FIGURE 140-1. Diagrammatic representation of sources of androgen and estrogen and the physiology of their actions. Most testosterone and approximately 15% of estradiol are secreted directly by the testes. Both bind to sex hormone–binding globulin (SHBG) and to a lesser extent albumin, while a small amount circulates in the free state. The free and albumin-bound steroids (the “bioavailable fraction”) enter extragonadal tissues, many of which contain the aromatase enzyme, which converts some of the testosterone to estradiol. This enzyme also converts androstenedione of adrenal origin to estrone, which may be further converted to the more potent estrogen, estradiol, through the action of 17β-hydroxysteroid dehydrogenase. The bioavailable testosterone, estradiol and estrone (derived from direct glandular secretion and extraglandular production) enter target tissues, where they bind to their respective receptors and initiate gene transcription. In addition, some of the testosterone is converted to the more potent metabolite, dihydrotestosterone, through the action of 5α-reductase. Dihydrotestosterone binds to the same androgen receptors as testosterone. Multiple processes can alter the pathways of estrogen and androgen production and action, resulting in gynecomastia from an enhanced estrogen effect or a diminished androgen effect at target-tissue levels.
(From Braunstein GD: Clinical practice. Gynecomastia, N Engl J Med 357:1229–1237, 2007.)
Physiologic Forms of Gynecomastia
NEWBORN GYNECOMASTIA
The high levels of estradiol and progesterone produced by the mother during fetal life stimulate newborn breast tissue, which persists for several weeks after birth in boys. Minimal breast discharge called “witch’s milk” can result.
PUBERTAL GYNECOMASTIA
Beginning at age 11, approximately 30% of boys develop detectable gynecomastia (i.e., glandular tissue > 0.5 cm in diameter). By age 14, gynecomastia is detectable in 65%.4 While usually bilateral, gynecomastia is unilateral in 20% of cases. Spontaneous regression occurs in the majority of boys after 1 to 2 years; long-term gynecomastia persists in less than 5%.4 Hormonal alterations in pubertal boys with gynecomastia are not found consistently and are variably reported as low free testosterone, increased estradiol, elevated estradiol/testosterone ratio, and increased TeBG levels.5–10
ADULT GYNECOMASTIA
Several studies11–15 document the prevalence of palpable breast tissue in normal volunteers and in hospitalized patients. One-third of normal military recruits have between 2 and 4 cm of palpable glandular tissue.11 Only 4% had tissue that measured greater than 4 cm. The percentage gradually increased with age from 17% in men younger than 19 years to 41% between ages 40 to 44.11 Three other studies in hospitalized patients12–14 reported a prevalence of gynecomastia which reached 57% in men aged 45 to 59. Even though palpable breast tissue exceeded 2 cm in diameter, these men had minimal awareness of the existence of the condition. Gynecomastia appears to increase with advancing age, with 72% of hospitalized men aged 50 to 69 having this condition in one study.14 As proof of the validity of these clinical observations, one autopsy study confirmed the impression of gynecomastia detected by physical exam in 60 consecutive cases and ruled out the possibility of pseudogynecomastia (i.e., the presence of subareolar fat tissue only).14 Another autopsy study independently corroborated the frequency with which gynecomastia occurs.16 Histologic examination of breast tissue at autopsy in men with asymptomatic, longstanding gynecomastia usually demonstrates dilated ducts with periductal fibrosis, stromal hyalinization, and increased subareolar fat.16–19 In those with more recent onset of gynecomastia, hyperplasia of the ductal epithelium, infiltration of periductal tissue with inflammatory cells, and increased subareolar fat is observed.
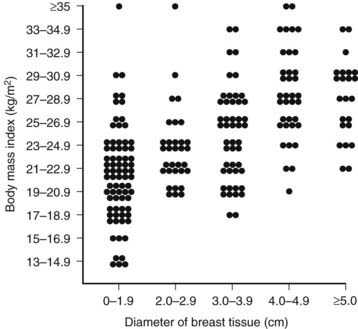
In 83% of hospitalized men with gynecomastia, breast tissue diameter did not exceed 5 cm.14 The etiology of this form of physiologic gynecomastia is not clear, but correlative evidence suggests increased aromatase activity. The total amount of aromatase activity in the body increases as a function of body fat, and the percentage of patients with gynecomastia appears to increase as a function of body mass index (Fig. 140-2). Notably, the diameter of breast tissue correlated significantly (r = 0.52) with body mass index in a group of 214 well-studied men (Fig. 140-3).20 Animal models support the concept that aromatase excess causes gynecomastia. Two different transgenic mouse strains with overexpression of aromatase in breast tissue exhibit substantial breast development which mimics gynecomastia in men.21,22
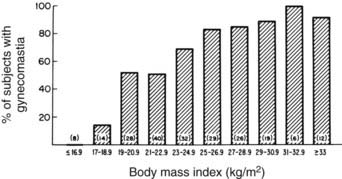
FIGURE 140-2. Correlation of percentage of subjects with gynecomastia and body mass index.
(From Niewoehner CB, Nutall FQ: Gynecomastia in a hospitalized male population, Am J Med 77:633, 1984.)
Pathologic Forms of Gynecomastia
Two subtypes of gynecomastia can be distinguished histologically.16,23,24 Glandular predominance occurs in 5% to 9% of patients and represents a process of recent hormonal stimulation of glandular elements. Stromal tissue predominates in 32% to 48% and reflects the aftermath of a prior hormonal imbalance with spontaneous resolution.
The distinction between physiologic and pathologic gynecomastia in adult men presents a challenge because of the common presence of breast enlargement in normal men and its association with obesity. Notably, not all experts agree that some forms of gynecomastia in adult men represent a normal finding.3,11,23 An operational definition of pathologic gynecomastia is provided here and is considered useful to the clinician in deciding the need for and the extent of evaluation. Accordingly, pathologic gynecomastia is arbitrarily defined as palpable tissue more than 4 cm in diameter, palpable tissue more than 2 cm which is tender, or palpable tissue more than 2 cm demonstrated to be gradually increasing in diameter on follow-up. True gynecomastia should be distinguished from pseudogynecomastia—fat tissue present underneath the nipple. Mammography may occasionally be necessary to make this distinction, particularly in obese patients (Fig. 140-4).25–27 Ultrasonography can also be used but is not considered as sensitive as mammography.28–31 Gynecomastia is considered physiologic if less than 2 cm in diameter or between 2 and 4 cm but nontender and not enlarging.
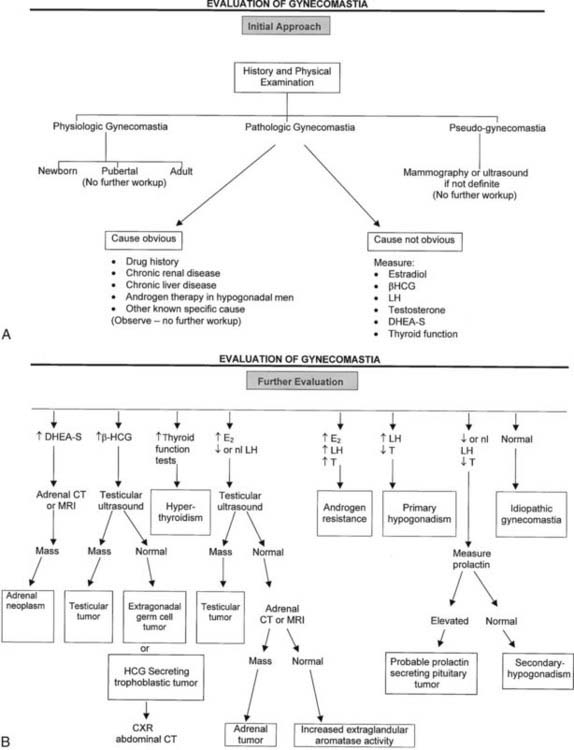
FIGURE 140-4. Algorithm depicting evaluation of patients with gynecomastia.
(Adapted from Braunstein GD: Aromatase and gynecomastia, Endocr Relat Cancer 6:315–324, 1999.)
A logical approach to the differential diagnosis is based on the physiologic framework represented in Fig. 140-1 and Table 140-1. An excess of estradiol, deficiency of testosterone, imbalance in the ratio of estradiol to testosterone, and abnormalities of regulatory hormones may be responsible for non–drug-related gynecomastia. A multitude of drugs which interact at each of these levels can also cause gynecomastia. The remainder of causes remains largely idiopathic or uncertain.
Table 140-1. Causes of Pathologic Gynecomastia
| Estradiol Excess |
| Testosterone Deficiency |
| Estradiol/Testosterone Imbalance |
| Regulatory Hormone Excess |
| Other Causes |
| Uncertain Causes |
| Idiopathic |
RELATED TO ELEVATED ESTRADIOL
Tumors
Adrenal tumors may secrete one or several hormones in excess. Adenomas or carcinomas of the adrenal may produce large amounts of estradiol directly.32,33 Alternatively, the adrenal tumor secretes androstenedione, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEAS), which are then aromatized in peripheral tissues to estradiol. These patients generally exhibit a rapid onset of gynecomastia in association with hypertension, elevated ketosteroids, elevated DHEA and DHEAS levels, and an adrenal mass.34
Testicular tumors of germ, sex cord, Leydig, or Sertoli cell origin can secrete an excess of estrogen or estrogen precursors. Tumors are large enough to be palpated in approximately half of patients,35–42 whereas the remainder require detection by testicular ultrasound. Choriocarcinomas of the testis may produce human chorionic gonadotropin (hCG) in situ and stimulate production of both estradiol and testosterone. Ectopic production of hCG by lung, kidney, liver and gastric carcinomas can also stimulate estradiol production and cause gynecomastia.
Aromatase Excess
Recent studies43 suggest that overexpression of the aromatase enzyme may be a unifying feature of several clinical causes of gynecomastia (Table 140-2). Obesity and hyperthyroidism are associated with an increase in total body production of aromatase.44,45 Aromatase also increases with aging, perhaps as a result of the increased percentage of total body fat.46
Table 140-2. Aromatase-Associated Causes of Gynecomastia
Adapted from Braunstein GD: Aromatase and gynecomastia, Endocr Relat Cancer 6:315–324, 1999.
Aromatase excess also explains published reports of familial gynecomastia. Early onset of gynecomastia is the cardinal clinical feature of these patients at presentation. Five families have been demonstrated to have marked overexpression of aromatase with up to 50% systemic conversion of androgens to estrogens.47–51 Five males in two generations of one family had gynecomastia and 10-fold higher levels of peripheral aromatization than normal. An additional kindred of familial gynecomastia from the time of the 18th Egyptian dynasty involved King Tutankhamen, his brother Smenkhare, his grandfather Amenophis III, and his uncle Akhenaton52 and could reflect a similar etiology. Familial gynecomastia and mental retardation also have been described in a syndrome linked to the DXS255 region of the X chromosome.53 Recent studies indicate that gain-of-function mutations cause aromatase excess and gynecomastia.51,54 In one of these families, regional rearrangements on chromosome 15q21 resulted in cryptic promoters of the aromatase gene as a mechanistic cause for aromatase overexpression.55 In another, re-arrangements between CYP19 (aromatase) and TRPM7 genes on chromosome 15q21.2 were implicated.51 Other familial disorders are associated with gynecomastia and testicular tumors. The Peutz-Jeghers syndrome is associated with aromatase excess, gynecomastia, Sertoli-cell tumors, pigmented lesions around the mouth, and colonic carcinoma.56–60 The testicular tumors contain amounts of aromatase which approach the levels in placenta, but levels of aromatizable substrate (i.e., testosterone and androstenedione) in testicular and peripheral vein samples are normal.56 The amount of aromatase enzyme present is sufficient to convert a large fraction of androgen to estrogen, resulting in estrogen excess. The Carney complex of primary, pigmented, nodular adrenal disease, myxomas, and lentigenes is familial and associated with calcified Sertoli-cell tumors of the testis.61,62
Several sporadic (rather than familial) neoplasms cause gynecomastia through overexpression of aromatase. Isolated Sertoli-cell tumors, hepatocellular carcinomas, and adrenocortical adenomas may overexpress aromatase, and melanomas contain aromatase and may be associated with gynecomastia.33,37–39,63,64 Choriocarcinomas contain high levels of aromatase as well, which may add to the effects of HCG in inducing gynecomastia.
Idiopathic gynecomastia may also reflect a sporadic increase in aromatase activity. Bulard et al.65 suggested that a mild increase in peripheral aromatase activity might be responsible for gynecomastia in subsets of patients with idiopathic gynecomastia. This conclusion was based on cultures of pubic-skin fibroblasts and demonstration of excessive aromatase activity in this tissue. Sasano et al. provided additional evidence by demonstrating that 11 out of 30 cases of gynecomastia had aromatase excess evidenced by immunochemical assessment of biopsy tissue using a monospecific antiaromatase antibody.66 A recent study examined aromatase polymorphisms in DNA from whole blood in 100 men with gynecomastia compared with 99 healthy controls.67 They found a marginally significant increase in T and TT single nucleotide polymorphisms (SNPs) at rs10046 in the 3′ untranslated region of exon 10 in the men with gynecomastia (40% versus 26.3%, P = 0.04). However, no direct evidence exists that these SNPs result in increased aromatase activity.
Recent molecular biological data suggest mechanisms whereby aromatase can be overexpressed. The aromatase gene utilizes nine alternate promoters to regulate transcription. Multiple different enhancers interact with DNA at the promoter sites to stimulate aromatase transcription. The tumors associated with aromatase overexpression commonly utilize promoters which respond to cyclic AMP as an enhancer. Taken together, the studies on tumors suggest a common metabolic abnormality associated with activation of a cyclic adenosine monophosphate (cAMP)-dependent signaling pathway. This gives rise to transcriptional transactivation of aromatase expression via alternate promoters I.3 and II in most tumors overexpressing aromatase.43
Excess Secretion of Precursors of Aromatase
Both congenital adrenal hyperplasia and adrenal tumors may produce gynecomastia through excess production of aromatizable androgens.68–70
Administration of Estrogen and Its Precursors
Estrogens
Several medications or topical creams provide estrogen or estrogenic substances for absorption and systemic action. Diethylstilbestrol (DES), given to patients with prostatic carcinoma or for other reasons, produces gynecomastia with a very high frequency.71 Epidemics of gynecomastia have occurred in which an excess of estrogens present in meats was ingested. Regulatory agencies inspect for illegal feeding of DES or other estrogens to animals prior to slaughter.72 However, the practice of estrogen use as an anabolic steroid continues in some areas and can result in gynecomastia if residual estrogens in meat remain sufficiently high. Inadvertent ingestion of oral contraceptives by boys from their mothers’ prescriptions or industrial exposure to oral contraceptives during the manufacturing process also can cause gynecomastia.73 Other drugs such as digitoxin may have intrinsic estrogenic properties and stimulate breast enlargement.74 Since this drug is administered to patients with severe systemic illnesses and often liver disease, other factors may be involved in the development of gynecomastia.
Environmental toxins such as embalming fluid may be estrogenic and produce gynecomastia.75 Unintended exposure to estrogen can occur from coital exposure to women using vaginal estrogen cream or even from women using estrogen-containing cosmetics.76 Exposure of prepubertal boys to estradiol creams utilized as a skin cream by their mothers can cause gynecomastia.77 In France, estrogen cream is distributed over large portions of the body as a means of transdermal estrogen delivery. Gynecomastia in their male partners could occur in this instance as well.
Estrogen Precursors
Exogenous androgens which can be converted to estrogen through aromatization are a common cause of gynecomastia. Testosterone enanthate and cypionate increase estradiol concentrations substantially78 and commonly result in transient gynecomastia. This is most common in hypogonadal boys during testosterone treatment, but adult athletes using anabolic steroids and men receiving testosterone as a contraceptive also may experience this problem.78,79 When hCG is given exogenously to hypogonadal patients, it raises both testosterone and estradiol levels and may be associated with gynecomastia but usually only for a transient period of several months.
RELATED TO TESTOSTERONE DEFICIENCY
Androgen levels gradually decline with aging in men, with free-testosterone levels falling to a greater extent than total. Notably, 50% of men in their 70s have a low free-testosterone concentration.80 Free-estrogen levels fall as well, but to a lesser extent than androgens. This results in an increase in estrogen/androgen ratio,81 a finding which could explain the high prevalence of gynecomastia in men aged 50 to 69.14 Patients with hypogonadotropic hypogonadism may have incomplete gonadotropin deficiency and relatively preserved follicle-stimulating hormone (FSH) secretion. Under these circumstances, androgen secretion may be impaired to a greater extent than that of estrogen, and gynecomastia may be present. More commonly, this is observed in the “fertile eunuch syndrome” of incomplete gonadotropin deficiency, but patients with panhypopituitarism also may have gynecomastia, presumably due to this mechanism.82 Fifty percent of anorchic patients also have gynecomastia, perhaps based upon absence of testicular androgens but adrenal secretion of aromatizable precursors of estrogen.23
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree