FIGURE 128-1. Diagram of plasma concentrations of pituitary gonadotropins (luteinizing hormone [LH], follicle-stimulating hormone [FSH]; top) and ovarian steroids (estradiol [E2], progesterone [P], and inhibins A and B; bottom) during an ovulatory menstrual cycle. The arrows above the figure represent pulses of gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus. For estradiol, divide by 3.7 pg/mL to convert to picograms per milliliter; for progesterone, divide by 3.2 ng/mL to convert to nanograms per milliliter.
The first few days of the follicular phase are characterized by plasma levels of FSH that are relatively high and by low levels of LH, estradiol, progesterone, and inhibins A and B. The initial predominance of FSH stimulation of the ovary is critical for the recruitment and maturation of a cohort of ovarian follicles, one of which is destined to ovulate.7–10 FSH stimulates follicular growth and inhibin secretion, induces the appearance of LH receptors on granulosa cells, and stimulates the activity of the aromatase enzymes that are required to convert androstenedione (from theca cells) to estradiol (see Chapter 126). The combined actions of FSH and LH stimulate secretion of estradiol, which by the middle to the late follicular phase is secreted predominantly by the follicle destined to ovulate, the dominant follicle. The exact mechanisms whereby one follicle achieves dominance while many others become atretic are not fully understood. FSH is important in primates, and suppression by exogenous estradiol during the early follicular phase delays development of the dominant follicle11 and prolongs the follicular phase in monkeys and humans. Administration of FSH to prolong the elevation in plasma FSH for 5 to 6 days results in several follicles being stimulated to develop in women and primates.12 If an antiestrogen is given to hypophysectomized rats along with FSH, this FSH-stimulated folliculogenesis is prevented. Thus, estrogens acting locally within the ovary are involved in follicular maturation initiated by FSH. In addition, regulatory proteins are secreted by granulosa cells, which can inhibit follicle growth and have been termed follicle regulatory proteins or oocyte maturation inhibitors.13 It is postulated that these compounds assist the dominant follicle to be selected by producing atresia and failure to survive in smaller follicles (see Chapter 125). An adequate FSH stimulus is also important for determining subsequent function of the corpus luteum inasmuch as women with short luteal phases have lower levels of serum FSH during the follicular phase of the cycle.
The magnitude and duration of follicular FSH elevation appear to be critical determinants of monofollicular development, and insight into the regulation of FSH secretion followed the development of specific assays for the inhibins A and B.6,7 Fig. 128-2 shows that inhibin B is the predominant form secreted during the follicular phase, and inhibin A is secreted by the corpus luteum. Inhibin B levels rise rapidly during the early to midfollicular phase and limit FSH secretion, which is also selectively inhibited by the late follicular rise in estradiol14 and inhibin A. This reduction in FSH may be causally related to atresia of the nondominant follicles. The increase in estradiol in the late follicular phase allows estradiol to exert a positive-feedback effect and enhance LH responsiveness to gonadotropin-releasing hormone (GnRH). Plasma progesterone is also rising and augments LH responses to GnRH,15 with the combined effects resulting in markedly enhanced LH release and the midcycle LH surge. GnRH secretion is also increased in sheep and monkeys, and estradiol can increase secretion of GnRH.16 Thus, this ovarian estradiol-progesterone signal from the mature follicle induces the GnRH-LH-FSH ovulatory surge. The LH surge persists for 40 to 48 hours and induces rupture of the mature follicle and release of the ovum some 16 to 24 hours after initiation of the LH surge. Concomitant with the abrupt rise in LH, serum estradiol falls precipitously and progesterone secretion increases, reflecting the altered function of the luteinized follicle. After ovulation, the luteinized follicle secretes progesterone, 17-hydroxyprogesterone, estradiol, estrone, and inhibin A, which all increase during the 7 to 8 days after ovulation. The elevated plasma inhibin A and estradiol suppress FSH secretion, which remains low during the luteal phase. LH appears to be essential for normal function of the corpus luteum. The nature of other factors that dictate the duration of corpus luteum function is uncertain, but in many species, prostaglandins of ovarian or uterine origin appear to be involved.17 If the ovum is fertilized, corpus luteum function is maintained by human chorionic gonadotropin (hCG), but in the absence of conception, plasma inhibin A, estradiol, and progesterone decline during the final days of the cycle. The fall in ovarian hormones allows an increase in plasma gonadotropins, particularly FSH, which initiates the recruitment and maturation of ovarian follicles for the next cycle.
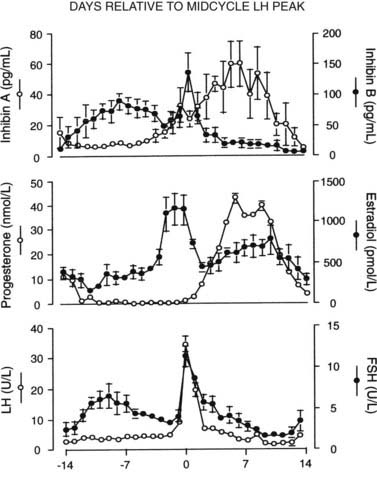
FIGURE 128-2. Plasma concentrations of inhibins A and B (top), progesterone and estradiol (middle), and luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (bottom) during ovulatory cycles in women. Data are aligned to the day of the midcycle LH peak (day 0). Mean ± SE is shown.
(From Groome NP, Illingworth PJ, O’Brien M, et al: Measurement of dimeric inhibin B throughout the human menstrual cycle, J Clin Endocrinol Metab 81:1401–1405, 1969. © The Endocrine Society.)
Mechanisms That Regulate Hormone Secretion
The mechanisms involved in interactions of the hypothalamic-pituitary-ovarian axis to produce the orderly sequential hormonal changes seen during the normal cycle are complex. Blood samples obtained every 10 to 20 minutes during the cycle have revealed patterns of LH and FSH secretion that suggest that alterations in pulsatile hypothalamic GnRH secretion are important in maintaining normal cyclicity. In humans, estimation of GnRH secretion has to be indirect, and changes in GnRH secretion are inferred from the patterns of LH or free α subunit in plasma.18 In animals, GnRH is secreted in an intermittent manner, and each episode of GnRH release from the hypothalamus is followed by an acute increase in plasma LH. Thus, the frequency and/or amplitude of LH pulses in peripheral blood can be used as an indirect measurement of GnRH secretion. FSH is less helpful in this regard because its longer half-life (about 3 hours) obscures pulsatile patterns. The frequency of LH pulses varies during different stages of the cycle.19–22 Fig. 128-3 shows patterns of plasma LH and FSH during cycles in two normal women.
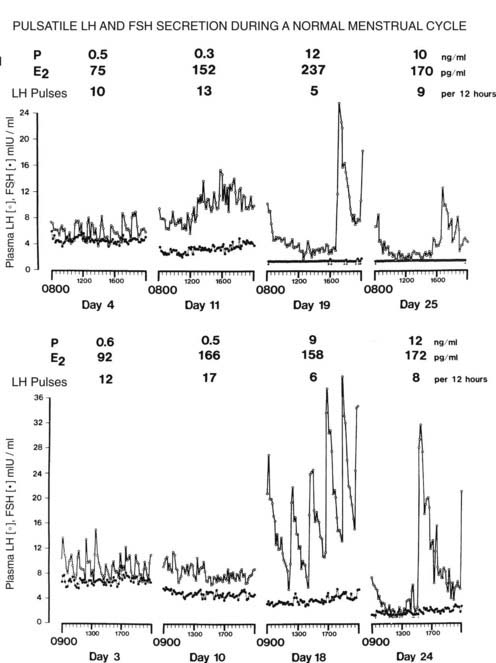
FIGURE 128-3. Pulsatile luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion in two women during ovulatory menstrual cycles. Blood samples were obtained at 10-minute intervals during the early and late stages of both the follicular and the luteal phases of the cycle. Mean values for ovarian steroids are shown. The number of LH pulses per 12 hours is shown for each day. E2, Estradiol; P, progesterone.
(From Reame N, Sauder SE, Kelch RP, et al: Pulsatile gonadotropin secretion during the human menstrual cycle: Evidence for altered frequency of gonadotropin-releasing hormone secretion, J Clin Endocrinol Metab 59:328–337, 1984. © The Endocrine Society.)
FOLLICULAR PHASE AND THE MIDCYCLE GONADOTROPIN SURGE
During the early follicular phase (days 1 to 5), plasma concentrations of FSH exceed those of LH, and LH pulses occur every 90 to 100 minutes. FSH stimulates the increase in inhibin B during the midfollicular phase,23 probably secreted by the small antral follicles which are rich in inhibin-α and inhibin-βB subunit mRNA,24 and the elevated plasma inhibin B suppresses FSH secretion by direct actions on the gonadotrope. In the late follicular phase, inhibin B levels decline and βB mRNA expression is markedly reduced in the preovulatory follicle.25 LH pulse frequency increases to about one pulse per hour and stimulates estradiol secretion by the maturing follicle.26,27 The increase in estradiol maintains suppression of FSH secretion together with the rise in inhibin A during the days before the LH surge. Granulosa cells of the dominant follicle contain both α and βA mRNA and are the probable source of inhibin A.24 The mechanisms involved in increasing the frequency of LH pulses in humans are unclear and may reflect the gradual loss of progesterone inhibition in the previous luteal phase28 and/or the rise in estradiol. Similar changes occur in sheep and appear to depend on the rise in estradiol.29,30 Measurement of portal blood GnRH in sheep after estradiol administration has shown that GnRH pulse frequency and amplitude increase, effecting a massive increase in GnRH secretion.16,29 The pulsatile release of GnRH is obscured, and GnRH remains elevated throughout and beyond the termination of the LH surge. Estradiol only appears to be required to initiate increased GnRH secretion and, once begun, GnRH secretion continues despite falling estradiol levels.31 In primates and humans, the requirement for a marked increase in GnRH secretion to induce the LH surge is less clear. Although GnRH is increased in rhesus monkeys,32 ovulatory LH surges can be induced by the administration of constant high amplitude GnRH pulses to GnRH-deficient monkeys and humans,33,34 though the magnitude of LH secretion is less than occurs spontaneously.
In addition to the increase in GnRH secretion, both estradiol and progesterone enhance LH responsiveness to GnRH. Plasma estradiol is maximal, and progesterone and 17α-hydroxyprogesterone concentrations rise immediately before the LH surge34,35 (Fig. 128-4). Thus, estradiol triggers increased GnRH secretion and enhanced LH responsiveness to GnRH, the latter augmented by the rise in progesterone resulting in the ovulatory LH surge.36 FSH also increases transiently during the midcycle surge, although levels are much lower than those of LH. This difference probably reflects increased GnRH secretion, although activin A has also been reported to increase.37 Inhibin B is also increased after the rise in FSH, but because βB mRNA is not detectable in corpora lutea, this may reflect release of inhibin B from the ruptured follicle.38 During the LH surge, progesterone continues to rise, and estradiol falls rapidly, reflecting the LH-induced luteinization of granulosa cells and altered steroidogenesis favoring progesterone secretion.39 The duration of the LH surge is probably limited by a combination of factors. The fall in estradiol results in loss of enhanced LH responses to GnRH, and progesterone is not effective in maintaining LH responsiveness in the absence of estradiol. GnRH secretion continues to be elevated during the declining part of the surge,39 and the reduced amplitude of LH pulse secretion may reflect gonadotrope desensitization after the prolonged rapid frequency or continuous stimulation by GnRH.
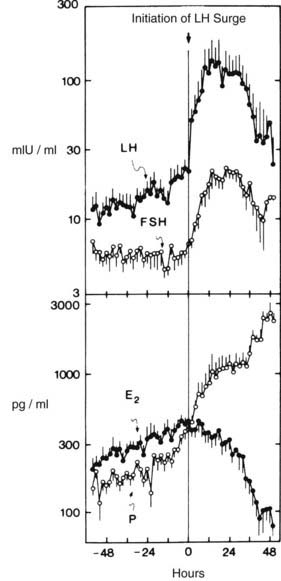
FIGURE 128-4. Plasma luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), and progesterone (P) concentrations in samples obtained at 2-hour intervals for 5 days at midcycle during seven cycles. Zero time represents initiation of the gonadotropin surge. Data are plotted on a logarithmic scale.
(From Hoff JD, Quigley ME, Yen SCC: Hormonal dynamics at mid cycle: a reevaluation, J Clin Endocrinol Metab 57:792–796, 1983. © The Endocrine Society.)
LUTEAL PHASE AND INITIATION OF THE NEXT WAVE OF FOLLICULAR RECRUITMENT
During the 3 to 4 days after ovulation, LH pulse frequency falls, to one pulse every 3 to 5 hours by the midluteal stage. The pattern of LH pulses also varies through the luteal phase. In the early luteal phase, large amplitude LH pulses occur regularly, whereas irregularity in both amplitude and frequency occurs in the midluteal to late luteal phase (see Fig. 128-3). The variable LH secretory patterns reflect altered hypothalamic GnRH secretion inasmuch as LH responsiveness to GnRH is not impaired.40 The elevation in progesterone is the main factor that reduces GnRH secretion in the luteal phase. Administration of progesterone during the follicular phase results in LH secretory patterns that resemble those of the normal luteal phase41,42 (Fig. 128-5).
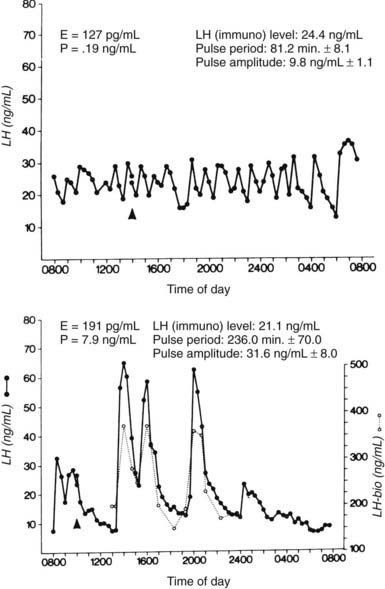
FIGURE 128-5. Patterns of plasma luteinizing hormone (LH) in samples obtained at 20-minute intervals during the late follicular phase (top) and during the late follicular phase after the administration of progesterone (P) for 8 days (bottom) in a normally cycling woman. The dotted lines in the lower panel represent serum LH measured by bioassay. E, Estradiol.
(From Soules MR, Steiner RA, Clifton DK, et al: Progesterone modulation of pulsatile luteinizing hormone secretion in normal women, J Clin Endocrinol Metab 58:378–383, 1984. © The Endocrine Society.)
The mechanism of progesterone slowing of GnRH secretion involves increased hypothalamic opioid activity. β-Endorphin is increased in hypothalamic portal blood in the luteal phase, and administration of naloxone, an opiate receptor blocker, increases LH pulse frequency during the luteal phase in both women and monkeys.43,44 Naloxone is ineffective during the follicular phase. The slowing of GnRH pulsatile secretion during the luteal phase may have important consequences for the lifespan of the corpus luteum, since LH is required for normal luteal function in primates. In the absence of LH secretion, the corpus luteum lifespan is shortened; in the presence of large doses of exogenous LH or the secretion of hCG (an LH-like hormone) in normal pregnancy, the corpus luteum lifespan is lengthened.45 In the early luteal phase, before LH pulse frequency has decreased, serum progesterone is stable and only minor fluctuations occur. During the midluteal to late luteal phase, progesterone secretion only occurs coincident with LH pulses.21,46 Thus reduced LH pulse frequency may play a role in demise of the corpus luteum. The slower frequency of GnRH pulses also modulates gonadotropin synthesis in the luteal phase. GnRH is essential for gonadotropin synthesis, and in rodents, faster-frequency GnRH pulses favor LH synthesis, and slower pulses favor FSH synthesis.47 The slow, irregular luteal GnRH stimulus would thus be expected to maintain FSH synthesis but may not be optimal for LH synthesis. Reduced LH synthesis, together with ongoing LH release, would result in depletion of pituitary LH stores during the luteal phase.
Plasma FSH remains low during the luteal phase (see Fig. 128-3), which reflects inhibition of release by estradiol and inhibin A secreted by the corpus luteum.5,47,48 With the demise of the corpus luteum, serum progesterone, estradiol, and inhibin A levels fall, and LH pulse frequency and plasma FSH levels increase during the last 2 to 3 days of the cycle. Detailed studies of the late luteal–early follicular phase transition in individual patients suggest that the fall in progesterone allows an increase in GnRH pulse frequency (Fig. 128-6), which continues to rise over the next 1 to 2 weeks.28 The increased GnRH stimulation results in predominantly FSH release, because the selective inhibition by estradiol and inhibin A is no longer present, and LH stores have been depleted. FSH release, together with the long half-life of FSH, effects a selective increase in plasma FSH. Studies in GnRH-deficient women have emphasized the role of increased GnRH secretion in the selective intercycle rise in FSH and subsequent follicular secretion of inhibin B. When a slow frequency (every 240 minutes) of GnRH pulses was continued during menses, a smaller increase in FSH and inhibin B occurred than when GnRH pulses were given at a physiologic, early follicular frequency of every 90 minutes.49 Thus, although FSH bioassay has suggested that some FSH may be present during the entire luteal phase,50 the critically important increase in plasma FSH to initiate recruitment of ovarian follicles for the next cycle occurs during the late luteal phase—the result of increased GnRH stimulation of a pituitary that is primed to release FSH.
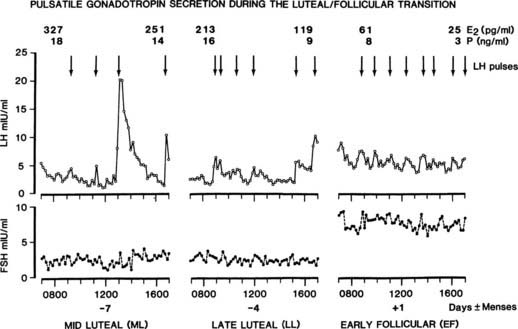
FIGURE 128-6. Gonadotropin secretion and gonadal steroids during the intercycle period of an ovulatory cycle. The transition of hormone secretion from the midluteal to the early follicular phase is shown. Arrows indicate luteinizing hormone (LH)/gonadotropin-releasing hormone (GnRH) pulses. E2, Estradiol; FSH, follicle-stimulating hormone; P, progesterone.
(From Marshall JC, Dalkin AC, Haisenleder DJ, et al: Gonadotropin-releasing hormone pulses: Regulators of gonadotropin synthesis and ovulatory cycles, Recent Prog Horm Res 47:155–189, 1991.)
ROLE OF GnRH SECRETORY PATTERNS IN CYCLE REGULATION
Regulation of normal menstrual cyclicity involves a complex series of timely interactions among the hypothalamus, pituitary, and ovaries. Changes in the pattern of GnRH secretion play an important role, as shown in Figs. 128-1, 128-3, and 128-6.
Follicular recruitment and maturation are initiated by FSH, which rises following the late luteal increase in GnRH pulse frequency in the presence of low levels of estradiol and inhibins A and B. Estradiol from the maturing follicle selectively inhibits FSH secretion and, in some species, stimulates the late follicular increase in GnRH pulse frequency. This in turn increases plasma LH, and subsequent estradiol secretion by the dominant follicle triggers markedly enhanced GnRH secretion at midcycle. The LH surge results from increased frequency and amplitude of GnRH secretion and augmentation of LH responses to GnRH. After ovulation, progesterone increases hypothalamic opioid activity, which slows GnRH frequency, thereby favoring FSH synthesis. The slow, irregular GnRH pulses do not release FSH (inhibited by elevated estradiol and inhibin A levels), and gonadotrope FSH stores are maintained. The fall in progesterone allows an increase in GnRH pulse frequency, which again effects FSH secretion to renew the cyclic process.
This synopsis of the role of GnRH secretion is based on patterns of hormone secretion consistently observed in women and several animal species. The importance of changes in GnRH secretion remains uncertain, however, because the administration of a fixed dose of GnRH at a fixed frequency can induce ovulation, both in women with GnRH deficiency and in GnRH-deficient monkeys (induced by hypothalamic lesions).33,51–53 This apparent contradiction may reflect the manner of GnRH administration. In most cycles in which ovulation has been induced with exogenous GnRH, the dose of GnRH used was supraphysiologic, which may override the need to change the frequency of the GnRH stimulus. Additionally, few studies have examined the efficacy of uninterrupted exogenous GnRH pulse regimens in the stimulation of ovulatory cycles over prolonged periods, and the ability to alter GnRH frequency may be important in the continued maintenance of normal cyclicity. Slowing of pulse frequency in the luteal phase appears to be important in this regard. The administration of GnRH at rapid frequencies in the luteal phase has led to deficient follicular development and corpus luteum function in subsequent cycles, perhaps a consequence of inadequate late luteal/early follicular FSH secretion.54–56 The fact that GnRH pulse frequency varies during the cycle appears well established and has led to the use of varied GnRH dose/frequency regimens for induction of ovulation, with excellent (>90%) ovulation rates in GnRH-deficient women.57 However, the exact role and the requirement for altered frequency to maintain ongoing cyclicity await clarification.
The changes in GnRH secretion during the cycle, particularly the increase in frequency from the luteal to the follicular phase, resemble changes in GnRH secretion during pubertal maturation.58 In prepubertal girls, LH (by inference, GnRH) pulse amplitude is low, and pulses occur infrequently (every 3 to 4 hours), with a minor augmentation during sleep.59–61 Pubertal maturation is initiated by increased GnRH secretion initially during sleep, subsequently throughout 24 hours, and responses to GnRH change from a predominant FSH response in prepubertal girls to the adult pattern of LH release. Thus, the increase in frequency of GnRH secretion during early puberty and during the luteal/follicular transition both result in FSH responses to GnRH evolving into LH-dominated responses. The ability to secrete GnRH at a rapid frequency (one pulse per hour) is reacquired at puberty (data suggest that fast-frequency secretion is present in infancy) and is necessary for the development of ovulatory cycles. A rapid frequency of GnRH secretion is needed to allow estradiol augmentation of GnRH secretion and LH responses to GnRH, critical events in the genesis of the LH surge. The luteal phase could then be viewed as a time when ovarian steroids restrict GnRH release to a pattern that is not optimal for LH secretion but allows ongoing FSH synthesis to stimulate the next wave of follicular development.58
Mechanisms of Anovulation
In view of the complex nature of the interrelations between the hypothalamus-pituitary and the ovary that are required for normal cyclic function, it is not surprising that disorders of any part of this axis can result in anovulation and amenorrhea. In many instances, however, anovulation occurs in the absence of recognized pathologic abnormalities. Before menarche, regular cycles with small LH surges occur, with the magnitude of LH and progesterone changes increasing over time.62–64 Anovulatory cycles frequently occur during the year after menarche, and because regular cycles subsequently ensue, this suggests that the adult hormonal interrelationships are established over time. Specifically, the ability of estradiol to induce positive feedback and increase LH release is absent in immature girls,65 which may in part account for the anovulatory cycles that occur soon after menarche.
In several instances, anovulation has been shown to be associated with abnormal patterns of LH (GnRH) pulsatile secretion, and these patterns are discussed in the sections that follow.
HYPOTHALAMIC AMENORRHEA
Hypothalamic amenorrhea, the most common form of amenorrhea, is a diagnosis made only after exclusion of pituitary and ovarian abnormalities. Conditions that often precede anovulation include marked weight loss, strenuous exercise such as gymnastics or competitive running, psychological stress, and occasionally the prior use of combination oral contraceptive preparations.66,67 In most women (about 70%), removal of these antecedent conditions results in the return of ovulatory menses within 12 months, but in the remainder, anovulation and amenorrhea persist. Basal hormone measurements show that plasma LH, FSH, and estradiol levels are often normal or low, leptin is low, prolactin is not elevated, and LH and FSH responsiveness to GnRH is usually preserved. Cyclic hormonal changes do not occur, and abnormalities of estrogen and progesterone feedback, with failure of positive feedback, have been described.68–72 Studies from several groups have shown that the frequency of GnRH pulsatile secretion is markedly reduced in most women with hypothalamic amenorrhea73,74 (Fig. 128-7). GnRH pulse frequency (one pulse every 3 to 4 hours) and the irregular amplitude of LH pulses resemble patterns seen during the luteal phase of ovulatory cycles, suggesting suppression of GnRH pulse frequency by increased hypothalamic opioid activity. Administration of the opiate receptor blocker naloxone results in rapid (within 1 to 2 hours) restoration of normal-frequency GnRH secretion in some 60% to 70% of women with hypothalamic amenorrhea75–77 (Fig. 128-8). Naltrexone (an orally active opiate receptor blocker) has been given to patients for 2 to 3 weeks and occasionally induced ovulation,78 although this action appears to be short lived, and repetitive ovulatory cycles rarely ensue. This temporary opiate antagonist–induced restoration of GnRH secretion suggests that some women are anovulatory on the basis of a persistent slow frequency of GnRH secretion that is inadequate to maintain the level of LH synthesis and secretion required for an ovulatory LH surge. Support for this view is found in studies in which GnRH pulses given at a slow frequency (every 3 hours) to GnRH-deficient primates did not maintain plasma LH concentrations.79
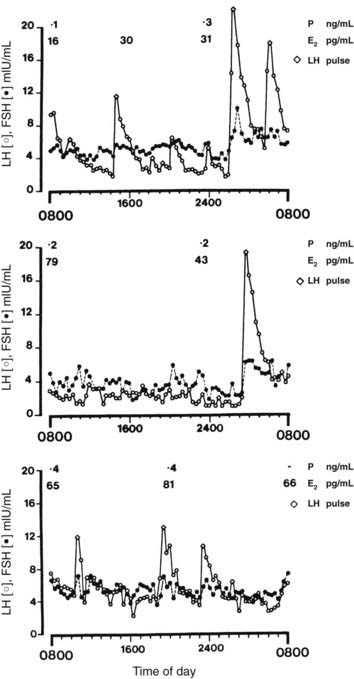
FIGURE 128-7. Patterns of pulsatile luteinizing hormone (LH) secretion over a 24-hour period in women with hypothalamic amenorrhea. Plasma estradiol (E2) and progesterone (P) levels are also shown. From top to bottom panels, patients showed 4, 3.5, and 5 pulses per 12 hours, respectively. FSH, Follicle-stimulating hormone.
(From Reame NE, Sauder SE, Kelch RP, et al: Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: Evidence for reduced frequency of GnRH secretion, J Clin Endocrinol Metab 61:851–858, 1985. © The Endocrine Society.)
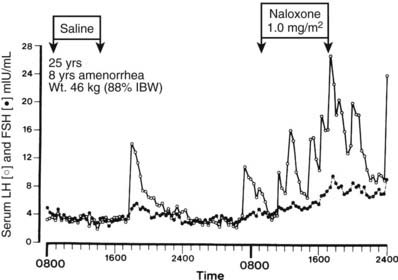
FIGURE 128-8. Effects of naloxone on pulsatile luteinizing hormone (LH, open circles) and follicle-stimulating hormone (FSH, closed circles) secretion in a woman with hypothalamic amenorrhea. The 25-year-old woman had a history of weight loss and amenorrhea for 8 years and, despite regaining weight to 90% of ideal 1 year ago, had remained amenorrheic. IBW, Ideal body weight.
(Data from Khoury SA, Reame NE, Kelch RP, et al: Diurnal patterns of pulsatile luteinizing hormone secretion in hypothalamic amenorrhea: Reproducibility and responses to opiate blockade and in α2-adrenergic agonist, J Clin Endocrinol Metab 64:755–762, 1987. © The Endocrine Society.)
The observation that patterns of GnRH secretion are variable but slower than normal pulse frequency would also explain observations that some women with hypothalamic amenorrhea ovulate after the administration of clomiphene citrate (Clomid). If pulse frequency were markedly impaired, clomiphene would not enhance GnRH and gonadotropin secretion to the levels required for an LH surge. On the other hand, lesser degrees of GnRH slowing may be overcome by clomiphene and permit follicular maturation and ovulation.
As noted, not all women with hypothalamic amenorrhea have slow-frequency GnRH pulses at the time of study, and not all those who do have slow-frequency pulses respond to opiate blockade. The mechanisms of amenorrhea in these women are uncertain, but some data have suggested that abnormalities of the hypothalamic-pituitary-adrenal axis may be involved.80,81 Stress elevates corticotropin-releasing hormone (CRH), which inhibits GnRH secretion and reproductive function in animal studies. Some women with hypothalamic amenorrhea have elevated plasma cortisol levels and blunted responses to CRH, which suggests stress-induced abnormalities in CRH secretion.82 In women where amenorrhea is associated with strenuous exercise, data suggest a negative energy balance is a precipitating factor, and plasma leptin levels are reduced.83,84 Studies in a primate monkey model have supported this concept, and provision of additional calories while maintaining the same level of exercise restored reproductive cyclicity.85,86
Hypoleptinemia appears to be an important factor in athletes and women at low body weight. Administration of recombinant human leptin for 3 months increased GnRH pulsatility within 2 weeks, and 3 of 8 women ovulated during treatment.72
HYPERPROLACTINEMIA
Amenorrhea and anovulation commonly occur when serum prolactin is elevated. Hyperprolactinemia may result from medications that reduce hypothalamic dopamine secretion or block dopamine action, or it may reflect the presence of a prolactinoma in the pituitary gland. Initial studies revealed slow, irregular patterns of GnRH secretion which were restored to normal follicular-phase patterns after suppression of serum prolactin by the dopamine agonist bromocriptine87,88 (Fig. 128-9).
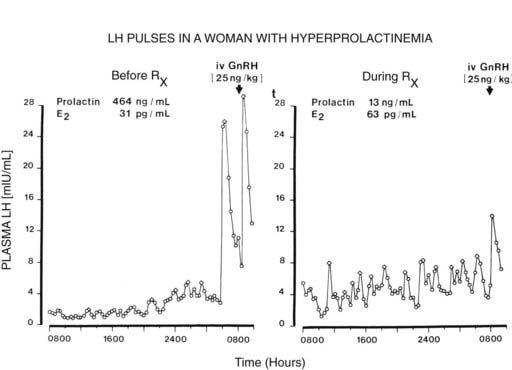
FIGURE 128-9. Patterns of pulsatile luteinizing hormone (LH) secretion before and after administration of bromocriptine in a woman with hyperprolactinemia. Note the initial irregular amplitude and infrequent LH pulses (6 per 24 hours) (left), which return to a more regular pulsatile pattern similar to that of the follicular phase (12 pulses per 24 hours) after the administration of bromocriptine and reduction in serum prolactin (right). Open diamond, LH pulses; E2, plasma estradiol; GnRH, gonadotropin-releasing hormone.
(From Sauder SE, Frager M, Case GD, et al: Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: Responses to bromocriptine, J Clin Endocrinol Metab 59:941–948, 1984. © The Endocrine Society.)
Of interest, the mechanisms of reduced GnRH pulsatile secretion in hyperprolactinemic patients also appear to involve a final common pathway of increased hypothalamic opioid activity. Administration of naloxone to hyperprolactinemic women (serum prolactin remains elevated) results in a rapid increase in pulsatile GnRH secretion in a manner similar to that seen in women with hypothalamic amenorrhea.89,90 This response suggests that an elevated prolactin level enhances hypothalamic opioid activity, which in turn reduces GnRH secretion by reducing pulse frequency. These data suggest that in both hypothalamic amenorrhea and hyperprolactinemia, anovulation depends on a continuing slow frequency of endogenous GnRH secretion. The inability to increase pulsatile GnRH secretion results in failure of follicular maturation and consequent estradiol/progesterone augmentation of LH release. In addition, the abnormal GnRH secretion may be variable,77 and women with apparent hypothalamic amenorrhea may have “normal” LH pulse secretion during some studies. If an underlying abnormality such as stress increases hypothalamic opioid activity, this response may be variable in both degree and duration. When such abnormalities are present for long enough to affect the hypothalamic mechanisms involved in normal follicular maturation and ovulation, anovulation would be expected to ensue.
POLYCYSTIC OVARY SYNDROME
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder of uncertain cause associated with anovulation, hirsutism, obesity, insulin resistance, and multiple cysts in the ovaries (see Chapter 132). The excess androgen secretion in PCOS has been shown to be predominantly of ovarian origin, but insulin resistance and adrenal abnormalities exist, and it is likely that the clinical syndrome encompasses several different etiologies.91,92 Ovarian abnormalities, including abnormal steroidogenesis and follicular maturation, may be the primary cause of excess androgen secretion in some patients.93 In most, however, the syndrome is associated with increased LH secretion, and some 75% of patients with PCOS have elevated mean LH levels, increasing to 90% when recent spontaneous ovulation is excluded.94 In obese subjects, plasma LH tends to be lower, and mean LH is inversely related to body mass index (BMI). LH and GnRH pulse frequency are elevated, however, in both lean and obese women with PCOS.95 Desensitization of LH secretion by a long-acting GnRH agonist is followed by reduced androgen secretion, thus confirming the importance of LH stimulation of the ovary.96 Studies have demonstrated that the frequency and amplitude of LH pulses are usually increased in patients with PCOS.97,98 This suggests that a persistent rapid frequency of GnRH secretion causes excess LH synthesis and secretion, which in turn enhances androgen production by the ovary and failure of follicular maturation. Abnormal GnRH secretion could be viewed as being secondary to anovulation and consequent reduced ovarian progesterone secretion and is a factor in anovulatory women. An alternative view is that the abnormal pulsatile GnRH secretion reflects an underlying abnormality in PCOS.99–101 In normal cycles, estradiol and progesterone inhibit pulse frequency in the luteal phase, and if a relative hypothalamic insensitivity to these steroids were present, persistently increased GnRH and LH secretion would result. Many patients with PCOS had an onset of symptoms soon after pubertal maturation, a time when the ability to secrete GnRH at a rapid frequency is reacquired. Thus, if adolescent girls destined to develop PCOS were relatively resistant to estradiol and progesterone slowing of GnRH secretion, the normal luteal-phase slowing may not occur. This lack of normal slowing would be expected to result in a relative deficiency in FSH secretion, with consequent impaired follicular maturation and infrequent ovulation. Over time, increased GnRH pulse frequency and LH secretion would increase ovarian androgen production and lead to cyst formation.
Evidence to support this concept is found in adolescents with hyperandrogenemia. In half of the adolescent girls with anovulation studied, LH pulse amplitude and frequency were increased, and this increase was associated with elevated plasma androgens, estradiol, and progesterone.102–104 A detailed study over 24 hours confirmed these findings. Compared with age-matched controls, hyperandrogenemic adolescent girls had higher LH pulse frequency (one pulse every 80 minutes) and mean LH during both waking and sleeping hours.105 It is uncertain whether changes consistent with PCOS will later develop in these adolescents, but only 40% subsequently established ovulatory cycles.104 Excess ovarian secretion of testosterone in adolescent girls appears to be related to pre- and peripubertal obesity. Obesity in adolescence has increased markedly during the past 30 years, and in 2004 some 18% of girls aged 6 to 19 years were > 95th percentile BMI for age. Testosterone is elevated in more than 50% of obese girls, and as sex-hormone binding globulin is suppressed, mean free testosterone may increase threefold across puberty.106 This was most evident in pre- and early-pubertal girls and was associated with marked hyperinsulinemia, with increased LH playing a major role in later puberty.107 Other evidence suggests that elevated androgens may interfere with normal regulation of GnRH secretion during puberty. In pre- and early-pubertal girls, testosterone exceeds estradiol concentrations in plasma by 10-fold.108,109 This exposure of the hypothalamus to androgens may reduce GnRH sensitivity to steroid inhibition and, as puberty progresses, lead to higher circulating levels of both LH and ovarian steroids.110 Studies in monkeys and sheep have shown that prenatal exposure to elevated androgens results in reduced GnRH inhibition by progesterone and elevated plasma LH during subsequent adolescence and adulthood.111,112
To explore these possibilities, the ability of physiologic luteal concentrations of estradiol and progesterone to suppress the rapid frequency of GnRH secretion has been assessed in women with PCOS.113 Administration of estrogen and progesterone initially slowed the frequency of GnRH secretion (by day 10), with later persistent slowing and marked reduction in LH pulse amplitude. Withdrawal of ovarian steroids was associated with increased GnRH pulse frequency, a selective increase in FSH, temporary normalization of the LH/FSH ratio to unity, with follicular maturation in all subjects and ovulation in some. These data show that luteal steroids can suppress the frequency of GnRH secretion in women with PCOS, but recent studies have indicated that the hypothalamic GnRH pulse generator is relatively resistant to the inhibitory effects of sex steroids. After a combined oral contraceptive, GnRH pulse frequency was higher in women with PCOS than in controls, both during treatment and after steroid use was discontinued.114 Similarly, administration of estradiol and progesterone for 7 days to normal controls and patients with PCOS showed that GnRH pulse frequency was suppressed to a greater degree in controls after exposure to similar levels of steroids.115 Lower plasma levels of progesterone (<10 ng/mL) were more effective in suppressing GnRH pulse frequency in normal controls than in women with PCOS, while higher concentrations of progesterone suppressed GnRH pulse frequency to a similar degree in both groups. These findings suggest that the GnRH pulse generator is relatively resistant to suppression by progesterone in women with PCOS.
Subsequent studies have indicated that the hypothalamic insensitivity to progesterone reflects the effects of hyperandrogenemia.116 When women with PCOS were pretreated with the androgen receptor blocker flutamide, the ability of lower concentrations of progesterone to suppress GnRH pulse frequency was restored to normal (Fig. 128-10). Plasma testosterone was not reduced, and basal LH pulsatility was not altered, suggesting that excess androgens selectively impair progesterone inhibition of the GnRH pulse generator. These data strongly support a role for hyperandrogenemia in the etiology of the elevated LH in PCOS, and data in adolescents support this concept. Similar insensitivity to progesterone is present in 50% of hyperandrogenic adolescents,117 which supports a role for hyperandrogenemia during puberty in the genesis of abnormal LH secretion in PCOS.118
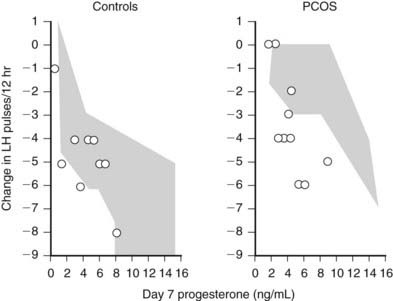
FIGURE 128-10. Effect of the androgen receptor blockade by flutamide on progesterone suppression of luteinizing hormone (LH) pulse frequency. Both normal control and women with polycystic ovary syndrome (PCOS) received 250 mg of flutamide twice daily for 5 weeks. During the last 7 days, estradiol (E2, plasma concn approx 120 pg/nL) and variable doses of progesterone (P) were also given. Blood was drawn every 10 minutes for 12 hours before and on day 7 of E2 + P treatment and pulsatile LH secretion analyzed. The data (circles) show the decrement in LH pulse frequency after 7 days of E2 + P as a function of plasma progesterone on day 7. The shaded areas indicate the range of response in the absence of flutamide pretreatment. Flutamide had little effect in controls but enhanced the ability of low doses of progesterone to inhibit LH/gonadotropin-releasing hormone [GnRH]) pulse frequency in women with PCOS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








