotropin deficiency.13 TSH deficiency sometimes may disappear after selective adenomectomy.15 Cranial radiotherapy for brain tumors causes hypothyroidism in 65%, depending on the radiation dose; the onset varies between 1 and 26 years after irradiation.16
Radiotherapy for pituitary tumors is followed by hypothyroidism in at least 15% (55% when combined with surgery).17 Less common causes include traumatic brain injury and subarachnoid hemorrhage,18 ischemic necrosis from postpartum hemorrhage (Sheehan’s syndrome) and severe shock, pituitary apoplexy (hemorrhage in a pituitary adenoma), infiltrative diseases, and lymphocytic hypophysitis.19 Lymphocytic hypophysitis most likely is an autoimmune disease that occurs predominantly in women during pregnancy and the postpartum period and is characterized by a pituitary mass and hypopituitarism.19 Despite the many known causes of central hypothyroidism, idiopathic cases are still encountered.
In critically ill patients receiving dopamine, serum TSH and the T4 production rate decrease by 60% and 56%, respectively, as a result of direct inhibition of pituitary TSH.20 Transient functional inhibition of TSH release is observed after withdrawal of long-term levothyroxine suppressive therapy, which may last 6 weeks.21 Glucocorticoid excess dampens pulsatile TSH release, which rarely results in decreased serum FT4.22 Octreotide therapy does not cause hypothyroidism despite its inhibition of TSH secretion. High doses of bexarotene, a specific retinoid X receptor agonist used in the treatment of cutaneous T cell lymphoma, cause central hypothyroidism by strongly inhibiting TSH secretion.23
CHRONIC AUTOIMMUNE THYROIDITIS
Hypothyroidism secondary to chronic autoimmune thyroiditis is caused mainly by destruction of thyrocytes. The goitrous variant (hypothyroid Hashimoto’s goiter) is characterized by massive lymphocytic infiltration of the thyroid with the formation of germinal centers, oxyphilic changes in thyrocytes called Hürthle or Askanazy cells, and some fibrosis. In the atrophic variant (atrophic myxedema), fibrosis is the predominant feature, along with lymphocytic infiltration. The less common goitrous variant is characterized by a diffuse goiter of firm “rubbery” consistency; the histology remained essentially unaltered after 20 years, and the goiter did not regress despite T4 treatment in 43% of cases.24 Many patients with chronic autoimmune thyroiditis are euthyroid, and a few have an initial transient hyperthyroid stage (labeled as Hashitoxicosis). Hashimoto’s disease is used by many authors as an umbrella term to indicate autoimmune-mediated destruction of thyrocytes, frequently but not always resulting in hypothyroidism, as opposed to Graves’ disease, in which TSH receptor–stimulating antibodies usually result in hyperthyroidism. The two disease entities overlap and can be viewed as opposite ends of a continuous spectrum of thyroid autoimmunity. Destruction of thyrocytes and development of hypothyroidism in Hashimoto’s disease are mediated by cytotoxic T cells and cytokines (especially interferon-γ and tumor necrosis factor) released by infiltrating T cells and macrophages. Humoral immunity appears less important in this respect, but (a subset of) thyroid peroxidase (TPO) antibodies may contribute via antibody-dependent, cell-mediated cytotoxicity, complement-mediated cytotoxicity, and inhibition of TPO enzymatic activity (see Chapter 79). TSH receptor–blocking antibodies enhance thyroid atrophy and hypothyroidism, possibly also by inducing apoptosis; their prevalence is low except in Japanese patients.25
Genetic and environmental factors enhance the susceptibility of individuals to develop the disease and may determine the direction of the evolving autoimmune reaction. Autoimmune thyroid disease runs in families (80% of patients have a positive family history) and is four to ten times more common in women. Autoimmune hypothyroidism in whites is weakly associated with HLA-DR and CTLA4 polymorphisms; other, still unidentified genes probably are involved. Iodine intake has been identified as an environmental factor because the prevalence of autoimmune hypothyroidism is higher in iodine-replete than in iodine-deficient areas,26 and the incidence increases after supplemental iodine is introduced. Smoking decreases the risk for developing TPO antibodies and hypothyroidism.27
REVERSIBLE AUTOIMMUNE HYPOTHYROIDISM
Chronic Autoimmune Thyroiditis
Autoimmune hypothyroidism may revert spontaneously into euthyroidism in connection with the disappearance of TSH receptor–blocking antibodies.28 The presence of a goiter and high thyroidal radioiodine uptake increase the likelihood of spontaneous recovery.29 The incidence of spontaneous recovery is about 5%,30 but in Japan—in the face of a high ambient iodine intake—iodide restriction alone restores euthyroidism in one third of patients.29 Autoimmune hypothyroidism, however, is permanent in most patients. Peculiar cases of alternating hypothyroidism and hyperthyroidism are explained by changes in coexisting TSH receptor–blocking and TSH receptor–stimulating antibodies.31
Silent and Postpartum Thyroiditis
Silent or painless thyroiditis and postpartum thyroiditis are variant forms of chronic autoimmune thyroiditis. Thyroid histology shows lymphocytic infiltration with no germinal centers or fibrosis. The autoimmune attack is intense (resulting mainly in T cell–mediated destructive thyroiditis) but transient, which explains the characteristic pattern of transient thyrotoxicosis followed by transient hypothyroidism in the recovery stage. Each stage lasts 2 to 8 weeks. Most patients remain asymptomatic and revert spontaneously to euthyroidism. Occurrence is common in the first year after delivery: The incidence of postpartum thyroiditis is 4% to 6% and 25% in patients with type 1 diabetes mellitus.32–34 Several patterns are recognized: Thyrotoxicosis alone occurs in 38%, thyrotoxicosis followed by hypothyroidism occurs in 26%, and hypothyroidism alone occurs in 36%. TPO antibodies in serum of 100 kU/L or greater at 12 weeks’ gestation predict to a certain extent postpartum thyroiditis (positive predictive value 0.50, negative predictive value 0.98).33 Thyroid antibody titers decrease in the second and third trimesters and increase postpartum. Women with postpartum thyroiditis are at risk for recurrent postpartum thyroiditis after delivery (about 40%) and for permanent hypothyroidism (20% to 30% after 5 years) related to higher antibody titers and absence of a thyrotoxic phase.
Cytokine-Induced Thyroiditis
Treatment for malignant tumors or for hepatitis C or B with interleukin-2 or interferon-α is causally related to the de novo occurrence of TPO antibodies and the development of thyroid dysfunction.35,36 Typical features are similar to features of silent and postpartum thyroiditis and include sudden onset, biphasic pattern of thyrotoxicosis followed by hypothyroidism (although hypothyroidism alone is most frequent), and spontaneous resolution after discontinuation of treatment. The incidence is about 6%; risk factors include female sex and preexisting TPO antibodies.36
POSTOPERATIVE AND POSTIRRADIATION HYPOTHYROIDISM
Surgery
Total thyroidectomy results in overt hypothyroidism within 1 month. Subtotal thyroidectomy for Graves’ hyperthyroidism is followed by hypothyroidism in 40% after 10 years37; risk factors include a small thyroid remnant, lymphocytic infiltration, and subsequent exposure to iodine. Most patients become hypothyroid in the first year after surgery; thereafter, the cumulative incidence of hypothyroidism increases by only 1% to 2% per year. Immediate postoperative hypothyroidism does not always indicate permanent hypothyroidism; it may resolve spontaneously by 6 months. Subtotal thyroidectomy for (toxic) nodular goiter carries a much lower risk (about 15%) for postoperative hypothyroidism.
Radioactive Iodine
Radioactive iodine (131I) treatment for Graves’ hyperthyroidism results in a cumulative incidence of hypothyroidism of 70% after 10 years,37 depending on the dose of 131I administered. Most cases occur in the first year (spontaneous return to euthyroidism is observed in some patients); thereafter, the annual incidence of hypothyroidism is 0.5% to 2%, also related to persisting chronic autoimmune thyroiditis. Hypothyroidism after 131I treatment for toxic nodular goiter is less common (6% to 13%).38 131I treatment for nontoxic goiter to reduce goiter size carries a cumulative risk of 58% for the development of hypothyroidism in 8 years, the risk being related to the (relatively high) dose of 131I and the presence of TPO antibodies.39 Hypothyroidism caused by ionizing radiation has been reported in subjects exposed to atomic or hydrogen bomb explosions.
External Irradiation
External radiotherapy of the neck for Hodgkin’s or non-Hodgkin’s lymphoma causes hypothyroidism in 25% to 50% of patients; the risk is related to the radiation dose, the use of iodine-containing contrast agents before radiotherapy, and the duration of follow-up.40 The risk is decreased when the thyroid is shielded during mantle field irradiation. External radiotherapy for head and neck cancer has an actuarial risk of 40% for the development of subclinical hypothyroidism and 15% for overt hypothyroidism 3 years after treatment.41 Another study with a median follow-up of 4.4 years reports a 5-year incidence rate of 48%, with a median time of 1.4 years (range, 0.3 to 7.2 years) to the onset of elevated TSH values.42 Total body irradiation with subsequent bone marrow transplantation for acute leukemia or aplastic anemia is associated with (mainly subclinical) hypothyroidism in about 25% and usually occurs after 1 year; it is transient in half of patients.43
INFILTRATIVE AND INFECTIOUS DISEASES
A rare cause of hypothyroidism is thyroidal infiltration by systemic disease.44 Hypothyroidism is observed in the course of invasive fibrous thyroiditis of Riedel’s (30% to 40%), cystinosis (86% in adults), progressive systemic sclerosis, and amyloidosis. Infections of the thyroid gland are rare and are associated with preexisting thyroid disease and immunocompromising conditions. Occasionally, damage to the thyroid causes hypothyroidism. In contrast, hypothyroidism in the recovery phase of subacute thyroiditis of de Quervain (related to previous viral infections) is a common event (see Chapter 84).
CONGENITAL HYPOTHYROIDISM
Congenital hypothyroidism can be permanent (incidence 1 in 3100 newborns) or transient. Causes include loss of functional thyroid tissue (thyroid dysgenesis), functional defects in thyroid hormone biosynthesis (related to loss-of-function mutations in genes encoding for the TSH receptor, Na+/I− symporter, thyroglobulin, TPO, dual oxidase 2, or dehalogenase 1), and thyroid hormone resistance (mutated TRβ) (see Chapters 92 and 94).
IODINE DEFICIENCY AND IODINE EXCESS
Hypothyroidism can be caused by iodine deficiency (see Chapter 88) or iodine excess. Inorganic iodide in excess of daily doses of 500 to 1000 µg inhibits organification of iodide, known as the Wolff-Chaikoff effect. Usually, the thyroid gland escapes the Wolff-Chaikoff effect after several weeks because autoregulatory mechanisms inhibit thyroid iodide transport, and the intrathyroidal iodine concentration consequently falls below the level required for inhibition of organification. Failure to escape results in hypothyroidism, which occurs in the presence of underlying thyroid disease, such as chronic autoimmune thyroiditis, previous subacute or postpartum thyroiditis, and 131I or surgical therapy.45 Iodide-induced hypothyroidism may be due to inorganic iodide or organic iodine compounds that are deiodinated in vivo. Sources of iodine excess include an iodine-rich diet (e.g., in Japan with high consumption of seaweed29) and iodine-containing medications, such as potassium iodide, vitamins, kelp, topical antiseptics, radiographic contrast agents, and amiodarone.45 The incidence of amiodarone-induced hypothyroidism in areas with high environmental iodine intake is higher than in areas with low iodine intake (22% and 5%)46; cases occur predominantly in the first 18 months of amiodarone treatment, especially in women with preexisting thyroid antibodies.47
DRUG-INDUCED HYPOTHYROIDISM
Drugs that cause hypothyroidism through interference with thyroid hormone production or release in the thyroid gland48 include thiouracils and imidazoles (used as treatment for thyrotoxicosis), lithium, cytokines (see Reversible Autoimmune Hypothyroidism), iodine (see Iodine Deficiency and Iodine Excess), and a variety of environmental and industrial goitrogenic chemicals. Examples of the latter group include naturally occurring goitrogens, such as flavonoids and resorcinol (present in watersheds of the coal-rich and shale-rich regions of Colombia and Kentucky), and industrial pollution with polychlorinated biphenyls. Lithium inhibits thyroidal iodide transport and release of T4 and T3. Long-term lithium treatment results in goiter in 50%, subclinical hypothyroidism in about 20%, and hypothyroidism in about 20%; goiter and hypothyroidism usually occur in the first 2 years of treatment, especially in patients with preexisting thyroid antibodies. Tyrosine kinase inhibitors like sunitinib induce hypothyroidism in about 50%; the responsible mechanism is incompletely understood.49,50
CONSUMPTIVE HYPOTHYROIDISM
Hepatic and cutaneous hemangiomas often express high levels of type 3 iodothyronine-5-deiodinase (D3), which catalyzes the conversion of T4 and T3 into biologically inactive rT3 and 3,3′-T2. In infants with large hemangiomas, hypothyroidism can occur as the result of D3-induced degradation of thyroid hormone at rates that exceed the synthetic capacity of the thyroid gland.51,52 Removal of the tumor restores euthyroidism.
Clinical Features
Systemic manifestations vary considerably, depending on the cause, duration, and severity of the hypothyroid state. The characteristic clinical finding is slowing of physical and mental activity and many organ functions. The characteristic pathologic finding is accumulation of hyaluronic acid and other glycosaminoglycans in interstitial tissue, which is related to loss of the inhibitory effects of thyroid hormone on the synthesis of hyaluronate, fibronectin, and collagen by fibroblasts.53 The hydrophilic properties of glycosaminoglycans lead to a peculiar mucinous nonpitting edema (myxedema) that is most obvious in the dermis but can be present in many organs.
ENERGY AND NUTRIENT METABOLISM
Thyroid hormone deficiency causes slowing of a wide variety of metabolic processes, which results in decreased resting energy expenditure, oxygen consumption, and use of substrates. Reduced thermogenesis is related to the characteristic cold intolerance of hypothyroid patients. The decline in metabolic rate and substrate use contributes to decreased appetite and food intake. Body weight increases on average by 10% because of an increase in body fat and retention of water and salt.
Serum leptin in some but not all studies is slightly low, returning to normal levels after treatment.54,55 Whether thyroid hormone regulates leptin secretion independent of body mass index and body fat remains controversial.56,57 The other adipocytokines have normal (adiponectin) or slightly low (resistin) serum concentrations.55 Hypothyroidism delays glucose absorption from the intestine. Insulin secretion in response to oral glucose is appropriate for the slightly flattened oral glucose tolerance curve. Hepatic gluconeogenesis and glucose use usually remain normal, and blood glucose levels are maintained within normal limits. The occurrence of hypoglycemia in hypothyroid patients should alert the physician to concomitant diseases (e.g., hypopituitarism). The development of hypothyroidism in patients with insulin-dependent diabetes mellitus may require lowering of the insulin dose to counteract the decreased rate of insulin degradation.
Synthesis and degradation of proteins are reduced in hypothyroidism; one of the obvious consequences during childhood is impaired growth. Biosynthesis of fatty acids and lipolysis also are reduced. An increase in total cholesterol in serum occurs, largely as the result of an increase in low-density lipoprotein (LDL)-cholesterol (explained by decreased expression of the T3-responsive liver LDL receptor, which is involved in LDL clearance), combined with an increase in apolipoprotein B, lipoprotein (a), and possible triglycerides.58 An increase in the oxidizability of LDL particles occurs,59 along with a decrease in the metabolism of serum remnant-like particles reflecting chylomicrons and very low-density lipoprotein (VLDL) remnants.60 HDL2 but not HDL3 is increased modestly with higher apoprotein AI but not AII. The changes in serum lipids result in an atherogenic lipid profile that is reversible upon treatment.
SKIN AND APPENDAGES
Skin changes are prevalent among hypothyroid patients. The skin is dry, pale, thick, and rough with scales, and it feels cold. Dryness is related to decreased function of sebaceous and sweat glands. Pallor is related to decreased skin blood flow and anemia. Yellowish discoloration of the skin may be present, especially on the palms and soles, because of the deposition of carotene, which is converted to a lesser extent to vitamin A. The thick rough skin with scales is caused by mucinous swelling of the dermis and hyperkeratosis of the stratum corneum in the epidermis. The nonpitting swelling is most marked in the extremities and the face and gives rise to the so-called myxedema face (Fig. 85-2). This classic appearance of primary hypothyroidism is seen less often nowadays, probably because of earlier diagnosis achieved by widespread use of the TSH assay. The hair becomes dull, coarse, and brittle. Hair loss occurs in 50%; it usually is diffuse and involves the scalp, beard, and genital hair and less often the eyebrows. Nail deformities also are common: The nails become thin and brittle, have grooves, and grow more slowly.
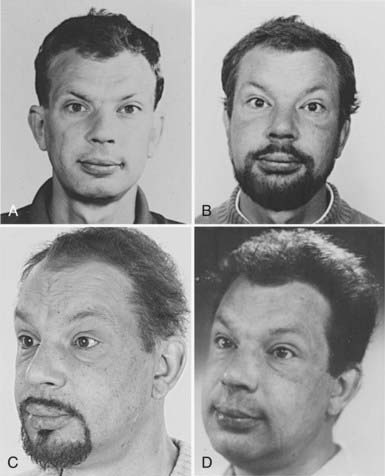
FIGURE 85-2. Appearance of a 47-year-old man 12 years (A), 5 years (B), and 3 years (C) before hypothyroidism secondary to atrophic myxedema (D) was diagnosed. Note the typical myxedema face characterized by puffy nonpitting swelling of the skin and coarse facial features.
(Reproduced with permission of the patient.)
NERVOUS SYSTEM
Thyroid hormones are essential for normal brain development; congenital hypothyroidism, if left untreated, results in mental retardation and neurologic abnormalities (see Chapters 108 and 112). In adult hypothyroid patients, a generalized decrease in regional cerebral blood flow and in cerebral glucose metabolism has been shown.61 Studies using phosphorus 32 nuclear magnetic resonance spectroscopy of the frontal lobe of adult hypothyroid patients reported reversible alterations in phosphate metabolism.62 The low-voltage electroencephalogram, prolonged central motor conduction time, and reduced visual and somatosensory-evoked potential amplitude with longer latency in adult hypothyroid patients are reversible with T4 treatment. These findings indicate that the adult human brain is a thyroid hormone–responsive organ and provide a biological basis for the prevalent neurobehavioral symptoms and cognitive impairment associated with adult hypothyroidism.61,63,64
Typically, a hypothyroid patient is slow in movement and thought, is less alert, and is less able to concentrate and memorize. Speech becomes slow and often hoarse. Hearing can be impaired. The patient sleeps longer and may fall asleep during the daytime. Hypothyroidism is listed as one of the rare but treatable causes of dementia.65 Patients may accept the limitations in physical and mental activity as part of the unavoidable aging process, but many become anxious or depressed. Rarely, severe anxiety and agitation occur, a condition known as myxedematous madness. Depression develops in more than 40%, most likely related to reduced synthesis and turnover of brain 5-hydroxytryptamine; central 5-hydroxytryptamine activity is reduced in hypothyroid patients.66
Thyroid hormone deficiency may give rise to several reversible neurologic syndromes. Cerebellar ataxia may occur, especially in elderly people, and is associated with an unsteady gait and intention tremor. More common (30%) is the carpal tunnel syndrome, which is linked to entrapment of the median nerve by thickening of the connective tissue of tendon sheaths.67,68 Complaints of paresthesias occur in 64%, and signs of sensorimotor axonal neuropathy are observed in 42%.67 Hashimoto’s encephalopathy is a vaguely defined condition in which otherwise unexplained clinical manifestations of central nervous system dysfunction are linked to the presence of TPO antibodies; serum TSH can be normal or slightly elevated.69 The condition responds to glucocorticoids, but the relationship to thyroid autoimmunity is currently uncertain.
MUSCULOSKELETAL SYSTEM
Muscles
Muscle symptoms are prevalent in hypothyroid patients and include myalgia, weakness, stiffness, cramps, and easy fatigability.67,68,70 The biochemical substrate of these complaints is provided in part by an increase in the inorganic phosphate-to-adenosine triphosphate (ATP) ratio in resting muscle and by an important decrease in phosphocreatine in working hypothyroid muscle with a greater decrease in intracellular pH than in controls.71 Impairment of mitochondrial oxidative metabolism also has been shown in subclinical hypothyroidism.72 Transition from white fast type II to red slow type I muscle fibers is involved in the change in muscle bioenergetics, which is probably multifactorial. The histopathology varies; most common is type II fiber atrophy, but fiber hypertrophy may be present along with interstitial edema and sarcoplasmic degeneration.70 Rarely, chronic hypothyroid myopathy results in increased volume of muscles (notably in the tongue and extremities), which may cause entrapment syndromes.73 Serum creatine kinase (MM fraction derived from skeletal muscle) is often elevated and correlates with the severity of hypothyroidism. The decreased rate of muscle contractility in hypothyroidism is evident from slow deep tendon reflexes. The half-relaxation time of the Achilles reflex is prolonged in many hypothyroid patients, but substantial overlap is seen in euthyroid subjects.
Joints
Arthralgia and joint stiffness are common complaints. Synovial effusions (usually of the knee) are rare.
CARDIOVASCULAR SYSTEM
Changes in cardiovascular dynamics in hypothyroidism include an increase (of 50% to 60%) in peripheral vascular resistance and a decrease (of 30% to 50%) in cardiac output.74,75 Aortic stiffness is increased.76 As a result, mean blood pressure is largely unaltered, although systolic pressure may decrease and diastolic pressure may increase. The increase in systemic vascular resistance is due to endothelial dysfunction and impaired vascular smooth muscle relaxation. The decrease in cardiac output is due to a decrease in stroke volume and heart rate. The pre-ejection time and isovolumetric contraction time are prolonged, and the ventricular relaxation rate during diastole is slower.75 The mechanism of reduced cardiac contractility with subnormal systolic and diastolic performance is multifactorial. Changes in T3-dependent myocardial gene expression are involved, especially in genes that code for calcium regulatory proteins.77 Blood volume is decreased. Edema may develop through albumin extravasation as a result of increased capillary permeability; it may give rise to pericardial, pleural, or peritoneal effusions.
Cardiovascular symptoms of hypothyroid patients include dyspnea and decreased exercise tolerance; the hemodynamic response to exercise usually is preserved. Physical examination may reveal a slow pulse rate, diastolic hypertension (in 20%), weak heart sounds, occasionally cardiac enlargement (caused by pericardial effusion or, rarely, by T4-reversible cardiomyopathy77), and peripheral nonpitting or pitting edema (rarely caused by heart failure except when cardiac disease is preexisting). The electrocardiogram may show bradycardia, low-voltage conduction disturbances, and nonspecific ST-T changes. Symptomatic ischemic heart disease with anginal complaints occurs in about 3%; the reduced need for oxygen in view of the hypometabolic state might give some protection.78 The atherogenic profile of serum lipids and the hyperhomocysteinemia79 in hypothyroidism suggest a greater prevalence of coronary atherosclerosis in these patients.80
RESPIRATORY SYSTEM
Respiratory symptoms of hypothyroidism include shortness of breath and sleep apnea. Shortness of breath can be caused by the cardiac effects of thyroid hormone deficiency, by weakness of respiratory muscles, by pleural effusion, or by impaired pulmonary function. In most nonobese hypothyroid patients, pulmonary function is nearly normal. Reduced ventilatory drive is observed in 34%; the depressed response to hypercapnia or hypoxia usually is restored rapidly on T4 treatment.81 Severe obstructive sleep apnea occurs in 7.7%,82 in part as a result of increased size of the tongue and pharyngeal muscles with a slow and sustained muscle contraction; the contribution of reduced ventilatory drive to sleep apnea is less marked but can be substantial in obese patients.
UROGENITAL SYSTEM
Kidneys and Fluid Metabolism
Renal plasma flow and glomerular filtration rate are reduced in hypothyroidism in accordance with the changes in cardiovascular hemodynamics. Serum creatinine is increased by 10% to 20%, and hyponatremia sometimes occurs.79,83 Hyponatremia is associated with increased total body water and sodium content in hypothyroidism, which is a result of the increased vascular permeability and extravascular accumulation of hydrophilic glycosaminoglycans. Free water clearance in hypothyroidism is diminished, regardless of the presence of hyponatremia. Plasma arginine vasopressin frequently is increased in hypothyroid patients; arginine vasopressin levels increase normally in response to hypertonic saline, but they are not suppressed normally after water ingestion. The syndrome of inappropriate antidiuresis in hypothyroidism is not fully understood,84 but a purely renal vasopressin-independent mechanism seems to be involved,85 presumably related to increased expression of aquaporin water channels in the kidney.86 The significance of low serum atrial natriuretic peptide concentrations in hypothyroidism is unclear.72
REPRODUCTIVE SYSTEM
Juvenile hypothyroidism results in delayed sexual maturation; it seldom results in precocious puberty (explained by spillover of the action of TRH on gonadotropes and the action of TSH on follicle-stimulating hormone receptors87,88). In adult hypothyroid men, semen analysis is usually normal; erectile dysfunction is common but fully reversible.89 Serum sex hormone–binding globulin, free testosterone, follicle-stimulating hormone, and luteinizing hormone levels most often are normal. In adult hypothyroid women, pulsatile gonadotropin release in the follicular phase is normal,90 but the ovulatory surge may not occur. Irregular—often anovulatory—cycles occur in 23% (three times as often as in the general population); oligomenorrhea and menorrhagia are most common.91 Some patients are seen initially with the galactorrhea-amenorrhea syndrome, which is due to hyperprolactinemia induced by thyroid hormone deficiency. Despite restricted fertility, conception may occur with a successful pregnancy outcome. Pregnancy-induced hypertension is two to three times more common in hypothyroid women. The prevalence of an elevated TSH among pregnant women is about 2%.92 The IQs of children born to affected mothers are 7 points lower than the IQs of controls.93 The spontaneous abortion rate is higher in hypothyroid mothers who do not receive adequate levothyroxine treatment than in mothers who do.94
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









