FIGURE 116-1. Schema of the structures of inhibin, the α subunit containing peptide pro-αC, αN, activin, and follistatin. Note that 31-kD inhibin is derived from the 58-kD form by proteolytic cleavage of αN from the α43 subunit. FSH, Follicle-stimulating hormone.
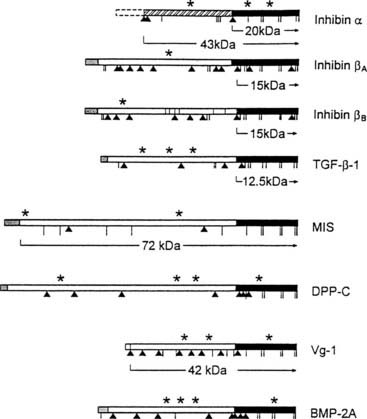
FIGURE 116-2. Schematic diagram of subunit precursor sequences of the transforming growth factor-β/inhibin family members as deduced from their cDNA structure. The darkened region corresponds to the region of highest homology between sequences and the region associated with biological activity. *, Potential glycosylation site; 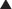 potential proteolytic site. Vg-1 mRNA and its protein are localized in the vegetal region of oocytes and embryos. Cysteine residues are denoted by vertical bars. BMP, Bone morphogenetic protein; DPP-C, decapentaplegic complex; MIS, müllerian inhibiting substance; TGF-β1, transforming growth factor beta 1.
potential proteolytic site. Vg-1 mRNA and its protein are localized in the vegetal region of oocytes and embryos. Cysteine residues are denoted by vertical bars. BMP, Bone morphogenetic protein; DPP-C, decapentaplegic complex; MIS, müllerian inhibiting substance; TGF-β1, transforming growth factor beta 1.
ACTIVINS
Activins are dimers of the β subunits and occur in three major forms, activin A (βAβA), activin AB (βAβB), and activin B (βBβB),10 although the additional forms βC, βD, and βE have now been identified (see later).
There is substantial interspecies homology between the amino acid sequences of these subunits.10 The α subunit shows 85% homology between the human, bovine, porcine, murine, and ovine forms and 100% homology between the βA subunits of human, bovine, porcine, and murine species, with a single amino acid substitution in the ovine. The βB subunits are 95% homologous among species, and 70% homology is found between the βA and βB subunits. The α and all β subunits are members of the transforming growth factor β (TGF-β) family of proteins.
Given the capacity of the α and β subunits to dimerize to form inhibin A and B and activins A, B, and AB, when all the subunits are present at one cellular site of production, the presence of the α subunit tends to drive production towards inhibin A and B, whereas deletion of the α subunit gene results in high levels of production of the activins.14
Castration of male or female animals leads to a rapid decline in circulating levels of the inhibins,15–17 whereas such experiments indicate that no significant decline in circulating activin A levels18 has been found, indicating, as discussed later, that the activins are produced at multiple sites and exert their major effects in a paracrine or autocrine manner.
Consequences of Deletion of the α, βA and βB Genes
Targeted disruption of the inhibin α subunit gene led to elevated levels of activin A concentrations, and the results of α subunit deletion have been reviewed in detail.14,19 The mice develop gonadal/sex cord stromal tumors in both sexes, accompanied by profound cachexia associated with elevated activin levels. Crossing the inhibin α subunit gene knockout mice with mice with targeted disruption of the gene for ActRII led to the development of tumors in the absence of cachexia, indicating that the wasting was the action of elevated activin levels.20 However, the development of the tumors supports the concept that the α subunit gene is a tumor-suppressor gene. From the point of view of reproductive function, the α-subunit-deleted male mice were capable of normal copulation and had normal spermatogenesis, relatively normal seminal vesicles, and fertility. These data were obtained from young mice before tumor development. In contrast, inhibin-deficient female mice showed disrupted folliculogenesis and absent corpora lutea. Mice in which granulosa-cell tumors developed showed evidence of elevated estradiol levels consistent with the capacity of activin to stimulate aromatase activity.14
Extensive studies have been reported on the consequences of deletion of the genes coding for the βA and βB subunits, as well as deletion of the genes coding for the activin receptors.14 Activin βB–deficient mice have developmental defects in eyelid closure,21 and males have normal reproductive capacity. The gestation time of activin βB–deficient females is prolonged, and live-born litters fail to survive postnatally, owing to the failure of mothers to nurse their young, consistent with the role of activin B in control of hypothalamic oxytocin secretion.22
Activin βA–deficient mice die within 24 hours of birth because of multiple craniofacial abnormalities that prevent suckling.23 The defects include cleft palate and other palatal abnormalities, absence of whiskers, and lack of lower incisors. It is thus clear that activin βA is essential for normal craniofacial development.23 Insertion of the βB subunit gene at the βA locus prevented the major palatal defects, but the mice had smaller testes and exhibited a delay in the onset of spermatogenesis.24 A conditional knockout of the βA gene in granulosa cells led to female infertility associated with the presence of multiple corpora lutea.25 Mice deficient in ActRII mostly develop normally to adulthood but have suppressed FSH levels and show a decrease in testis size and some delay in the acquisition of fertility.26 Quantitative studies of spermatogenesis show a decrease in Sertoli cell number and diminished germ cell counts.27 ActRII receptor–deficient female mice are infertile. It is not clear whether this infertility results from reduced serum FSH levels, absent activin signaling in the ovaries, or both.
The fact that mice that lack both activin βA and activin βB, ActRII, or follistatin28 show normal mesoderm formation and neurulation suggests that activins are not required for mesoderm induction or neural development in mammals.
FOLLISTATIN
Follistatin is a glycosylated protein which was originally isolated on the basis of its ability to suppress FSH secretion by pituitary cells in vitro, but it shows no structural similarity to the inhibins.5,6 There are differing forms, with molecular weights ranging from 31 to 44 kD, based on alternative splicing mechanisms of the five exons of the follistatin gene and the degree of glycosylation.29,30 However, two major forms are recognized because of their physiologic implications. Follistatin 288, a 288-amino-acid form has the capacity to bind avidly to heparan sulfate proteoglycans, whereas follistatin 315 does not demonstrate this property and represents the circulating form31,32 (reviewed in ref. 33). Follistatin 315 can be proteolytically processed in the ovary to follistatin 300 (300 amino acids). The capacity of follistatin to suppress FSH relates to its capability to bind and neutralize the biological activity of the activins, which in the context of the pituitary, is their ability to stimulate FSH.34 They have the capacity to neutralize all the actions of the activins.
The amino acid sequences of rat, mouse, human, bovine, ovine, and porcine follistatin are highly homologous (97%). No follistatin receptor has been identified. Because of the binding of follistatin to heparan sulfate proteoglycans, administration of heparin can release significant quantities of follistatin and bound activin into the circulation in both humans and sheep35 (Fig. 116-3). These studies indicate the presence of significant quantities of tissue stores linked to basement membranes, some of the activins being targeted through this pathway to intracellular degradation via lysosomal pathways.31
FIGURE 116-3. Changes in the plasma concentrations of follistatin (•) and activin A ( ) in the serum of adult ewes given a variety of treatments: A, saline only; B, heparin (100 units/kg); C, premixed heparin and protamine; D, protamine (2 mg/kg). Note the release of both activin and follistatin by heparin, the attenuation of the release when heparin and protamine are premixed, and the release of follistatin only by protamine given alone.
) in the serum of adult ewes given a variety of treatments: A, saline only; B, heparin (100 units/kg); C, premixed heparin and protamine; D, protamine (2 mg/kg). Note the release of both activin and follistatin by heparin, the attenuation of the release when heparin and protamine are premixed, and the release of follistatin only by protamine given alone.
(Data from Jones KL, de Kretser DM, Phillips DJ: Effect of heparin administration to sheep on the release profiles of circulating activin A and follistatin, J Endocrinol 181:307–314, 2004.
The heparin-binding site is critical to the capacity to neutralize the actions of endogenous activins, since a related molecule, follistatin-like protein 3, lacks this binding site and is a weak antagonist of endogenous activin despite being only slightly less potent in neutralizing exogenous activin.36
Consequences of Targeted Disruption of the Follistatin Gene
Knockout of the follistatin gene results in death within hours of birth due to respiratory failure related to weak diaphragmatic and thoracic musculature caused by the absence of follistatin’s neutralization of the actions of myostatin.28 A more recent study indicates the existence of lung pathology involving thickened alveolar-capillary membranes.37
Knockout mice also showed shiny, taut skin, similar to that observed in mice overexpressing TGF-β1 in the epidermis.28 The transgenic mice had abnormal whiskers, a cleft hard palate, and abnormal lower incisors. Defects in axial skeletal components were noted in the ribs and vertebrae. This phenotype is consistent with the possibility that follistatin modulates the activities of multiple members of the TGF family as discussed previously.38
Further studies have identified that the prenatal development of their ovaries is abnormal, with a substantial decrease in the ovarian follicular pool.39 These data are consistent with the tissue-specific targeted disruption of the follistatin gene in granulosa cells.40 In an attempt to rescue the follistatin knockout phenotype by crossing these mice with mice transgenic for the human follistatin gene expressed under the native promoter and engineered to express either follistatin 288 or follistatin 315, several novel actions of follistatin were identified, including a role in angiogenesis affecting tail growth and the potential prevention of corpus luteum formation in the ovary and abnormal Müllerian duct development, in that the oviduct failed to develop.34
MÜLLERIAN INHIBITING SUBSTANCE
Müllerian inhibiting substance (MIS) is a dimer of two 72-kD subunits, the C-terminal domain of which shows homologies with the α, βA and βB inhibin subunits and other members of the TGF-β family of proteins.41 Crystallographic studies indicate that all members of the TGF-β superfamily share a similar three-dimensional structure characterized by a central cysteine knot generated by disulfide bridges between six of the seven conserved cysteine residues in the C-terminal portion of the proteins.42
RECEPTORS AND BINDING PROTEINS
The activins and other members of the TGF-β family of proteins signal through a combination of type II and type I receptors which are members of a family of transmembrane serine-threonine kinases.43 The activins signal by binding to ActRIIA, which initiates recruitment of the type I receptor, termed ALK4, in turn leading to its phosphorylation by ActRII. The activated type I receptor in turn phosphorylates Smads (sons of mothers against decapentaplegia) 2 and 3 to form a complex with Smad-4 that is transported into the nucleus to regulate specific gene expression.44,45 The intracellular pathway utilizing Smads 2, 3, and 4 is common to signaling induced by TGF-βs, myostatin, GDF11, and nodal.46 However, the TGF-β family use a specific type II receptor (TGF-βRII) and a different type I receptor (ALK5), whereas the activin II receptors are shared by activin and GDF11 (ActRIIA), myostatin (ActRIIB), and nodal (ActRIIB). There is also sharing of the ALK4 type I receptor between the activins and GDF11, whereas nodal (ALK7) and myostatin (ALK5) have specific type I receptors. To add to the complexity, some of the bone morphogenetic proteins (BMPs) also signal through ActRIIA and ActRIIB but have distinct type I receptors and specific Smads (Smad 1, 5, and 8). A pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI), which acts as a type I receptor but lacks an intracellular serine-threonine kinase domain, can bind these ligands but cannot signal.46
Further regulation of the actions of these proteins occurs through the binding protein follistatin, which binds activins with a very high affinity and can block all their actions.34 The availability of the crystal structure of the activin-follistatin complex indicates that two follistatin domains encircle activin and bury one third of its residues and its receptor binding sites.47 Given that myostatin and some BMPs (4, 6, 7, and 15) signal through the activin receptors, the crystallographic data provide an explanation of the capacity of follistatin to block their actions, although the affinity of follistatin for activin is at least 10-fold higher.38 Finally, since the nodal co-receptor Cripto can bind both nodal and the activins, it can modulate some of the actions of the activins.46
Inhibin A binds to ActRII with 10- to 20-fold lower affinity than activin A does, and inhibin B binds with even lower affinity. The signaling pathway for the inhibins is less well understood. No specific receptor has been isolated. Inhibin antagonizes the binding or signaling of activin to the activin receptor through the capacity of inhibin to bind to betaglycan, the type III TGF-β receptor, which in turn facilitates its binding to ActRII.48 Studies of the patterns of glycosylation of inhibin A and B indicate that differently glycosylated forms may have different capacities to bind to betaglycan and may differ in their bioactivity.49,50 Mechanisms of inhibin action may, however, vary depending on the ligand and receptor types involved.51 The MIS receptor system functions similarly to the activin system.46
The Inhibins
SITES OF PRODUCTION
Inhibin subunit mRNAs have also been demonstrated in the placenta52 and the decidua,53 as well as in both fetal and adult adrenal glands.54 Inhibin subunit α mRNA is present in extracts of early pregnancy placenta and is much more abundant than inhibin βA mRNA, with βB mRNA being detected at low levels only in term placenta.55 Inhibin α subunit was demonstrable in the cytotrophoblast and βB in the syncytial layer of the villi; βA was widely distributed. Partial characterization of human placental inhibin, activin, and follistatin has been described.56 Regulation of placental inhibin secretion has been reviewed.57,58
In the rat, gonadotrophs contain immunoreactive inhibin α and inhibin βB subunits and their mRNAs,59 and in male monkeys, inhibin-like immunoreactivity was noted in clusters of chromophobe cells, frequently lying close to gonadotrophs.60 Inhibin β subunits (but not α subunits) were reported in monkey pituitary,61 and the entire range of inhibin/activin subunit genes and the ActRII gene have been demonstrated in normal and adenomatous human pituitary tissue.62–64 However, neither inhibin bioactivity nor immunoreactivity has been detected in media from cultured rat anterior pituitary cells, and incubation of such cells in medium that contains anti-inhibin antiserum does not alter basal FSH secretion.
Finally, current studies in prostatic diseases, particularly prostate cancer, suggest that progression to malignancy is accompanied by loss of the α subunit in prostatic epithelium, a topic considered further in Chapter 144.65,66
ASSAYS
Bioassays
In vitro bioassays for inhibin using rat anterior pituitary cells and measurements of FSH secretion are compromised because of the concomitant production of both activins and follistatin.67–69 These bioassays have a place in the validation of newly developed radioimmunoassay (RIA) and enzyme-linked immunosorbent assay (ELISA) systems and in the characterization of inhibin and related molecules synthesized by recombinant techniques.
Radioimmunoassays
A number of early RIAs for human inhibin used antibodies directed to the NH2-terminal region of the α subunit, but the presence of very significant cross-reactivity with the α subunit precursor pro-αC, which is present in follicular fluid and peripheral blood, leads to overestimation of inhibin levels.16,70–75 Thus, in the follicular phase of the human menstrual cycle, these assays provided an accurate measure, but in the luteal phase, the corpus luteum secreted predominantly α subunit products, providing a poor index of inhibin biological activity.76 Despite the limitations, the major findings with respect to inhibin physiology in humans were consistent with the concept that inhibin was a major component of the closed-loop feedback regulating system involved in the control of FSH secretion.76,77 The development of ELISAs specific for each of the inhibin dimers provided specific measures of inhibin A and B78,79 and were particularly important with regard to inhibin physiology in the male. Inhibin A is undetectable by ELISA in the male; the major species of inhibin secreted in the male is inhibin B.79,80 Both males and females secrete significant quantities of α subunit–related peptides.11 Whereas use of the specific assays is now regarded as mandatory in the definition of inhibin physiology, nonspecific RIAs or more recently developed assays of similar specificity still appear to be the method of choice in the application of inhibin assays to the field of ovarian malignancies (see later).
PHYSIOLOGY
Male
Castration decreases circulating inhibin levels to undetectable levels,15,16 indicating that the testis is the principal source of inhibin in the male. The availability of specific assays for inhibin A and B has established that inhibin B is the principal form in men and most mammals except the sheep. The specific inhibin B ELISA showed that in fractionation studies of male serum, the predominant biologically active inhibin species in the male is inhibin B, with activity concentrated in two molecular weight species of 66 and 32 kD.11 However, in addition to inhibin B, large amounts of α subunit–related peptides circulate in the male and are measured in inhibin RIAs.11
The Sertoli cell is the major testicular source of both immunoreactive and biologically active inhibin, as established by in vitro secretion from Sertoli cell cultures and the localization of both α and β subunits by immunohistochemistry, together with evidence of in vivo production.81–83 In addition, Leydig cells secrete immunoreactive inhibin, and both α and β subunit expression has been demonstrated in those cells and cell cultures,82,84 but as yet the function of secreted Leydig-cell inhibin remains unclear. Sertoli cells secrete inhibin both from their apical surfaces into the seminiferous tubule lumen and from their basal surfaces into interstitial fluid. The relative importance of each route of secretion is controversial.85 As demonstrated by studies in the rhesus monkey, inhibin B secretion is governed by the inhibitory and stimulatory actions of luteinizing hormone (LH) and FSH, respectively,58 with the actions of LH due to testosterone inhibition of βB gene expression.
Fetus
There is evidence that the fetal testis expresses both the α and β subunits in many species including humans.86,87 Significant concentrations of both bioactive and immunoactive inhibin have been identified, with the predominant source being the Sertoli cell. Castration of fetal sheep confirm that in the fetus, the testis is the major source of inhibin B in the circulation and fetal fluids such as amniotic fluid.88
Early Postnatal Period and Prepuberty
In boys, the first few months of life are characterized by low FSH levels compared to adult levels, and inhibin B concentrations are above the adult male level at 3 months of age and remain elevated until about 15 months.89,90 A nadir in levels occurs at 6 to 10 years of age. In prepubertal boys, inhibin B correlates with age but not with FSH.91
Puberty
Inhibin B levels are low from the age of 2 or 3 years to 10 or 11 years, possibly due to constitutive secretion, but rising inhibin B levels occur during pubertal development.92–94 In early puberty, inhibin B correlates with testosterone, whereas from midpuberty, the correlation with FSH is inverse, consistent with the establishment of negative feedback.93 The two peaks of inhibin B in infancy and early puberty reflect secretory phases and also reflect periods of Sertoli-cell proliferation.
Adulthood
The early studies in men using the inhibin RIA, which detected inhibin dimers but also free α subunits, did not demonstrate the postulated inverse relationship between immunoreactive inhibin and serum FSH in men with testicular disorders, since the testis secretes α-subunit products in normal or even elevated levels in patients with severe seminiferous tubule damage, including men with Klinefelter syndrome.95 Several authors have demonstrated positive correlations between spermatogenesis and inhibin B concentrations.96–98 The most striking demonstration that inhibin B is the specific gonadal peptide regulator of FSH was in a study of 12 men with a variety of lymphomas and other hematologic malignancies evaluated before and during chemotherapy with agents toxic to the seminiferous epithelium. Serum inhibin B levels fell rapidly after administration of these agents, with an inverse rise in serum FSH levels and no significant change in the levels of testosterone.99 However, pro-αC–containing peptides rose in response to the induction of testicular damage.
Administration of exogenous FSH leads to a delayed, but clear-cut increase in circulating serum inhibin B, whereas suppression of the pituitary by using a combination of testosterone and a synthetic progestin resulted in suppression of inhibin B levels.96,97 In men, with increasing age, FSH levels rise associated with falling inhibin B levels.94 In pathologic states characterized by either gonadotropin deficiency or seminiferous tubule damage, serum inhibin B levels reflected the expected alteration in Sertoli-cell function.96–100 The relative roles of inhibin B and testosterone in FSH regulation are discussed in Chapter 136.
Female
The ovary is the principal source of circulating inhibin, and castration leads to a rapid decline of inhibin levels.15,16 Inhibin is also undetectable in most normal postmenopausal women, as well as in castrate men, indicating that the gonads are the major source of the circulating molecule.15–17 Granulosa cells in vitro produce inhibin and are the main source of inhibin during the follicular phase of the reproductive cycle.101–104 This is supported by immunohistochemical analysis and in situ hybridization.86,105–108
In primates, the corpus luteum is also a site of inhibin production, as shown by inhibin production in vitro by luteinized human granulosa cells,109 and cells from isolated human corpora lutea secrete inhibin in vitro under basal conditions and increase their inhibin production in response to human chorionic gonadotropin (hCG).110 Further, the α and βA subunits were demonstrable in human and monkey corpora lutea.111–113
Fetus
Although it has been demonstrated that the fetal gonad in sheep secretes immunoreactive inhibin when FSH is administered in a pulsatile manner,114 and that administration of porcine follicular fluid treated with charcoal leads to decreased circulating FSH but not LH levels in ovine fetuses,115 no data are available regarding the secretion of inhibin or activin by the human or primate fetal gonad. Rabinovici and co-workers116 showed weak immunostaining for the βA subunit in some of the follicles that had formed at mid-gestation (16 to 23 weeks) but were unable to demonstrate α or βB subunit staining. In late-gestation fetal rhesus monkey ovaries, granulosa cells surrounding oocytes showed positive immunostaining for all three inhibin subunits. Inhibin levels were undetectable in media from cultures of mid-gestation human ovaries, and no studies were reported on rhesus monkey fetal ovary cultures. In the fetal bovine ovary, both inhibin bioactivity and immunoactivity have been detected, as has follistatin.117
Early Postnatal Period
As in the male, the specific dimeric inhibins appear to participate in activation of the pituitary-gonadal axis in the first few months of life. In an extensive study of 473 unselected, healthy, 3-month-old girls, a marked intraindividual variation in inhibin concentrations was noted corresponding to those observed in puberty, suggesting a period of ovarian activity similar to that noted in the infantile testis.118
Another study of girls aged 2 months to 2 years noted that immunoreactive inhibin levels were in the low adult follicular-phase range, accompanied by midfollicular estradiol levels in the youngest girls. The presence of adult FSH levels, elevated in some into the postmenopausal range, suggest that this early ovarian activity is stimulated by the pituitary. They noted that beyond the age of 1 year, all four hormones were at extremely low concentrations.89
Puberty
In a large study of 345 girls aged 0 to 18 years, median inhibin B levels were low until age 6, subsequently rose slightly, and increased further after age 10. The finding of increased levels of both inhibins in some individual samples, together with their positive relationship with FSH, provided evidence to support the occurrence of sporadic follicular development throughout infancy and childhood under the influence of FSH.119
Serum immunoreactive inhibin concentrations in girls were low before the onset of puberty and rose in parallel with levels of FSH, LH, and estradiol.92 Levels of inhibin A and B behaved similarly to those of immunoreactive inhibin.93,119 In children treated with cytotoxic therapy for childhood leukemia,99,120 some undergo puberty at a significantly earlier age than normal, and many of these patients had undetectable inhibin concentrations, presumably reflecting the ovarian damage caused by chemotherapy. Further, in prepubertal girls with solid tumors or acute lymphoblastic leukemia, inhibin B decreased to undetectable levels in the majority during treatment, without any change in FSH or LH. Posttreatment recovery of inhibin B was variable, suggesting cancer chemotherapy may cause arrest of follicle development. Sustained suppression of inhibin B may indicate permanent ovarian damage. In boys, inhibin B was normal for age and sex, with no significant change during or after treatment, suggesting that in prepubertal boys, chemotherapy had little immediate effect on Sertoli-cell production of inhibin B.121
Menstrual Cycle
The endocrinology of the menstrual cycle is described comprehensively in Chapter 128. Only a brief summary of inhibin physiology is given here.
Immunoreactive serum inhibin levels and dimeric inhibin A remained relatively constant throughout most of the follicular phase, rose immediately before the midcycle gonadotropin surge, fell transiently, and rose again to reach their highest levels during the midluteal phase.78,122
In contrast, inhibin B78 levels rise during the luteal follicular transition, in close correlation with levels of FSH, follow the early follicular phase rise and fall in serum FSH, show a small midcycle peak, with a progressive decline thereafter throughout the luteal phase. The inhibin B pattern strongly suggests that it is a product of the pool of small FSH-responsive antral follicles from which the dominant follicle is selected and that it is not a product of the corpus luteum. Further data suggest that the intercycle rise in inhibin B is dependent on rising levels of FSH.123
Different regulatory mechanisms appear to be involved in the control of inhibin secretion during the follicular and luteal phases. During the follicular phase, the inhibins are predominantly under FSH control. Urinary gonadotropin preparations that contain both FSH and LH given for the purpose of ovarian hyperstimulation124 or biologically purified FSH given to women with polycystic ovarian disease undergoing ovulation induction125 led to increased serum immunoreactive inhibin and estradiol, and dose-response relationships were demonstrated between immunoreactive inhibin levels and FSH given as single injections in the early follicular phase of the menstrual cycle.126 Both inhibin A and inhibin B participate in this response, the dose-response curve for inhibin B being steeper than that for inhibin A.127 In studies of ovarian hyperstimulation using recombinant FSH, serum inhibin B levels provided an early indicator of the number of recruited follicles destined to form mature oocytes.128
LH appears to regulate inhibin levels during the luteal phase. Human granulosa lutein cells in long-term culture responded to LH and testosterone with increased inhibin production,109 and a gonadotropin-releasing hormone antagonist given to normal women in the midluteal phase decreased serum inhibin levels, an action prevented or reversed by hCG but not FSH.129 A gonadotropin-releasing hormone antagonist given to macaques produced a similar decrease in inhibin which was reversed by hCG but not FSH.130 However, FSH can stimulate inhibin A secretion during the luteal phase of the cycle.131 It should be noted that immunoreactive inhibin α and βA subunits have been demonstrated in human endometrium,132 but the major products are the activins,133 and their physiologic role is discussed later in this chapter.
Pregnancy and Lactation
The circulating levels of immunoreactive inhibin rise throughout normal pregnancy,134 an early rise paralleling increasing hCG levels in women pregnant after embryo transfer following in vitro fertilization.135 It is clear that the major form of bioactive, dimeric inhibin circulating in pregnant women is inhibin A.136,137 Inhibin A levels in early pregnancy peak at approximately 8 weeks, declining subsequently at approximately 16 weeks, remain low throughout the second trimester, and increase fivefold to peak around 36 weeks. Inhibin B concentrations remain near the detection limit of the assay throughout pregnancy. The levels subsequently fall in a biexponential manner after delivery.138 An increase in inhibin was noted relatively early in pregnancy in women without endogenous ovarian function,139 indicating that the ovary was not essential for the early inhibin increase in pregnancy. A fetoplacental source of inhibin A in early pregnancy is consistent with the detection of both bioactive and immunoactive inhibin in the placenta and with hCG capable of stimulating inhibin secretion from cultured placental cells.140 Physiologic doses of hCG given to normal women during the midluteal phase stimulate increases in both progesterone and inhibin, compatible with the corpus luteum as a significant source of inhibin early in pregnancy.141 Although the decidua in pregnancy expresses the α, βA, and βB inhibin subunits,52 the major product is activin A.133 Higher inhibin A levels are found in multiple pregnancies than in singleton pregnancies, and levels in missed abortions are very low. These observations suggest a prognostic role for inhibin A measurements, particularly in the management of early pregnancy in patients who conceive as a result of in vitro fertilization.142
Inhibin A (and activin A) concentrations are increased in preeclampsia, and it has been proposed that women who have increased inhibin A concentrations at 16 weeks’ gestation are at higher risk of preeclampsia.143 Studies using both inhibin α–directed assays and dimeric inhibin A assays have shown that measurement of maternal serum inhibin A in the second trimester can make a contribution to current screening tests for Down syndrome.144 A recent analysis indicates that with combined nuchal-thickening assessments, free β-hCG, dimeric inhibin A, and pregnancy-associated plasma protein A measurements in the late first trimester, 83% to 85% detection rates are achieved.145
Reproductive Aging and the Menopausal Transition
Serum FSH levels increase in women older than 40 years who continue to have regular menstrual cycles. Lenton and associates146 found that the increase in FSH occurred 5 to 6 years earlier than the LH increase when related to the time of onset of the menopause. They further reported that follicular-phase immunoreactive inhibin concentrations from older women (mean age 44.2 years) were lower than those of younger women (mean age 27.4 years) in cycles in which pregnancy did not occur but were similar to those with a mean age of 29.7 years when sampled during a conception cycle.147 This contrasted with the failure of estradiol and progesterone levels to change as a function of increasing age.148
Given that the major regulator of early-follicular-phase serum FSH levels is inhibin B, its serum levels (but not inhibin A or estradiol levels) were significantly lower in older, regularly cycling women with elevated follicular-phase serum FSH levels, compared with a younger control group.149 Inhibin B was inversely correlated with early-follicular-phase serum FSH in women aged 20 to 50 years, particularly those in the 40- to 50-year age group.150 A decrease in serum inhibin B appears to be the earliest endocrine marker of the onset of the menopausal transition, the time when menstrual cycle irregularity becomes manifest. Early perimenopausal subjects were shown to have significantly lower levels of inhibin B in the presence of a small, statistically nonsignificant increase in FSH and no change in estradiol and inhibin A. A recent review indicated that in view of the erratic characteristics of menstrual cycles in women approaching the menopause, there was no value in measuring FSH and estradiol levels in an attempt to stage an individual and noted that AMH levels showed a correlation with follicle numbers and showed a marked age-related decline.151,152 Further, Sowers et al. noted the decline of AMH prior to the final menstrual period and concluded that it provided a better predictor of the time of final menstrual period and the age at which it occurred.153 Comprehensive reviews of the endocrinology of the menopausal transition are available.151,152
There is evidence that a variant of the human inhibin α-subunit gene involving the substitution of alanine by a threonine (INHA A257T) is linked to premature ovarian failure, since this variant was more common in a population of women with premature ovarian failure.154 Recent data indicate that this variant exhibits impaired inhibin B bioactivity, supporting this hypothesis.155
CLINICAL APPLICATIONS OF INHIBIN ASSAYS
The growing use of the specific dimeric assays, as well as the less specific assays for immunoreactive inhibin, point to a number of diagnostic possibilities. In the male, the demonstration that inhibin B is the major gonadal peptide regulator of FSH and that it reflects Sertoli-cell function has suggested that inhibin B measurements may provide evidence regarding seminiferous tubule function, in addition to that provided by serum FSH measurement. Thus, inhibin B may be a sensitive marker of spermatogenesis156 and may help resolve some of the uncertainties regarding the diagnostic classification in men with severe oligospermia or azoospermia and normal serum FSH levels.96
In women, inhibin B measurements may be of prognostic value in the management of those undergoing ovarian hyperstimulation for the purposes of in vitro fertilization.128,157 Early studies suggested that low serum inhibin B in the early follicular phase before ovarian hyperstimulation may indicate a poor prognosis for the outcome of in vitro fertilization cycles, but a recent study indicated that AMH correlated better than age, FSH, LH, inhibin B, and estradiol with the number of retrieved oocytes and predicted ovarian responsiveness to controlled ovarian stimulation.158 The clinical applications of inhibin A assays have been mentioned previously with reference to the monitoring of early pregnancy, prediction of preeclampsia, and screening for Down syndrome.144,145
The major area of diagnostic promise is in the monitoring of patients with stromal and epithelial tumors of the ovary.159 An initial report indicated that circulating immunoreactive inhibin concentrations were markedly elevated in patients with granulosa cell tumors of the ovary160 (Fig. 116-4) and that, at least in some patients, rising inhibin levels were observed up to 20 months before clinical evidence of tumor recurrence was obtained. Inhibin assays are clearly of greatest value in patients who have previously undergone bilateral oophorectomy or in those who are postmenopausal, both situations in which endogenous inhibin levels are expected to be undetectable. Serum inhibin concentrations are elevated in most postmenopausal women with mucinous cystadenocarcinoma of the ovary and in approximately 15% of patients with nonmucinous epithelial cancers. Successful surgical removal of such tumors leads to a rapid fall in serum inhibin to nonsignificant levels by 1 week postoperatively.161 In postmenopausal women with proven ovarian cancer, serum inhibin is inversely correlated with FSH and positively correlated with estradiol and progesterone, particularly in those with granulosa cell tumors. In women with mucinous tumors, the inverse correlation with FSH is lost; one possible explanation for this is that mucinous tumors may secrete α subunit–related peptides. In addition to their production of α subunit–related peptides, granulosa cell tumors produce both inhibins A and B.162 Mucinous epithelial tumors also have the capacity to secrete the dimeric inhibins, more frequently inhibin B than inhibin A, but the levels are less consistently elevated than in granulosa cell tumors. Some patients with serous cystadenocarcinomas produce the dimeric inhibins. Current studies are directed at establishing whether a combination of a nonspecific inhibin measurement with a standard marker such as CA125 may have value for screening in addition to diagnostic value for ovarian tumors.163,164
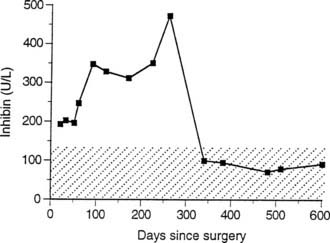
FIGURE 116-4. Serum inhibin concentrations in an 80-year-old patient with a stage 2 granulosa cell tumor of the right ovary. Despite apparent clinical remission, serum inhibin levels rose steadily for 9 months after cytoreductive surgery. Second-look laparotomy 272 days after primary surgery confirmed residual granulosa cell tumor that was successfully removed, after which the patient remained clinically disease free. The serum inhibin range seen in functionally agonadal postmenopausal women is shown as a hatched band.
The Activins and Follistatin
SITES OF PRODUCTION
The Activins
Activins are homodimers or heterodimers of the inhibin β subunits, βA and βB, with three isoforms being recognized: activin A (βA βA), activin B (βB βB), and activin AB (βA βB).3,4 Three further β subunits have been cloned, including mammalian βC and βE subunits and a Xenopus βD subunit.165–167 There is evidence to suggest that activin C can dimerize with activin A and activin B to form inactive proteins.168 Activins are synthesized as precursor proteins and are characterized in particular by having a bioneutralizing binding protein, follistatin,5,6 that modulates activin activity.34 Activins also bind to the broad-spectrum protease inhibitor α2-macroglobulin.169 Although the activins signal through serine-threonine kinase receptors, there are numerous other mechanisms that can regulate their biological activity.43–45 Given the widespread actions of these proteins, these mechanisms serve to limit their actions to specific tissues and cell types. Activin β subunits can be identified by immunohistochemistry and in situ hybridization in a wide variety of tissues, thus reflecting the evidence that they are local regulators of growth and differentiation in a number of organs.
All three species of activin have been isolated from porcine follicular fluid.3,4,170 The βA subunit monomer is present at 25% to 60% of the level of activin A dimer in bovine follicular fluid.13 The erythroid differentiation factor isolated from the culture medium of a human leukemia cell line is activin A.171 Activins may play fundamental roles in embryonic differentiation, specifically in mesoderm induction.172 Exposure of animal pole cells of the Xenopus embryo to activin A or activin B leads to the formation of a miniature embryo complete with head and rudimentary trunk. Activin B is transcribed very early in embryonic development, at the blastula stage, several hours before activin A.172 Related studies in the chick embryo showed that activins can induce the formation of organized axial structures from epiblasts and that activin B is expressed in the hypoblast, which normally induces axial differentiation in the epiblast. Other diverse effects of activin include the stimulation of insulin secretion by rat pancreatic islets and glucose production in isolated rat adipocytes. The effects on pituitary cells other than gonadotrophs have included a reduction in growth hormone–releasing factor–mediated growth hormone release and thyrotropin-releasing hormone–mediated prolactin release, together with inhibition of somatotroph growth and growth hormone biosynthesis and secretion.173 There are widespread actions on many tissues, including the induction of apoptosis in the liver and B lymphocytes, as well as other roles in bone biology, wound healing, inflammation, and neuronal survival.174–180 These multifunctional actions of the activins indicate that these proteins function as growth factors or cytokines rather than hormones because, in many states, the circulating levels are bound with high affinity to follistatin, thereby neutralizing its actions.33 Given the capacity of follistatin to bind activin A34 and in turn also bind to heparan sulfate proteoglycans,31,32 there are significant quantities of both activin A and follistatin that can be released by heparin from cell surfaces and basement membranes.35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








