FIGURE 123-1. Mammary gland of human female at birth formed by several excretory ducts, ending in terminal ducts. A, Detail of the inset showing the club-shaped terminal end bud from which lengthening and further divisions of the virginal ducts originate. B, Cross section at the level shown in “A”; the duct is limed by the two layers of cells. Proliferation takes place chiefly in the basal cells. The inner cells have secretary properties from which the “witch milk” is formed. Toluidine blue ×25.
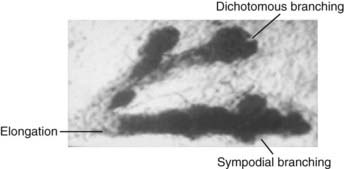
FIGURE 123-2. Before the onset of puberty in human females, the ducts grow and divide on a dichotomous and sympodial basis, a ball-shaped end bud sprouts from the duct, and new branches and twigs develop from the terminal and lateral end buds. Toluidine blue ×25.
The ducts grow, divide, and form club-shaped terminal end buds. These structures give origin to new branches, twigs, and small ductules or alveolar buds (see Fig. 123-2). We have coined the term alveolar bud to identify those structures that are morphologically more developed than the terminal end bud but yet are more primitive than the terminal structure of the mature structure, which is called an acinus. Alveolar buds cluster around a terminal duct, forming the type 1 lobule or virginal lobule (Fig. 123-3). Each cluster is composed of approximately 11 alveolar buds. Lobule formation in the female breast occurs within 1 to 2 years after onset of the first menstrual period. Full differentiation of the mammary gland is a gradual process that takes many years; in some cases, if pregnancy does not supervene, it is never attained.
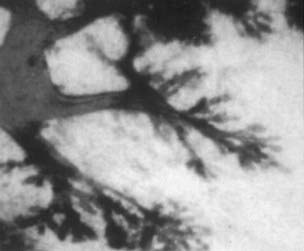
FIGURE 123-3. Whole mount preparation of breast tissue from an 18-year-old nulliparous woman showing lobules type 1. Toluidine blue ×25.
The Mature Breast
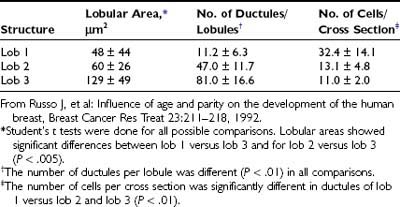
Study of normal breast tissue in adult women identifies two additional and more mature types of lobule, designated types 2 and 3 (Figs. 123-4 and 123-5). The transition from type 1 to the more mature types 2 and 3 represents a gradual process with sprouting of an increased number of new alveolar buds. In type 2 and 3 lobules, these are now called ductules; they increase in number from approximately 11 in the type 1 lobule to 47 in the type 2 lobule and 80 in type 3, respectively (Fig. 123-6 and Table 123-1). The increase in number results in a concomitant increase in the size of the lobules and a reduction in the size of each individual structure.3,8
FIGURE 123-4. Whole mount preparation of human breast tissue from a 24-year-old nulliparous woman showing lobules type 2. Toluidine blue ×25.
FIGURE 123-5. Whole mount preparation of human breast tissue from a 35-year-old parous woman containing lobules type 3. Toluidine blue ×25.
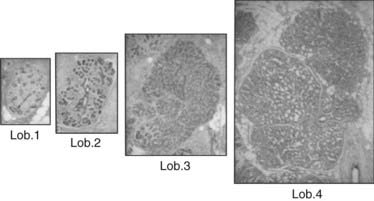
FIGURE 123-6. Lobules type 1, 2, 3, and 4 taken at the same magnification (2.5×) and stained with hematoxylin and eosin (H&E).
NULLIPAROUS WOMEN
In these patients, breast tissue contains more undifferentiated structures such as terminal ducts and type 1 lobules, although occasional type 2 and 3 lobules are seen. This pattern remains constant throughout the reproductive years unless pregnancy ensues. Type 2 lobules are present in moderate numbers during the early reproductive years but sharply decrease after age 23, whereas the number of type 1 lobules remains significantly higher. This observation suggests that a certain percentage of type 1 lobules might have progressed to type 2 lobules, but the number of type 2 lobules that progress to type 3 is significantly lower than in parous women.
PAROUS WOMEN
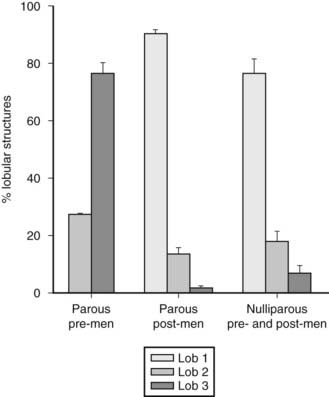
In this group, the predominant structure is the most differentiated lobule (i.e., type 3). The number of type 3 structures peaks during the early reproductive years and decreases after the fourth decade of life. A history of parity between the ages of 14 and 20 years correlates with a significant increase in the number of type 3 lobules that remain present as the predominant structure until the age of 40. At that time, the number of type 3 lobules decreases owing to their involution to predominantly type 1 lobules (Fig. 123-7).9
Pattern of Distribution of Cells Positive for Estrogen Receptor and Progesterone Receptor in Relation to Proliferating Cells in the Mammary Gland
Even though the breast is influenced by a myriad of hormones and growth factors,10–17 estrogens are considered to play a major role in promoting the proliferation of both normal and neoplastic breast epithelium.18–24 Estrogens may influence the proliferative activity of mammary epithelial cells by one of at least three different mechanisms: direct receptor-mediated stimulation,19,25–32 indirect autocrine/paracrine loops,22–23 or interruption of negative feedback factors (i.e., the effect of estrogen in removing one or more inhibitory factors present in the serum).21,33 Unfortunately none of these mechanisms has been precisely defined with regard to its role in the normal development and differentiation of the breast, or in the initiation and progression of the neoplastic process.
A greater degree of complexity emerged recently with the cloning of a gene encoding a second type of ER, the ER beta. This receptor is present in the mouse, rat, and human and has an affinity for estradiol similar to that of the classical ER (now identified as ER alpha). In addition, it has now become possible to localize modulators of estrogen and progesterone receptor function in tissue. ER- and PR-mediated transcription often involves binding of co-activators to the transcription complex, a mechanism that enhances receptor function. One of these co-activators, SRC-1, has been cloned and can be localized with specific antibodies. Knockout of SRC-1 causes decreased growth and development of the uterus, prostate, testis, and mammary gland.34 Examination of its localization in mammary tissue can provide insight into the local regulation of ER functionality.35 These new findings have prompted a reevaluation of the estrogen signaling system in mammary tissue.36–38
Cell proliferation is indispensable for the normal growth and development of the breast. The fact that the normal epithelium contains receptors for both estrogen and progesterone lends support to the receptor-mediated mechanism as a major player in the hormonal regulation of breast development. The essential role for ER alpha and PR in mammary gland development has recently been confirmed by knockout mice lacking functional receptors. ER alpha knockout mice display grossly impaired ductal epithelial proliferation and branching. PR knockout mice display significant ductal development but decreased arborization and absence of alveolar differentiation.39,40
Clinical studies have defined the relationship of cell proliferation to estrogen and progesterone production by correlation with phases of the menstrual cycle in women. The breast epithelium of sexually mature, normally cycling women does not exhibit maximal proliferation during the follicular phase of the menstrual cycle,14–18,41–46 when estrogens reach peak levels of 200 to 300 pg/mL and progesterone is less than 1 ng/mL.47 Maximal proliferative activity occurs during the luteal phase, when progesterone reaches levels of 10 to 20 ng/mL and estrogen levels are two- to threefold lower than during the follicular phase.47 In breast cells grown in vitro, or when breast tissues are implanted in athymic nude mice, however, estrogens alone stimulate cell proliferation, and progesterone has no effect or even inhibits cell growth.22,25,45,46
Our studies of the proliferative activity of the mammary epithelium in both rodents and humans have demonstrated that cell division varies with the degree of differentiation of the mammary parenchyma.10–13,18,48–50 In women, the highest level of cell proliferation is observed in the undifferentiated type 1 lobules (lob 1), which are present in the breasts of young nulliparous subjects.10–1318 The progressive differentiation of type 1 lobules into type 2 and 3 results in a concomitant reduction of the proliferative activity of the mammary epithelium.9–13 Further differentiation into type 4 lobules, characteristic of breast tissue during end of pregnancy and during lactation, further lowers the rate of proliferation.
Understanding of the relationship between lobular differentiation, cell proliferation, and hormone responsiveness of the mammary epithelium is just beginning to be unraveled. An important finding is that ER alpha and progesterone receptor (PgR) content in the lobular structures of the breast is directly proportional to the rate of cell proliferation. Proliferation and receptor content is maximal in the undifferentiated type 1 lobule and decreases progressively in types 2, 3, and 4.
Distribution of Proliferating Cells in Relation to the Presence of Steroid Receptors and Their Co-activators
Use of Ki67 immunohistochemical techniques allows detection of proliferating cells in tissue. The highest percentage of positive cells occurs in type 1 lobules (Fig. 123-8 and Table 123-2); a threefold lower percentage is found in type 2 lobules and a tenfold decrement in type 3 (see Figs. 123-8 and 123-9A and 123-9B; see also Table 123-2). The proliferating cells are almost exclusively found in the epithelium-lining ducts and lobules, and only occasional positive cells are found in the myoepithelium, or in the intralobular and interlobular stroma. The same pattern of reactivity is observed in tissue sections incubated with ER and PgR antibodies. Positive cells are found exclusively in the epithelium, with type 1 lobules containing the highest number of positive cells. Their number decreases progressively in type 2 and 3 lobules (see Fig. 123-8 and Table 123-2).
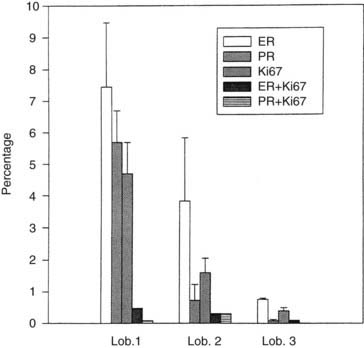
FIGURE 123-8. Percentage of cells positive for estrogen receptor (ER), progesterone receptor (PgR), Ki67, and of cells positive for both ER and Ki67 (ER+Ki67) or PgR and Ki67 (PgR+Ki67) (ordinate). Cells were quantitated in lobules type 1 (lob 1), type 2 (lob 2), and type 3 (lob 3) of the breast (abscissa).
Table 123-2. Distribution of Ki67-, ER-, and PgR-Positive Cells in the Lobular Structures of the Human Breast
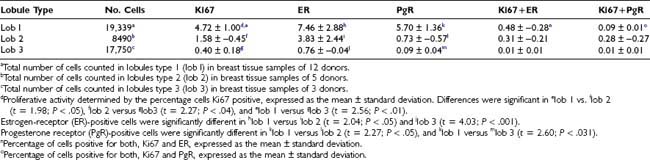
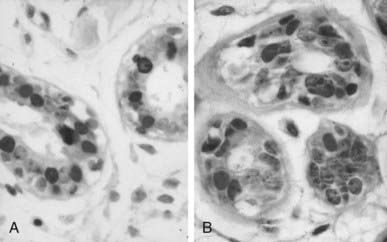
FIGURE 123-9. Lobules type l (lob 1) ductules of the human breast. A, The single-layered epithelium lining the ductule contains Ki67-positive cells (brown nuclei) and estrogen receptor (ER)-positive cells (red-purple nuclei) (×40). B, The single-layered epithelium lining the ductule contains brown Ki67-positive cells and red-purple progesterone receptor (PgR)-positive cells. Sections were stained with 3,3′-diaminobenzidine (DAB)/alkaline phosphatase-vector red, with light hematoxylin counterstain, and were photographed at ×40.
Use of double-staining procedures for Ki67 and ER or PgR has allowed quantitation in the same tissue sections of the spatial relationship between proliferating and receptor-positive cells. This can be done with techniques using two colors or through immunofluorescent methods. The percentages of ER- and PgR-positive cells in type 1 lobules do not differ significantly, at 7.5% and 5.7%, respectively (see Table 123-2). In type 2 lobules, the percentages of ER- and PgR-positive cells are reduced to 3.8% and 0.7%, respectively; in type 3, their numbers become negligible (see Table 123-2). Similarities have been noted in the relative percentages of Ki67-, ER- and PgR-positive cells and in the progressive reduction in percentage of positive cells as the lobular differentiation progressed. However, those cells positive for Ki67 are not the same as those that reacted positively for ER or PgR (see Figs. 123-9A and 123-9B and Table 123-2). Very few cells—less than 0.5% in type 1 lobules, and even fewer in type 2 and 3—appear positive for both Ki67 and ER (Ki67+ER) (see Table 123-2). Double reactivity with this technique is identified by darker staining of the nuclei, which appear dark purple-brown. The percentage of cells exhibiting double labeling with Ki67 and PgR antibodies (Ki67+PgR) in type I lobules is lower than the percentage of double-labeled ER-positive cells (see Table 123-2).
The content of ER and PgR in normal breast tissue, as detected immunocytochemically, varies with the degree of lobular development in a linear relationship with the rate of cell proliferation of the same structures. The utilization of a double-labeling immunocytochemical technique has allowed determination of whether the receptor-positive cell population is the same population that is proliferating (i.e., Ki67-positive cells). Clearly this was not the case, as was reported by other authors.44 The findings that proliferating cells are different from those that are ER and PgR positive support data that indicate that estrogen controls cell proliferation through an indirect mechanism. A likely explanation is that ER-positive cells respond to estrogen with an increase in growth factor production, which acts in a paracrine fashion on neighboring ER-negative cells. This paracrine phenomenon has also been demonstrated using supernatants of estrogen-treated ER-positive cells that stimulate the growth of ER-negative cell lines in culture and in vivo in nude mice bearing ER-negative breast tumor xenografts.51,52 A paracrine mechanism can also explain inhibition of cellular proliferation. ER-positive cells treated with antiestrogens secrete transforming growth factor (TGF) alpha, which inhibits the proliferation of ER-negative cells.29
The proliferative activity and the percentage of ER- and PgR-positive cells are highest in type 1 lobules in comparison with types 2 through 4, which compose the normal breast. These findings provide a mechanistic explanation for the higher susceptibility of these structures to transformation by chemical carcinogens in vitro,53,54 supporting as well the observations that type 1 lobules are the site of origin of ductal carcinomas.55 However, the relationship between ER-positive and ER-negative breast cancers is not clear.56–58 It has been suggested that ER-negative breast cancers may result from loss of the ability of cells to synthesize ER during clinical evolution of ER-positive cancers, or that ER-positive and ER-negative cancers may be different entities.56,58 It is postulated that type 1 lobules contain at least three cell types: ER-positive cells that do not proliferate, ER-negative cells that are capable of proliferating, and a small proportion of ER-positive cells that can proliferate as well (Fig. 123-10). Therefore, estrogen might stimulate ER-positive cells to produce a growth factor that in turn stimulates neighboring ER-negative cells capable of proliferating (see Fig. 123-10). In the same fashion, the small proportion of cells that are ER positive and can proliferate could be the source of ER-positive tumors. The possibility exists as well that the ER-negative cells may convert to ER-positive cells. Conversion of ER-negative to ER-positive cells has been reported.59,60 The newly discovered ER beta opens the possibility that those cells traditionally considered to be ER alpha negative might be ER beta positive.36,37 It has been found recently that ER is expressed during the immortalization and transformation of ER-negative human breast epithelial cells, supporting the hypothesis of conversion from a negative to a positive receptor cell.61
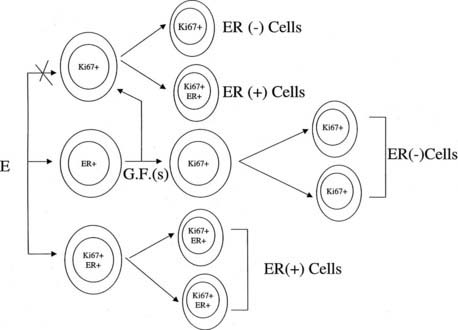
FIGURE 123-10. Schematic representation of the postulated pathways of estrogen actions on breast epithelial cells. Cells expressing three different phenotypes might be present in the epithelium: Estrogen receptor (ER)-negative Ki67-positive cells that are capable of proliferating, ER-positive cells that do not proliferate, and a small proportion of ER- and Ki67-positive cells. Estrogen might stimulate ER-positive cells to produce a growth factor that in turn stimulates neighboring ER-negative cells capable of proliferating. ER+Ki67-positive cells can proliferate and could be stimulated by estrogen to originate ER-positive daughter cells and probably tumors. ER-negative cells may convert to ER-positive cells during neoplastic transformation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree