FIGURE 117-1. Schematic representation of the hormones and feedback control mechanisms in the hypothalamic-pituitary-ovarian axis. Solid lines indicate established and dotted lines indicate putative regulatory pathways. Pos shows stimulatory and neg shows inhibitory regulation. DA, Dopamine; EOP, endogenous opioid peptides; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
Gonadotropin-releasing hormone (GnRH) is a decapeptide secreted by hypothalamic neurons that stimulates the synthesis and secretion of both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by pituitary gonadotrope cells. Secretion of GnRH is regulated by neurotransmitters, and, in general, glutamate increases GnRH release, whereas endogenous opioid peptides such as beta endorphin inhibit GnRH secretion. LH and FSH are glycoprotein hormones composed of two subunits: a common alpha subunit and structurally dissimilar beta subunits that convey specificity to the hormones. Both LH and FSH are secreted by the same gonadotrope cells, which constitute 7% to 10% of cells in the anterior pituitary gland. Metabolic clearance rates of the two hormones are different, with the plasma half-life of LH (30 to 90 minutes) being shorter than that of FSH (120 to 240 minutes). FSH and LH act sequentially in concert to produce ovarian follicular maturation, ovulation, and secretion of estradiol and progesterone. Prolactin regulates ovarian function in rodents but does not appear to be important in humans. The inhibins are proteins with a common α subunit linked to either a βA subunit (inhibin A) or a βB subunit (inhibin B). The activins are homodimers or heterodimers of the β subunits, activin A (βA, βA) and activin B (βB, βB). Follistatin is an unrelated single-chain protein that binds to and inactivates activin. Inhibins, activins, and follistatin were originally obtained from gonadal extracts but are widely distributed and exert multiple actions in different tissues (inhibins and activins are related to transforming growth factor beta compounds). In relation to gonadotropins, inhibin and follistatin reduce and activin increases FSH synthesis and secretion. They are active in both sexes, with effects being more marked in females and immature males. Plasma inhibin and FSH are inversely related, which indicates an endocrine role for inhibin of ovarian origin. The exact roles of circulating activin and follistatin are less clear, and because both compounds are also present in the pituitary, they exert paracrine or autocrine actions.
Evidence from animal studies has shown that the suprahypothalamic portion of the central nervous system (CNS) can exert a major influence on reproductive function. In rodents, odors from a male mouse can coordinate the timing of estrous cycles in female mice housed together (Whitten effect). These effects are mediated by pherohormones,1 and similar mechanisms may be active in primates. If vaginal secretions from a female rhesus monkey at midcycle are placed on the perineum of a castrate female, males are attracted to the castrate animal.2 Exposure to light and, in particular, the duration of light exposure are important factors in some species, and the effects vary with the species. Regulation of light exposure (constant day-night length) can regularize the estrous cycle of rats, and constant light can advance puberty in female rats. The duration of light exposure is a major factor in the regulation of reproductive function in seasonal breeding species. In female sheep, LH secretion is inhibited during summer (long days), and during fall, gonadotropin secretion increases and cyclic estrous function continues during the winter breeding season (short days).3 The transition from anestrus (summer) to the breeding season is associated with an increase in the frequency of pulsatile LH secretion, which suggests that the duration of light exposure regulates secretion of GnRH. The exact CNS mechanisms mediating these effects remain uncertain, and little evidence is available that similar factors play any role in reproductive function in women. Higher CNS regulation of reproductive function exists, however, as evidenced by the anovulation and amenorrhea seen in some women under conditions of stress or after weight loss or intensive exercise (see Chapter 128).
GnRH is the major regulator of LH and FSH synthesis and secretion. Thus, in this summary of regulation of gonadotropin secretion, we review the secretion and actions of GnRH and examine the factors that modulate GnRH actions on the pituitary gonadotrope.
Gonadotropins
GONADOTROPIN PHYSIOLOGY
LH and FSH are members of a family of glycoprotein hormones that are composed of two dissimilar subunits. The α subunit is common to both LH and FSH, as well as to thyrotropin (TSH) and chorionic gonadotropin (CG), which is selectively expressed in the placenta of primates and horses.4 The β subunits are unique and confer biological activity and specificity.5 LH and FSH act in a synergistic manner to stimulate male and female gonads, thereby regulating sexual maturation and reproductive function. More specifically, LH stimulates steroidogenesis within the ovarian theca cell, resulting in the induction of ovulation and luteinization.6 In the male, LH stimulates androgen production in the testicular Leydig cell, which plays a critical role in inducing and maintaining spermatogenesis and secondary sexual characteristics.5,7 FSH is essential in the development of ovarian follicles, the recruitment of dominant follicles, and the induction of granulosa cell LH receptors.8 In the male, FSH plays a similar role as a stimulator of spermatogenesis.7
GONADOTROPIN STRUCTURE
In all species studied to date, the α subunit is coded by a single gene and contains 4 exons and 3 introns.5 In primates and equine species, the α subunit is expressed in the placental trophoblast, as well as in the pituitary.9 The size of the α subunit gene varies from 8 to 16.5 kilobases (kb), largely as the result of variation in size of the first intron. However, the coding region is highly conserved across species.5 Mature α subunit mRNA is 700 to 800 nucleotides in length and codes a polypeptide that is 96 amino acids in most species, but 92 amino acids in the human.10,11 LH-β and CG-β apoproteins are similar in sequence and likely are derived from a single ancestral gene.12 The LH-β gene contains three exons and two introns and is considerably smaller (≈1.5 kb) than the α subunit gene, encoding an mRNA that is approximately 700 nucleotides in length. In the human, the LH-β subunit is 122 amino acids in length.4,5 FSH-β is also a single gene, with three exons and two introns, which encodes for a 117 amino acid polypeptide.4,13 FSH-β mRNA is much larger (≈1.7 kb) than α and LH-β, owing to a large 3′ untranslated region, which may play a role in the regulation of mRNA stability by GnRH, steroids, and gonadal peptides.14–16 In contrast to other species, the human FSH-β is unique in expressing four mRNA size variants, owing to different transcription start and polyadenylation sites.17
The α and β subunits are glycosylated, with N-linked carbohydrate chains attached to specific asparagine residues. Published reports reveal considerable heterogeneity in the carbohydrate moieties present in LH and FSH subunits, and data show that the quantity and type of oligosaccharide present can affect bioactivity, intracellular sorting, and clearance from the circulation.18,19 Multiple disulfide bonds play a major role in α and β tertiary structure, and the location of critical cysteine residues is highly conserved across gonadotropins.4,19 The α and β subunits, which have no known biological action alone, form a heterodimer via noncovalent association.
GONADOTROPIN SYNTHESIS AND SECRETION
The human α subunit gene is located on chromosome 6, and the FSH-β gene on chromosome 11.20 In contrast, the human LH-β and CG-β genes are contained within a cluster of seven β subunit–like genes on chromosome 19, but only two transcripts encode viable hormone subunits.12,20 Published reports show that α subunit mRNA concentrations are significantly higher than LH-β or FSH-β mRNAs,21,22 and free α subunit is synthesized in excess and secreted into the circulation, in contrast to β subunits.4 Based on these findings, it is generally believed that β subunit production is the rate-limiting step for LH and FSH synthesis. The level of subunit glycosylation, particularly sialic acid residues, plays an important role in LH and FSH serum half-life and bioactivity.18 Studies reveal that the higher sialic acid content in FSH is responsible for its longer half-life in circulation (2 to 4 hr) versus LH (≈30 to 90 min).19
Dynamic regulation of pituitary gonadotropin secretory activity is essential for mammalian reproduction, and over the course of reproductive life, major alterations in LH/FSH release are seen. Gonadotropins increase during the fetal/neonatal stage, playing a role in gonadal development.6 In most species, gonadotropin secretion remains restrained during postneonatal development, until the initiation of puberty, at which time nocturnal increases in LH and FSH stimulate gonadal steroid production.23 In adults, LH and FSH secretion is stable in males but changes markedly during the female reproductive cycle.6 Alterations in LH and FSH secretion generally reflect similar changes in subunit gene expression, but in some physiologic states (e.g., rat or sheep estrus cycle), secretion and synthesis/gene expression diverge.22,24 LH and FSH are released in a coordinate or differential manner.25 GnRH is the primary regulator of gonadotropin secretion, with gonadal steroids playing a modulatory role, acting on the hypothalamus or pituitary (see Fig. 117-1). In general, both androgens and estrogens inhibit LH and FSH through effects on GnRH pulsatile release; however, direct actions on the pituitary have been described.10 Activin and inhibin also have direct actions on the gonadotrope, through selective effects on FSH.14,26–28 Because of the complexity of regulatory stimuli from the hypothalamus, gonads, and autocrine/paracrine factors within the pituitary, dissecting out the roles played by specific input signals to the gonadotrope has been challenging. This investigation has been made more difficult by the fact that no gonadotrope-derived cell line completely mimics primary gonadotrope physiology.29 However, the use of in vitro perfusion systems has served as an important research model in recent years, allowing the administration of pulsatile GnRH stimuli and removing the influence of steroids and gonadal peptides in a selective and controlled manner.28,30,31
GnRH Secretion and Mechanisms of GnRH Action
PATTERNS OF GnRH SECRETION
Early studies of LH secretion in humans revealed that LH was released into the circulation in a series of pulses,32,33 and that similar secretory patterns are present in all species. These data suggested that each LH pulse resulted from the episodic release of GnRH by the hypothalamus. Direct evidence is lacking in humans, but data from sheep, rodents, and primates support this view. Simultaneous measurement of GnRH in portal blood and LH in jugular venous blood34 has shown good concordance between GnRH and LH pulses, which suggests that the patterns of GnRH secretion can be inferred from measurement of LH pulses in peripheral plasma. FSH is also secreted in a pulsatile manner, but FSH peaks are often obscured because the long half-life of FSH often exceeds the interval between pulses. Regulation of GnRH secretion is complex and consists of stimulatory and inhibitory actions of steroids and neurotransmitters acting to modify the intrinsic pulsatile secretion of GnRH neurons (see Chapter 139). The final pathway in GnRH release involves the peptide kisspeptin,35,36 which acts on the GPR-54 receptor on GnRH neurons to affect GnRH release.37,38 Estradiol exerts complex actions with initial inhibition and subsequent stimulation of GnRH release. Endogenous opioid peptides (β-endorphin), progesterone, testosterone, and prolactin exert inhibitory actions on GnRH secretion. Pulsatile secretion of GnRH is essential for maintenance of normal gonadotropin synthesis and secretion. The crucial importance of a pulsatile GnRH stimulus was initially observed in a GnRH-deficient castrate monkey model.39 If GnRH is given in a pulsatile manner, LH and FSH secretion is maintained for prolonged periods, whereas subsequent continuous GnRH infusion results in a decline in serum LH and FSH (Fig. 117-2).
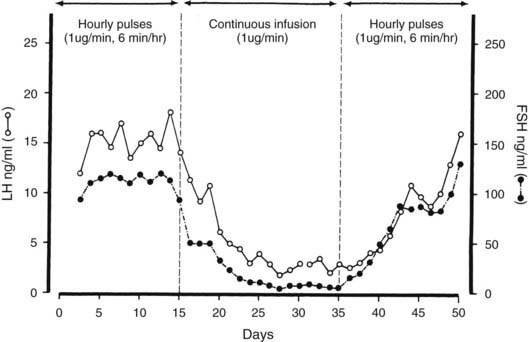
FIGURE 117-2. The effects of pulsatile or continuous administration of gonadotropin-releasing hormone (GnRH) to ovariectomized monkeys rendered GnRH deficient by placement of a lesion in the hypothalamus. Gonadotropin secretion was restored by hourly GnRH pulses, reduced during a continuous GnRH infusion, and again increased after reinstitution of pulsatile GnRH administration. FSH, Follicle-stimulating hormone; LH, luteinizing hormone.
(Data from Belchetz PE, Plant TM, Nakai Y, et al: Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone, Science 202:631–633, Copyright 1978 by the American Association for the Advancement of Science.)
These observations have been confirmed in humans, and pulsatile administration of GnRH to patients with isolated GnRH deficiency can stimulate gonadotropin secretion and induce pubertal maturation,40,41 reproducing the hormonal changes seen during the menstrual cycle and inducing ovulation.42,43 The frequency of the pulsatile GnRH stimulus is also important in determination of gonadotropin secretion. Increasing the frequency of GnRH pulses from one per hour to two or three per hour reduces plasma gonadotropins in monkeys with hypothalamic lesions, and slower frequencies of GnRH stimulation (one pulse every 3 to 4 hr) do not maintain serum LH concentrations.44 These data indicate that a narrow range of GnRH pulse frequency is required to maintain normal gonadotropin secretion. The frequency of GnRH stimulation also regulates differential release of LH or FSH by the gonadotroph. GnRH pulse frequencies of one per hour induce the release of both LH and FSH in monkeys, sheep, and humans. Slower frequencies—one pulse every 3 to 4 hours—increase serum FSH, but LH declines. The fall in LH may reflect the short half-life of LH, but the rise in FSH suggests that a slow frequency of GnRH stimulation favors FSH release by the pituitary.44,45 In rats, a GnRH pulse frequency of one every 30 minutes was optimal for maintaining LH responses, whereas FSH release continued after pulses were given every 60 to 120 minutes.46 GnRH pulse frequency may also determine the amount of LH released in response to a GnRH pulse. The amplitude of LH release is small when the GnRH pulse frequency is high, and it increases after a slower frequency of GnRH stimulation.45 This finding may explain in part the differences in LH release seen during the follicular (faster frequency) and luteal phases of the menstrual cycle (slower frequency).47,48
These data demonstrate the critical importance of a pulsatile GnRH stimulus in the maintenance of normal gonadotropin secretion and indicate a role for GnRH pulse frequency in the differential secretion of LH and FSH. The exact frequencies and amounts of GnRH required to produce optimal gonadotrope stimulation vary in different species, but all share a requirement for an intermittent stimulus.
MECHANISMS OF GnRH ACTION
GnRH acts on pituitary gonadotropes to stimulate the acute release and synthesis of both LH and FSH. The mechanisms involved in GnRH action are shown in Fig. 117-3 and have been reviewed.10,49–51
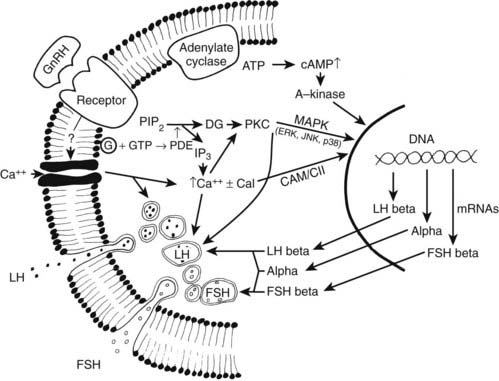
FIGURE 117-3. Mechanisms involved in gonadotropin-releasing hormone (GnRH) action on the gonadotroph. Dashed lines indicate pathways where the mechanisms are uncertain. ATP, Adenosine triphosphate; cal, calmodulin; CAMK II, calcium/calmodulin-dependent kinase II; cAMP, cyclic adenosine monophosphate; DG, diacylglycerol; FSH, follicle-stimulating hormone; G, G protein; GTP, guanosine triphosphate; IP3, inositol triphosphate; JNK, c-jun N-terminal kinase; LH, luteinizing hormone; MAPK, mitogen-activated protein kinase; PDE, phosphodiesterase (phospholipase C); PIP2, phosphatidylinositol 4,5-phosphate; PKC, protein kinase C.
GnRH action is initiated by binding to its receptor on the gonadotrope plasma membrane. The number of GnRH receptors varies in different physiologic situations, such as during sexual maturation and during the estrous cycle.52 The number of GnRH receptors is highest when responsiveness to GnRH is maximal, thus suggesting that the number of receptors plays a role in modulating GnRH action. GnRH itself has been shown to be the main factor regulating receptor concentration, and the number of receptors is elevated when endogenous GnRH secretion is increased, such as during the rodent preovulatory gonadotropin surges on proestrus,52 or after castration.53 Thus, the number of GnRH receptors reflects endogenous GnRH secretion, but the pattern of GnRH stimulation determines receptor response. In rats, GnRH pulses given every 30 minutes produce a maximum increase in receptor concentration,46 and faster or slower GnRH frequencies result in smaller responses. The number of GnRH receptors may play a role in differential activation of intracellular signaling pathways and subunit gene expression. LH-β expression is favored in the presence of elevated GnRH-R, suggesting preferential activation of signaling cascades, which activate LH-β transcription.29,54
After GnRH binding to the membrane receptor, multiple steps appear to be involved in GnRH-stimulated LH and FSH secretion and subunit gene expression. GnRH receptor binding activates several members of the GTP-associated protein family, including Gq and G11, leading to phospholipase Cβ hydrolysis of phosphatidylinositol 4,5-diphosphate to inositol triphosphate (IP3) and diacylglycerol in the plasma membrane.55 Inositol triphosphate activates the IP3 receptor, stimulating the rapid release of calcium (spike phase) from intracellular stores; this transient calcium increase affects the initial burst of LH release, lasting 1 to 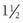 minutes. This first phase of elevated intracellular calcium is followed by a secondary (sustained) phase mediated by opening of voltage-gated (L-type) calcium channels within the plasma membrane.56 Recent findings suggest that intracellular calcium concentrations are restored via a GnRH-mediated negative feedback loop. More specifically, GnRH stimulates IP3 receptor degradation57 and increases the expression of Gem—a small G protein that inhibits L-type channels.58 The elevation in intracellular diacylglycerol and/or calcium induces the activation and translocation of various protein kinase C (PKC) isoforms (both conventional and calcium-independent novel isoforms) from the cytosol to the membrane.59,60 GnRH receptor binding also activates adenylate cyclase via coupling to the Gs protein, stimulating an increase in cyclic adenosine monophosphate (cAMP) production.61 Activated PKC phosphorylates proteins involved in initiating LH and FSH secretion, as well as members of the mitogen-activated protein kinase (MAPK) family, which are involved in stimulating gonadotropin subunit transcription.29,56,62,63 The GnRH-induced increase in intracellular calcium, together with the effects of PKC, maintains the sustained phase of gonadotropin secretion (over several minutes). Calcium and PKC also activate phospholipase A2 (PLA2), leading to the production of arachidonic acid, lipoxygenase, and/or epoxygenase products of the fatty acids, which play a role in gonadotropin exocytosis.50,51
minutes. This first phase of elevated intracellular calcium is followed by a secondary (sustained) phase mediated by opening of voltage-gated (L-type) calcium channels within the plasma membrane.56 Recent findings suggest that intracellular calcium concentrations are restored via a GnRH-mediated negative feedback loop. More specifically, GnRH stimulates IP3 receptor degradation57 and increases the expression of Gem—a small G protein that inhibits L-type channels.58 The elevation in intracellular diacylglycerol and/or calcium induces the activation and translocation of various protein kinase C (PKC) isoforms (both conventional and calcium-independent novel isoforms) from the cytosol to the membrane.59,60 GnRH receptor binding also activates adenylate cyclase via coupling to the Gs protein, stimulating an increase in cyclic adenosine monophosphate (cAMP) production.61 Activated PKC phosphorylates proteins involved in initiating LH and FSH secretion, as well as members of the mitogen-activated protein kinase (MAPK) family, which are involved in stimulating gonadotropin subunit transcription.29,56,62,63 The GnRH-induced increase in intracellular calcium, together with the effects of PKC, maintains the sustained phase of gonadotropin secretion (over several minutes). Calcium and PKC also activate phospholipase A2 (PLA2), leading to the production of arachidonic acid, lipoxygenase, and/or epoxygenase products of the fatty acids, which play a role in gonadotropin exocytosis.50,51
GnRH stimulates transcription of the α, LH-β, and FSH-β genes, and several intracellular pathways appear to be involved in transmitting the GnRH signal. After receptor activation, prominent calcium oscillations occur in gonadotrope cells.64 Experimentally induced episodic increases in intracellular calcium at different frequencies differentially increase α, LH-β, and FSH-β mRNAs in a manner similar to GnRH pulses62 (see below), suggesting a postreceptor site of action for frequency signal transduction and a role for calcium. Calcium/calmodulin-dependent kinase II (CAMK II) is an important mediator of calcium signaling within the pituitary. Recent studies reveal that CAMK II is activated by GnRH and plays a significant role in regulating gonadotropin subunit transcription.30,65 PKC activation also increases α and LH-β mRNA expression,63 and GnRH is relatively ineffective in stimulating LH-β mRNA in PKC-depleted cells.66 PKC plays an important role in GnRH activation of members of the MAPK pathway (ERK, JNK, and p38).59,67–69 Pulsatile GnRH is required to maintain ERK activation, and slower frequency pulses are optimal for phosphorylation and translocation to the nucleus.70,71 GnRH also stimulates the expression of MAPK phosphatase-2, one of the primary inhibitors of ERK activity in the pituitary, suggesting an important GnRH-mediated negative feedback loop.72 Although results differ depending on the species and the experimental paradigm, blocking ERK activation in the rat abolishes α and FSH-β, but not LH-β mRNA responses to GnRH.70,73 JNK plays a role in GnRH-stimulated gonadotropin subunit transcription in various species,68,74 and we have shown recently that JNK activation is essential for LH-β responses to pulsatile GnRH.75 In contrast to ERK and JNK, the role played by p38 appears to be limited to mediating the synergistic interactions between GnRH and activin on FSH-β transcription.76
Studies of the α subunit promoter have suggested that ERK acts in part via an Ets (E twenty-six specific) domain protein that binds to the GnRH response element.10 More recent data reveal that both ERK and JNK mediate GnRH stimulation of the human α promoter through activation of ATF-3,61 and the α promoter contains one or more (depending on the species) cAMP response elements.29 GnRH stimulates steroidogenic factor-1 (SF-1) expression, which plays an essential role in basal α, LH-β, and FSH-β transcriptional activity.10,77 The LH-β gene is responsive to both GnRH stimulation and steroid feedback. The rat LH-β promoter includes two GnRH responsive regions—the distal region contains binding sites for Sp-1 and a CArG box element; the proximal region contains binding sites for Egr-1, SF-1, and Ptx-1 (pituitary homeobox-1).56,78 The LH-β promoter may be an important site for GnRH pulse frequency regulation. Egr-1 expression is stimulated by 30 minute pulses in gonadotrope-derived LβT2 cells, whereas the expression of its co-repressors (Nab-1 and Nab-2) is increased by 120 minute pulses.79 Also, SF-1 mRNA is stimulated by 30 minute pulses,77 but expression of the SF-1 co-repressor (Dax-1) is selectively stimulated by 120 minute pulses.79 Thus, the rise in co-repressor expression associated with slow-frequency GnRH pulses may suppress LH-β transcription. Chromatin uncoiling due to histone subunit modification (i.e., acetylation, phosphorylation, ubiquitinylation, or methylation) allows DNA binding by transcription factor complexes, and the histone acetyltransferase (HAT EP300) associates with the LH-β promoter in response to GnRH.80 The FSH-β promoter contains SF-1, NFY, AP-1, and Ptx-1 (pituitary homeobox 1) sites, in addition to Smad response elements (activin stimulated).10,56 Of interest, androgens stimulate FSH-β transcription via androgen response elements (AREs) within the promoter,81 suppression of follistatin expression,82 and androgen stimulation of ERK.83
In sum, GnRH receptor binding activates several intracellular pathways, including calcium, PKC, PKA, CAMK II, and MAPK, which are involved in regulating gonadotropin subunit gene expression. Although recent findings have provided insights into mechanisms responsible for differential gonadotropin subunit responses to pulsatile GnRH, additional studies are needed to fully characterize critical sites of action in observed α, LH-β, and FSH-β divergence.
SELF-PRIMING ACTION OF GnRH
In addition to stimulating LH and FSH release, GnRH enhances pituitary responsiveness to subsequent stimulation by GnRH.84 When a second GnRH injection is given within 1 to 2 hours, enhanced secretion of LH occurs in response to the second stimulus. This phenomenon is termed a self-priming effect and depends on protein synthesis. The self-priming effect of GnRH is enhanced in the presence of estradiol, such as during the late follicular and midluteal phases of the menstrual cycle,85 and is one component of the positive feedback effect of estradiol in enhancing pituitary responses to GnRH (see below).
cAMP and progesterone also augment responses to subsequent GnRH stimulation. These effects are blocked by the progesterone receptor antagonist RU 486, which suggests that GnRH activation of protein kinase A may interact with the progesterone receptor (PR) and mediate GnRH self-priming. In pituitary cells transfected with a progesterone response element linked to a reporter gene, GnRH increases reporter gene activity, and GnRH self-priming is blunted in PR knockout mice. These data suggest that GnRH self-priming may result from GnRH-stimulated intracellular mechanisms (? cAMP) acting by way of the progesterone receptor to enhance responsiveness to subsequent GnRH stimuli.86,87
Factors That Modulate Gonadotrope Responses to GnRH
GnRH stimulation of LH and FSH release can be modified by other hormones, which may enhance or inhibit gonadotrope responses. Evidence points to estradiol, progesterone, and inhibin as the most important hormones regulating gonadotrope responsiveness in females. The hypothalamic peptides neuropeptide (NPY) and galanin, as well as prolactin, and adrenal steroids may play roles in modulating responses to GnRH.
HYPOTHALAMIC PEPTIDES
NPY is a 36 amino acid member of the pancreatic polypeptide family that is present in high concentrations in the hypothalamus. NPY enhances GnRH binding to gonadotropes and augments LH responses to GnRH.88,89 Immunoneutralization of NPY attenuates the proestrus LH surge, and NPY is elevated in portal blood during the surge, thus suggesting a role in augmenting GnRH action on proestrus.
Galanin is a 29 amino acid peptide that is present in GnRH neurons and is co-secreted with GnRH. Galanin augments LH responses to GnRH in the presence of estradiol and, together with NPY, is involved in mediating the marked increase in LH secretion that occurs during the preovulatory LH surge.90,91
Kisspeptin is a newly discovered neuropeptide that is present in the arcuate nucleus.92 Recent studies in humans found that a mutation within the kisspeptin receptor (GPR-54) results in hypogonadotropic hypogonadism and absent pubertal maturation.93 Thus, kisspeptin and its receptor GPR-54 play essential roles in the induction of pulsatile GnRH secretion.
PROLACTIN
The major action of prolactin on LH and FSH secretion is exerted by inhibiting secretion of GnRH, but prolactin also inhibits gonadotrope responses. In vitro, GnRH-stimulated LH release is impaired in the presence of elevated prolactin, and this action is reversed by bromocriptine.94 Similar results are observed in vivo,95 and prolactin inhibition of responses to GnRH contributes to the low gonadotropin levels seen in hyperprolactinemic states.
ESTRADIOL
The administration of estradiol to women or female animals is followed by an initial suppression of plasma LH and FSH, but both gonadotropins, particularly LH, are subsequently increased.96 The time course of this biphasic action depends on the species and the dose of estradiol used. In women, the inhibitory effects persist for 2 to 3 days and are followed by augmentation of LH secretion—“positive feedback.” With the use of exogenous GnRH, the inhibitory and the stimulatory effects of estradiol have been shown to be exerted on the gonadotrope cell. In women, LH responses to GnRH are suppressed during the first 36 hours but are augmented after 48 hours, and enhanced responses persist for several days.97,98 The temporal relationships in sheep, together with the pituitary site of estradiol action,99 are shown in Fig. 117-4. Estradiol initially inhibits LH release at each GnRH pulse, but responses then are augmented and mean plasma LH increases. This positive action of estradiol accounts in part for variations in LH responsiveness to GnRH during the menstrual cycle. LH responses are augmented during the late follicular and midluteal phases of the cycle, when plasma estradiol is elevated. In vitro studies have confirmed that the positive action of estradiol is exerted directly on the gonadotrope cell. In rat pituitary cells, LH responses to GnRH are augmented after 12 hours of exposure to estradiol. The mechanisms of estradiol action include enhancing GnRH receptor upregulation by GnRH and increasing both the amount of LH released by each cell and the number of cells releasing LH.100
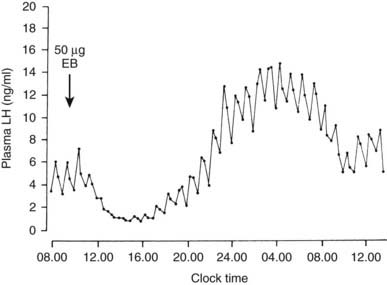
FIGURE 117-4. Inhibitory and stimulatory effects of estradiol on luteinizing hormone (LH) responsiveness to gonadotropin-releasing hormone (GnRH). Ovariectomized ewes with surgical disconnection of the hypothalamus from the pituitary were given GnRH pulse injections (500 ng every hour). LH responses to each GnRH pulse were measured after injection of 50 µg of estradiol benzoate (EB). LH responses were diminished between 12 noon and 4:00 pm and subsequently were augmented between 8:00 pm and 9:00 am.
(Data from Clarke IJ, Cummins JT: Direct pituitary effects of estrogen and progesterone on gonadotropin secretion in the ovariectomized ewe, Neuroendocrinology 39:267–274, 1984.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








