FIGURE 81-1. Onset of eye symptoms and diagnosis of Graves’ ophthalmopathy relative to the time of diagnosis of hyperthyroidism (0 on the horizontal axis). A, The number of patients who first experienced eye symptoms within a given 6-month period is expressed as a percentage of the entire group. B, The number of patients in whom Graves’ ophthalmopathy was first diagnosed within a given 6-month period.
(From Bartley GB, Fatourechi V, Kadrmas EF, et al: The chronology of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol 121:426–434, 1996. Published with permission from the American Journal of Ophthalmology. Copyright by the Ophthalmic Publishing Company.)
The concept of a pathophysiologic link between Graves’ thyrotoxicosis and ophthalmopathy has been challenged on the basis of the existence of patients with euthyroid ophthalmopathy or unilateral eye disease and a poor numeric correlation between thyroid-stimulating antibody levels and the presence of eye disease. However, these arguments have been superseded by the demonstration of subtle thyroid abnormalities in most “euthyroid” ophthalmopathy patients,6,7 the finding of contralateral eye muscle abnormalities in most patients with apparent unilateral disease,1 and the demonstration of a definite qualitative association between the severity of ophthalmopathy and other peripheral manifestations of Graves’ disease and thyroid-stimulating antibody titers.8,9
Studies investigating the pathogenesis of Graves’ ophthalmopathy have served to expand the understanding of ophthalmopathy beyond early purely mechanical descriptions. These efforts have focused on the identification of orbital fibroblasts as active participants in the retrobulbar immune response and on the elucidation of immune mechanisms responsible for the activation of these cells. In addition, recent studies have identified thyrotropin (the thyroid-stimulating hormone receptor [TSHR]) as an important orbital autoantigen.
Management of Graves’ ophthalmopathy requires a carefully integrated approach involving the endocrinologist and the ophthalmologist, with the goal of preserving the patient’s vision and restoring favorable self-perception and quality of life.10 In this chapter, we provide an examination of the present state of knowledge regarding the pathogenesis of Graves’ ophthalmopathy and an up-to-date review of current methods in the diagnosis and management of this disorder.
Epidemiology
Clinically evident ophthalmopathy occurs in 10% to 25% of unselected patients with Graves’ disease if lid signs are excluded as a diagnostic feature, in 30% to 45% if lid signs are included,3,11 and in approximately 70% of patients without overt eye disease if computed tomography (CT) or increased intraocular pressure on upgaze is used to establish the diagnosis.12–15 Magnetic resonance imaging (MRI) reveals extraocular muscle enlargement in 71% of patients without overt findings on physical examination.16 Fortunately, fewer than 5% of patients with Graves’ disease have severe ophthalmopathy.17 The age-adjusted incidence of Graves’ ophthalmopathy in the population of Olmsted County, Minnesota, was found to be 16 cases per 100,000 population per year for women and 2.9 for men.18 A bimodal distribution was noted, with peak incidence in the age groups 40 to 44 years and 60 to 64 years in women and 45 to 49 years and 65 to 69 years in men. Additional peripheral manifestations of Graves’ disease such as dermopathy and acropachy occur with lower frequency. Thyroid dermopathy is found in 4% to 15% of patients with clinically evident Graves’ ophthalmopathy, and 7% of dermopathy patients will also have thyroid acropachy.4
Pathogenesis
MECHANICAL FACTORS
Orbital imagining in Graves’ ophthalmopathy reveals an increase in the volume of soft tissue within the orbit, including the extraocular muscles and adipose and connective tissues. The bones of the orbit are unyielding in response to the pressure generated by this increase in tissue volume. Therefore, forward displacement of the globe, or proptosis, may result and can serve as a natural means of orbital decompression. The degree of proptosis is limited, to varying degrees, by the orbital septum and tethering action of the extraocular muscles on the globe. Although CT scans show that the increased volume of orbital tissues is due to enlargement of both the orbital fat and the extraocular muscles in most patients, some patients appear to have predominantly adipose tissue or extraocular muscle involvement.19 In addition, the orbital fatty tissue volume appears to be more closely correlated with the degree of proptosis than is the extraocular muscle volume. Besides resulting in forward displacement of the globe, the increase in orbital tissue volume causes impairment of venous and lymphatic outflow from the orbit, leading to periorbital and conjunctival edema.
PREDISPOSING INFLUENCES
Genetic Influence
The autoimmune thyroid diseases, including Graves’ disease and Hashimoto’s thyroiditis, are genetically complex and develop as a result of interactions between susceptibility genes and nongenetic factors. Studies performed in the past decade have identified several genes that either contribute directly to the development of these diseases or appear to be linked to important susceptibility genes.20–23 However, no unique gene associations distinct from those predisposing to Graves’ disease itself have been convincingly identified in patients with severe ophthalmopathy. A study of the families of 114 consecutive patients with Graves’ ophthalmopathy did not support a major role for familial factors in the development of severe ophthalmopathy.24 These findings suggest that environmental factors, rather than major susceptibility genes, are likely to predispose certain individuals with Graves’ disease to the development of ophthalmopathy.
Tobacco Smoking
A striking association between cigarette smoking and Graves’ ophthalmopathy has been noted in several studies. One group of researchers found that current smokers or ex-smokers represented 64% of patients with Graves’ disease and ophthalmopathy, compared with 47.9% of those without overt ophthalmopathy, 23.6% of patients with toxic nodular goiter, 30.4% of individuals with nontoxic goiter, 33.5% of patients with Hashimoto’s thyroiditis, and 27.8% of normal controls25 (Fig. 81-2). These effects do not appear to be related to behavioral changes associated with thyrotoxicosis, nor do they seem to be due to differences in the age, gender, or educational background of the study patients and their controls.26 Although the mechanisms underlying this association remain unknown, smokers have larger thyroids and higher thyroglobulin levels than do nonsmokers. Other contributors might include the effect of orbital hypoxia27 or the action of free radicals contained in tobacco smoke on orbital fibroblast proliferation.28 Smokers have lower levels of interleukin-1 receptor antagonists than do nonsmokers,29 which could lead to enhanced negative effects of interleukin-1 on the orbital inflammatory process.30 Smokers in one study were more likely to experience aggravation of ophthalmopathy after radioiodine therapy than were nonsmokers.17,31 Finally, smoking was shown to adversely influence the course of the eye disease during treatment with corticosteroids and orbital radiotherapy.32 The strong association between smoking and ophthalmopathy might provide important clues to the pathogenesis of this disorder in at least a subset of patients.
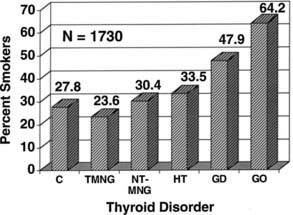
FIGURE 81-2. The prevalence of past and present cigarette smokers among patients with various thyroid disorders. Percentages shown (on the vertical axis) represent the prevalence of cigarette smokers in the group. Normal controls (C), toxic nodular goiter (TMNG), nontoxic goiter (NT-MNG), Hashimoto’s thyroiditis (HT), Graves’ disease without eye involvement (GD), and Graves’ disease with ophthalmopathy (GO) are shown. Smokers represented significantly increased proportions among patients with diagnoses of Graves’ disease and Graves’ ophthalmopathy.
(From Burch HB, Wartofsky L: Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev 14:747–793,
Age and Gender
Patient age and gender may also affect the prevalence and severity of Graves’ ophthalmopathy. The female-to-male ratio in series of ophthalmopathy patients has ranged from 1.8 to 1 to 2.8 to 1, which is considerably lower than the ratio of 8 to 1 generally cited for Graves’ disease in general.33 Men also appear to be disproportionately represented among patients with severe ophthalmopathy. One study found a female-to-male ratio of 9.3 to 1 in patients with mild disease, 3.2 to 1 in patients with moderate disease, and 1.4 to 1 in patients with severe ophthalmopathy.34
Therapy for Thyrotoxicosis
An area of considerable controversy concerns the impact of the choice of therapy for hyperthyroidism on the subsequent course of ophthalmopathy in Graves’ disease.35–37 Several retrospective studies have examined this issue, often with conflicting results.3 More recently, prospective trials have focused on this area.38–42 Two studies allow a direct comparison between the effects of radioiodine, thyroidectomy, or antithyroid drug therapy.39,42 In the first of these studies, 114 patients aged 35 to 55 years were randomized to receive radioiodine, thyroidectomy, or methimazole.39 As assessed with an ophthalmopathy index, new or worsened eye involvement occurred in 10% of patients treated medically, 16% of those treated surgically, and 33% of those treated with 131I. Interpretation of this small study was hampered by a higher prevalence of cigarette smokers among the radioiodine-treated patients, a period of hypothyroidism before thyroid hormone therapy was started in patients treated with radioiodine, and a requirement for multiple doses of radioiodine in nearly half the patients receiving this therapy, which suggests both refractory disease and a mechanism by which repeated release of thyroid antigen may have contributed to the autoimmune response.1,43 However, these authors later showed that elevated TSH levels after 131I treatment did not correlate with worsening eye status,44,45 and that eye changes in smokers were no more frequent than eye changes in nonsmokers. Finally, the authors noted that most patients receiving multiple doses of 131I had worsening of eye status before the second dose of radioiodine was given.46
Another randomized trial compared eye changes in 150 patients treated with radioiodine, 148 patients receiving methimazole alone, and a third group of 145 patients receiving both radioiodine and prophylactic prednisone.42 Patients were monitored for 1 year and were assessed for change by largely objective criteria, as well as an activity score and patient self-assessment. The groups were similar with regard to percentages of smokers or patients with preexisting ophthalmopathy. Hypothyroidism or persistent hyperthyroidism was corrected within 2 to 3 weeks of testing performed every 1 to 2 months. Worsening of eye disease occurred within 6 months after radioiodine therapy in 15% of patients versus 2.7% of patients who received antithyroid drugs alone. Seventy-four percent of the patients who experienced worsening eye status after radioiodine therapy had preexisting ophthalmopathy. The eye changes that occurred were largely mild and returned to baseline within 2 to 3 months in 65% of cases. However, eight patients (5%) in the radioiodine group required orbital radiation or high-dose corticosteroids as compared with one patient in the methimazole group and no patients in the combined prednisone and radioiodine group. Patients who had preexisting ophthalmopathy and those who were smokers were more likely to have progression after radioiodine administration.
A logical interpretation of the two prospective controlled studies in this area is that patients with Graves’ disease who are treated with radioiodine therapy have an increased risk for worsening eye status compared with those who are treated with antithyroid drugs alone. An alternative explanation for these findings is that patients who are treated with antithyroid drugs experience beneficial effects from this therapy47 rather than harmful effects from the radioiodine.48 In any event, the ocular worsening that was noted was generally mild, reversible, and short-lived. Patients with preexisting eye disease and those who smoke or have severe thyrotoxicosis appear to be more likely to experience this complication, but concurrent use of corticosteroids negates this risk.1,49,50
Despite continuing uncertainty concerning the use of radioiodine thyroid ablation, results of these studies appear to have influenced management practices in patients with Graves’ hyperthyroidism. A recent survey of members of the European Thyroid Association revealed that 60% of respondents believe that their choice of therapy would be influenced by the presence of ophthalmopathy, two thirds of whom would avoid radioiodine in the presence of severe eye disease.51 However, many members of this organization would not chose radioiodine as the first therapy even in the absence of eye disease.52
The authors use an approach that is tailored to the individual patient. Thyrotoxicosis in patients without ophthalmopathy or with mild inactive ophthalmopathy generally is managed with radioiodine without concurrent corticosteroids. Patients with active eye disease are managed with antithyroid drugs alone or the combined use of radioiodine and corticosteroids (commencing with 0.3 to 0.5 mg of prednisone/kg bw per day orally 1 to 3 days after radioiodine and tapering the dose until withdrawal about 3 months later).1 Near-total thyroidectomy is occasionally the treatment of choice, such as in a patient who is allergic to thionamides and has progressive ophthalmopathy. Thyroidectomy and total thyroid ablation share the theoretical advantage of removing thyroid antigen, which could potentially serve to bolster the autoimmune response directed against this antigen. However, evidence at present is insufficient to support this as a routine approach. The most important factors in prevention or successful treatment for Graves’ eye disease appear to be early and accurate control of hyperthyroidism (with avoidance of subsequent hypothyroidism) with the use of radioiodine, antithyroid drugs, or surgery, and counseling the patient to refrain from smoking.1,17 Frequent monitoring of thyroid status (every 4 to 6 weeks) is important in the initial stages of treatment to ensure that euthyroidism is restored promptly and maintained stably.1
THE ORBITAL AUTOIMMUNE RESPONSE
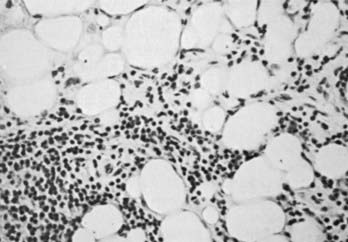
The autoimmune response within the orbit can be dissected into contributions from constituent cells of the inflamed orbit, including extraocular myocytes, connective tissue (fibroblasts, adipocytes, and intercellular matrix), and “professional” immune effector cells and their products.2,3,53 Histologic analysis has revealed largely intact muscle fibers, an expanded extracellular compartment, and infiltration by macrophages, activated T lymphocytes, and to a lesser extent B lymphocytes, as well as natural killer cells54 (Fig. 81-3). Further characterization of the activated retro-ocular T lymphocytes reveals increases in both CD4+ and CD8+ lymphocytes and restriction in the T cell receptor repertoire.55 T cell phenotyping has shown the presence of Th156 or Th257 profiles for cytokine gene expression or no clear preponderance of either phenotype.58,59 Additional evidence suggests that the profile that is found might depend on the stage of disease, Th1 being predominant in early disease and the Th2 profile appearing in later stages.60 Enlargement of the connective tissue compartment can be ascribed in part to a proliferation of retro-ocular fibroblasts and an attendant increase in the secretion of hydrophilic glycosaminoglycans by these cells.53 In addition, recent studies suggest that adipogenesis is active in the orbits of patients with Graves’ ophthalmopathy and results in expansion of the orbital adipose tissues.2,61,62 The increase in orbital tissue volume caused by edema and increased orbital fat causes forward displacement of the globe and venous compression, followed by orbital congestion and further edema.
ORBITAL IMMUNE TARGETS
Although extraocular muscle enlargement and subsequent fibrosis play a central role in the mechanics of Graves’ ophthalmopathy, the retro-ocular fibroblast and the embryologically related preadipocyte may have a greater role in the molecular events contributing to orbital autoimmunity. Retro-ocular CD8+ T cells proliferate in response to autologous retro-ocular fibroblasts but not eye muscle extracts, which suggests that fibroblasts might contain antigenic targets for activated T lymphocytes.63 Similar results were found recently with the use of retro-ocular CD4+ T cells against autologous orbital fibroblast protein.64 Retro-ocular fibroblasts respond to various cytokines with proliferation, release of glycosaminoglycan, and expression of several immunomodulatory proteins, including HLA class II molecules, lymphocyte adhesion molecules such as intercellular adhesion molecule-1, and heat shock proteins.2,53 Orbital fibroblasts also appear to have unique characteristics and responses to cytokines that facilitate their participation in the autoimmune response, as opposed to fibroblasts derived from other sources.65–67
Coexpression of thyroid autoantigen in the retro-ocular tissue has received considerable attention in studies investigating the pathogenesis of Graves’ ophthalmopathy. Investigators in studies of this sort have difficulty distinguishing a secondary immune response against previously sequestered antigens released during tissue damage from a primary autoimmune response. It now is generally believed that antibodies against such retro-ocular proteins as the 64 kDa extraocular muscle protein,68 protein 1D,69 and the 23 kDa fibroblast protein70 are secondary phenomena.
Because the autoimmune response against the TSHR is responsible for the hyperthyroidism of Graves’ disease, numerous studies have examined retro-ocular tissue for expression of the TSHR or antigenically related proteins. Most studies show at least a low level of TSHR gene expression in retro-ocular fibroblasts,71,72 preadipocytes, or retro-orbital fat73,74 and either a TSHR or an antigenically related protein in these cells.75–77 In addition, some studies have found higher levels of TSHR gene expression in Graves’ orbital adipose tissues than in normal orbital tissues or tissues from Graves’ patients with inactive eye disease.73,78,79 Insight into the role of TSHR as a thyroid and orbital antigen has come from animal models of Graves’ disease, including one in which increased TSHR expression was noted in the orbits of mice vaccinated with TSHR cDNA or receiving T cells that were primed in animals immunized with a TSHR fusion protein.80 Recent studies of cultured orbital preadipocytes have shown enhanced TSHR expression following stimulation of adipogenesis in these cells,81 suggesting a link between increased TSHR antigen expression and the expanded orbital adipose tissues characteristic of the disease.62 A current model for the pathogenesis of Graves’ ophthalmopathy is shown in Fig. 81-4.
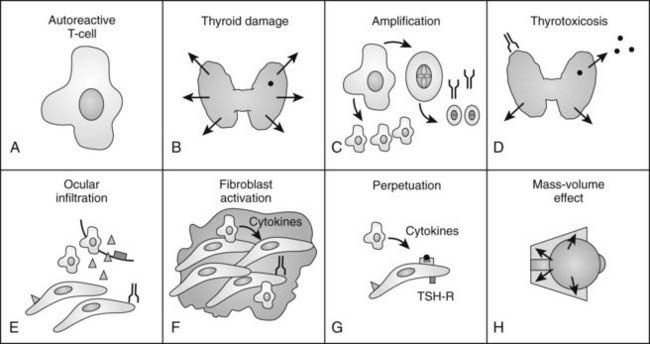
FIGURE 81-4. The authors’ current concept of the pathogenesis of Graves’ ophthalmopathy. A, In the presence of a permissive immunogenetic milieu and given appropriate environmental stimuli, autoreactive T cells directed against thyroid antigens emerge. B, Thyroid damage releases thyroid antigens, including the thyroid-stimulating hormone receptor (TSHR), thyroglobulin, and thyroid peroxidase. C, Release of thyroid antigens leads to further activation of autoreactive T lymphocytes, which results in amplification of both the cellular and humoral immune response against thyroid antigens. D, Thyrotoxicosis results from activation of the TSHR by circulating antibodies against this receptor. E, Circulating activated T lymphocytes infiltrate the orbit in response to the elaboration of specific lymphocyte adhesion molecules by orbital connective tissue cells. F, Activated T lymphocytes synthesize and release cytokines that stimulate fibroblasts to proliferate and to produce glycosaminoglycans. In addition, orbital preadipocytes are stimulated to differentiate into adipocytes that express increased levels of TSHR. G, Presentation of thyroid-eye cross-reactive antigens, such as hTSHR, leads to further activation of the local autoimmune response within the orbit. Enhanced orbital fat cell production and local edema caused by glycosaminoglycan-associated hydrophilic forces result in increased orbital tissue volume. H, The resulting mass-volume mismatch within the orbit produces venous congestion and forward displacement of the globe and leads to periorbital and conjunctival edema, extraocular muscle dysfunction, and proptosis.
History and Examination
In diagnosing the clinical features of Graves’ ophthalmopathy, it is helpful for the physician to be familiar with a problem-focused ophthalmic history and examination.82,83
HISTORY OF THE PRESENT ILLNESS
A complete eye history should be taken for each new patient with Graves’ disease at the initial evaluation and periodically thereafter. Does the patient have frequent injection, tearing, foreign body sensation, or photophobia from exposure keratitis? Is diplopia present, and if so, is it intermittent or constant? Has the patient experienced visual blurring or blind spots (scotomas)? If visual blurring is present, is it relieved by blinking, such as occurs with dryness, or by covering one eye, as occurs with unilateral neuropathy or extraocular muscle dysfunction? Is pain or a sense of pressure felt behind the eyes? Does the patient smoke?
THE PAST OPHTHALMIC HISTORY
Patients should be questioned about previous ophthalmic surgical procedures or treatments to help determine whether recent eye complaints are related to previous disorders. Specifically, has the patient undergone cataract extraction, strabismus operations, retinal detachment repair, or laser surgery? Some patients with a history of strabismus might not recall that they had crossed eyes during childhood or had extraocular muscle surgery; this historical detail may imply the presence of amblyopia (lazy eye), of which many patients are surprisingly unaware until unrelated ophthalmic problems develop in adulthood. A history of ocular trauma or treatment for glaucoma is of obvious importance, and the past or present use of topical ophthalmic medications, several of which have systemic side effects, should be recorded.
EXAMINATION OF THE EYE: AN OPHTHALMIC EVALUATION FOR THE NONOPHTHALMOLOGIST
Objective measurement and recording of eye findings are essential for assessing both the severity of ophthalmopathy and the response to therapy.84 In addition, an assessment of disease activity and the patient’s self-assessment of the disease state are important.85 The following nine areas should be included in the eye evaluation.
Visual Acuity
Visual acuity usually is measured as a Snellen fraction (e.g., 20/30) for distance vision. During bedside or office examinations, however, one may use a near-vision acuity card, several of which are commercially available. In the absence of a standardized card, the patient may be asked to read any available printed material, for example, the smallest type possible in a newspaper; the size of the print can be recorded, or the material itself can be taped in the patient’s record. Of course, patients should wear their glasses when visual acuity is being checked. Because loss of color perception can be an early sign of optic neuropathy, color vision evaluation is an important diagnostic test.86,87 One simple method for detecting possible early optic neuropathy is to check whether the patient perceives a difference between the two eyes in the color intensity of a red object; the top of a bottle of mydriatic eye drops is commonly used for this purpose. More advanced color vision testing should be performed by an ophthalmologist.
Pupils
Direct and consensual pupillary responses should be checked. An afferent pupillary defect (Marcus Gunn pupil) may indicate optic neuropathy.
Eyelids
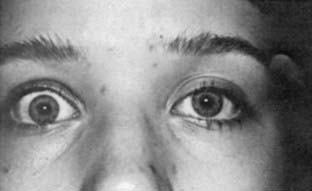
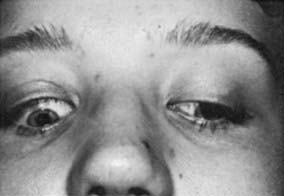
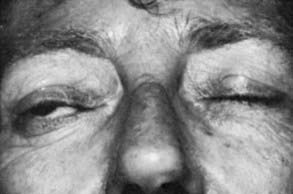
Upper eyelid retraction is a common finding in patients with Graves’ ophthalmopathy.5,88–90 Early in the course of Graves’ disease, eyelid malposition may result from increased sympathetic activity. With chronicity, the eyelid retractors (levator palpebrae superioris and Müller’s muscle) become hypertrophic, eventually fibrotic, and adherent to orbital tissues.91 Lid retraction may be unilateral or bilateral and may be subtle in some instances. The upper lid usually rests 1 to 2 mm below the junction of the cornea and sclera; therefore, if the white of the eye is seen above the corneoscleral limbus, eyelid retraction of at least 1.5 mm is present. The level of the lower eyelid is typically at the inferior corneoscleral limbus. Lower eyelid retraction is a less constant and specific finding and usually is not seen in patients with Graves’ ophthalmopathy without concomitant retraction of the upper lids. Lid lag (Figs. 81-5 and 81-6), which is diagnosed by asking the patient to look down and then observing delayed or restricted excursion of the upper eyelids as they follow the globes, and lagophthalmos (Fig. 81-7), which is an inability to close the eyelids completely, are additional stigmata of Graves’ ophthalmopathy. Eyelid retraction, lid lag, and lagophthalmos frequently interfere with the maintenance of an adequate tear film on the eye. Results include ocular irritation and dryness, reflex tearing, photophobia, and corneal scarring or even ulceration in severe cases.
Conjunctiva and Cornea
The conjunctiva is the clear, thin tissue that covers the sclera. It is normally transparent, except for the small blood vessels that course within it. In Graves’ ophthalmopathy, the conjunctiva may become hyperemic (usually termed injection) or edematous (chemosis) from exposure or from decreased venous drainage secondary to orbital suffusion. A characteristic conjunctival finding in patients with Graves’ eyes is focal injection over the insertions of the lateral or medial rectus muscles (Fig. 81-8). Engorged blood vessels do not extend to the corneoscleral limbus.
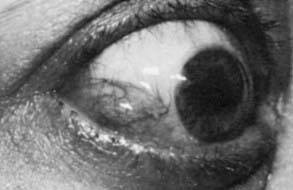
FIGURE 81-8. Chemosis (conjunctival edema) and focal injection (conjunctival blood vessel dilation) over the lateral rectus muscle insertion in a patient with Graves’ ophthalmopathy.
The cornea normally appears transparent and lustrous. Dryness resulting from exposure is difficult to detect without slit-lamp biomicroscopy. Corneal ulceration, which is an ophthalmic emergency, usually can be seen grossly with a penlight and typically is accompanied by severe pain.92
Exophthalmometry
Proptosis may be quantitated with an exophthalmometer, an instrument that measures the position of the globes in relation to the lateral orbital rim. The device is easy to use and is helpful in documenting the results of treatment. Exophthalmometry measurements in most adult eyes are 22 mm or less, and the difference between the patient’s two eyes usually does not exceed 2 mm.93 Caucasians tend to have Hertel exophthalmometry measurements of less than 18 to 20 mm, which is higher than that of Asians (16 to 18 mm) and lower than those seen in many blacks (20 to 22 mm).94–96
Ocular Motility
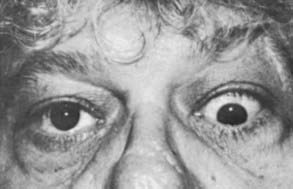
The range of movement should be evaluated for each eye separately and then for the eyes together, during which time the patient should be asked to state whether double vision is noted. Diplopia is most likely to occur in upgaze or in the extremes of lateral gaze because of restriction of the inferior or medial recti. Any or all of the extraocular muscles may be involved in Graves’ ophthalmopathy, however, and unusual patterns of strabismus may occur (Fig. 81-9).
Visual Fields
As was noted earlier, scotomas may appear in the visual field from optic neuropathy. Gross visual field defects may be detected with careful confrontation testing. An Amsler grid, a handheld card with a pattern of perpendicular crossed lines, may be used in the office or at the bedside as a simple screening tool. Formal perimetry testing should be performed by an ophthalmologist.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree