FIGURE 98-1. Glucocorticoid receptor (GR) domain structure and signaling pathway. A, GR is composed of an amino-terminal transactivation domain (NTD), DNA-binding domain (DBD), hinge region (H), and ligand-binding domain (LBD). Regions involved in transactivation (AF1 and AF2), dimerization, nuclear localization, and hsp90 binding are indicated. The location of residues posttranslationally modified by phosphorylation (P) (Ser-113, Ser-141, Ser-203, Ser-211, and Ser-226); ubiquitination (U) (Lys-419); sumoylation (S) (Lys-277, Lys-293, and Lys-703); and acetylation (A) (Lys-494 and Lys-495) are also depicted. Numbers are for the human glucocorticoid receptor. B, The unliganded GR resides in the cytoplasm of cells in a complex with chaperone proteins. Upon binding glucocorticoids ( ), the receptor undergoes a change in conformation, dissociates from accessory proteins, and translocates into nucleus via the nuclear pore complex (NPC). Nuclear GR regulates gene expression in three primary ways: (a) GR binds directly to DNA and enhances or inhibits transcription of target genes. (b) GR interacts with DNA-bound transcription factors without itself binding to DNA and enhances or inhibits transcription of target genes. (c) GR binds directly to DNA and interacts with transcription factors bound to neighboring sites and enhances or inhibits transcription of target genes. BTM, Basal transcription machinery; TBP, TATA-box binding protein.
), the receptor undergoes a change in conformation, dissociates from accessory proteins, and translocates into nucleus via the nuclear pore complex (NPC). Nuclear GR regulates gene expression in three primary ways: (a) GR binds directly to DNA and enhances or inhibits transcription of target genes. (b) GR interacts with DNA-bound transcription factors without itself binding to DNA and enhances or inhibits transcription of target genes. (c) GR binds directly to DNA and interacts with transcription factors bound to neighboring sites and enhances or inhibits transcription of target genes. BTM, Basal transcription machinery; TBP, TATA-box binding protein.
GR Regulation of Transcription via Direct Binding to DNA
Unliganded GR is located in the cytoplasm of cells as part of a multiprotein complex that includes two molecules of heat shock protein 90 (hsp90) (see Fig. 98-1B).9 These chaperone proteins maintain the receptor in a transcriptionally inactive state that favors high-affinity ligand binding. Upon binding glucocorticoids, GR undergoes a conformational change resulting in the dissociation of hsp90, exposure of the nuclear localization signals, and translocation of the receptor into the nucleus via the nuclear pore. The receptor then regulates gene transcription by binding directly to specific DNA sequences known as glucocorticoid response elements (GREs) and/or by binding other transcription factors and modulating their activity. Global gene expression analyses indicate that up to 20% of the genome is induced or repressed by glucocorticoids.5,10
The consensus GRE is an imperfect palindrome, GGTACAnnnTGTTCT, that is usually found in the promoter region of target genes.11,12 The interaction of ligand-bound GR with the GRE stimulates the transcription of numerous genes, including the metabolic enzymes tyrosine amino transferase, phosphoenolpyruvate carboxykinase, and glucose-6-phosphatase (see Fig. 98-1B[a], upper scheme). High-affinity GRE binding requires receptor dimerization and is short lived because the receptor rapidly cycles on and off target sites every few seconds.13,14 Upon binding DNA, GR undergoes a conformational change that results in the coordinated recruitment of three general types of coactivators necessary for stimulating transcription of the target gene: the ATP-dependent complex BRG1 (SWI/SNF) that mediates large noncovalent disruptions in chromatin structure; CBP, p300, and members of the SRC/p160 family of proteins that modify chromatin structure locally through their intrinsic histone acetyltransferase activity; and components of the DRIP/TRAP complex that assist in the recruitment of the basal transcription machinery.15 The alterations in chromatin structure result in DNA unwinding, thereby permitting promoter access to transcription factors and cofactors that enhance target gene expression. The specific coactivators recruited by GR determine the gene induction profile, and this assembly is dependent on the promoter context, the bound glucocorticoid, and the availability and activity of the coactivators themselves.
Negative GREs (nGREs) are the transcription-repressing counterpart of positive GREs.16 These response elements bear little resemblance to positive GREs, are highly variable, and a consensus sequence has not been determined. How ligand-bound GR represses transcription via nGREs is unclear and likely involves multiple mechanisms dependent upon the promoter context. For some genes, such as osteocalcin, DNA-bound GR may sterically interfere with binding of positively acting transcription factors to elements that overlap the nGRE (see Fig. 98-1B[a], lower scheme).17 The situation is more complex for other repressed genes, since both binding to DNA and interacting with neighboring transcription factors appear to be required of the receptor (see Fig. 98-1B[c], lower scheme). Referred to as a composite GRE, nGREs of this nature are found in the promoters of the proliferin and corticotropin-releasing hormone (CRH) genes. Here, DNA-bound GR interacts with the activator protein-1 (AP-1) transcription factor occupying an adjacent site to repress transcription.18,19 Finally, since nGREs correspond poorly to the GRE consensus sequence, negative regulation may be achieved at some target genes by GR interacting with DNA-bound positive transcription factors without itself binding to the promoter. This “tethering” mechanism of repression (described in more detail later) appears to mediate the glucocorticoid-dependent inhibition of gonadotropin-releasing hormone receptor and pro-opiomelanocortin expression via the interaction of GR with the promoter-bound Oct1 and Nur77 transcription factors, respectively.20,21
GR Regulation of Transcription via Protein-Protein Interactions
In addition to transcriptional regulation by direct binding to DNA, ligand-bound GR can also interact with other transcription factors and modulate their activity on glucocorticoid-responsive promoters without itself binding to DNA. The two most studied examples of this form of regulation involve the transcription factors AP-1 and nuclear factor κB (NF-κB). These two proteins are central mediators of the inflammatory and immune responses, and their inhibition by GR is thought to underlie the major antiinflammatory and immunosuppressive actions of glucocorticoids.22,23 When activated by stress signals such as proinflammatory cytokines, bacterial and viral infectious agents, or pro-apoptotic stimuli, AP-1 and NF-κB bind their cognate response elements and induce the expression of many proinflammatory genes, including cytokines, cell adhesion molecules, and enzymes involved in tissue destruction. Glucocorticoids indirectly antagonize the actions of AP-1 and NF-κB at multiple levels by inducing the expression of other regulatory proteins. Activated GR stimulates expression of the IκB protein, which sequesters NF-κB in the cytoplasm,24 MAPK phosphatase 1, which dephosphorylates c-Jun N-terminal kinase to prevent activation of AP-1,25 and tristetraprolin, which destabilizes the mRNA of many AP-1 and NF-κB-induced genes.26
The primary way, however, by which hormone-bound GR represses the activity of AP-1 and NF-κB is through a direct interaction with the c-Jun subunit of AP-1 and the p65 subunit of NF-κB (see Fig. 98-1B[b], lower scheme).22,23 Interestingly, the antagonism is reciprocal; the association of these proteins with GR represses its activity on target genes. Multiple mechanisms appear to underlie the antagonism, suggesting the mode of action may vary in a promoter, cell-type, and/or signal-dependent manner. In early studies, the receptor was reported to sequester AP-1 and NF-κB in the cytoplasm and/or nucleus and prevent DNA binding. More recent findings, however, suggest the receptor is tethered to DNA-bound AP-1 and NF-κB and alters the subsequent assembly and/or activity of recruited transcriptional proteins. For example, on several toll-like receptor genes, GR represses NF-κB by disrupting the interaction of p65 with the promoter-specific coactivator IRF3 (interferon regulatory factor 3).27 At the interleukin 8 (IL-8) and intercellular adhesion molecule 1 (ICAM-1) promoters, the association of GR with NF-κB impairs the phosphorylation of the C-terminal domain of RNA polymerase II.28 GR-mediated repression of the AP-1-responsive collagenase-3 promoter and the NF-κB-responsive IL-8 promoter have both been shown to be potentiated by receptor-dependent recruitment of the coactivator GRIP1, indicating coactivators can actually function as corepressors in the appropriate configuration with GR.29 Finally, GR has been reported to interact with histone deacetylase 2 (HDAC2) and to repress the histone acetyltransferase activity of NF-κB, suggesting that GR antagonizes NF-κB by effects on chromatin structure.30
In contrast to AP-1 and NF-κB, the physical association of GR with the signal transducer and activator of transcription (STAT) family of proteins enhances their activity on target genes.31 STAT transcription factors are activated by a range of cytokines through induction of the Janus kinase pathway (JAK) and tyrosine phosphorylation. Upon binding their cognate response elements, STATs regulate genes involved in the immune response, differentiation, survival, and apoptosis. GR has been shown to interact with several members of the STAT family, including STAT3 and STAT5, and to synergistically enhance their activity on target genes in a promoter-dependent fashion.31 The association of GR with STAT3 at the γ-fibrinogen and α2-macroglobulin promoters, which lack identifiable GREs, super-induces their expression (see Fig. 98-1B[b], upper scheme).32–34 Similarly, the synergistic activation of the β-casein and toll-like receptor-2 genes results from the association of GR with promoter-bound STAT5.35–38 However, the observed synergy at these STAT5-responsive genes may also require GR binding to DNA and more accurately reflect a composite GRE (see Fig. 98-1B[c], upper scheme).37,38 How GR synergistically activates STAT-regulated genes is poorly understood but may involve GR enhancing STAT nuclear localization,39 prolonging the promoter occupancy of STAT by inhibiting its tyrosine dephosphorylation32,40 and/or promoting the co-utilization of certain coactivators.31 Interestingly, the synergism of these two transcription factors is not always mutual, since GR activity is inhibited or stimulated depending on the particular STAT isoform employed.34,35,41,42
Multiple GR Isoforms Derived from Single Gene
The human GR gene is located on chromosome 5q31-32 and comprises 9 exons.43–46 Alternative splicing in exon 9 generates two receptor isoforms, termed GRα and GRβ, that are identical through to amino acid 727 but then diverge at their carboxyl-termini (Fig. 98-2).45–47 The classic, full-length GRα contains an additional 50 amino acids, whereas GRβ has an additional, nonhomologous 15 amino acids. Because of its unique carboxyl-terminus, GRβ does not bind glucocorticoids and resides constitutively in the nucleus of cells.45,48 GRβ has been shown to function as a dominant negative inhibitor and repress the transcriptional activity of GRα45,49–51; therefore, alterations in GRβ expression may contribute to changes in glucocorticoid responsiveness. Indeed, expression of GRβ is selectively increased over GRα in cells exposed to proinflammatory cytokines and microbial superantigens, leading to reduced sensitivity to glucocorticoids.52–56 In addition, glucocorticoid-resistant forms of inflammatory diseases (e.g., asthma, rheumatoid arthritis, and ulcerative colitis) have been associated with elevated expression of GRβ.50 Conversely, methotrexate, an effective drug for treating autoimmune and inflammatory diseases, promotes a selective increase in GRα at the expense of GRβ and improves glucocorticoid sensitivity of lymphocytes.57
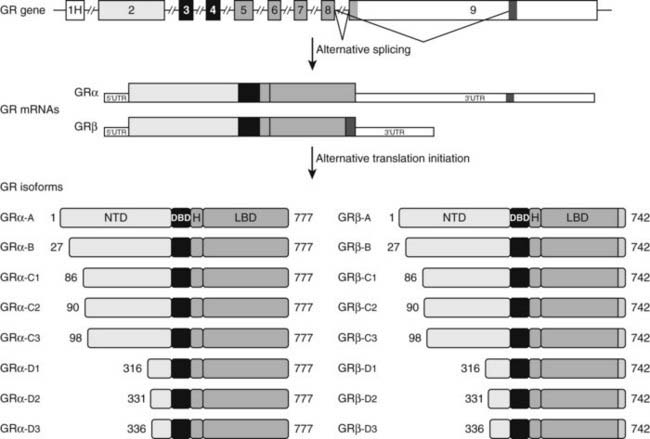
FIGURE 98-2. Alternative processing of the single glucocorticoid receptor (GR) gene generates multiple GR isoforms. The human GR gene comprises 9 exons. Alternative splicing at the 3′ end of the primary transcript generates GRα and GRβ mRNAs, which encode GRα and GRβ proteins differing only at their carboxyl-terminus. Alternative translation initiation from 8 different AUG start codons derived from exon 2 generates additional protein isoforms with progressively shorter NTDs. Numbers shown denote the first and last residues for the human GR isoforms. For simplicity, only the most proximal of 9 alternate exon 1s (1H) is shown.
Elevated GRβ levels also result from a naturally occurring ATTTA to GTTTA polymorphism (A3669G) in the 3′ untranslated region of GRβ. This nucleotide substitution disrupts an mRNA destabilization motif and leads to increased stability of the GRβ mRNA and enhanced protein expression.58,59 The A3669G allele has been associated with reduced central obesity in women and a more favorable lipid profile in men,60 suggesting the increase in GRβ might antagonize some of the undesirable effects of GRα on fat distribution and lipid metabolism. The A3669G-directed rise in GRβ may also compromise the immunosuppressive actions of GRα. Persons harboring the A3669G allele have an elevated risk of the autoimmune disease rheumatoid arthritis and a reduced risk of bacterial nasal infection.58,61 Moreover, A3669G carriers exhibit an increased risk of myocardial infarction and coronary heart disease, two pathologies with inflammatory underpinnings.62
A broader role for GRβ in cell signaling has recently emerged with the demonstration that this isoform can modulate gene expression apart from its effects on GRα. In a genome-wide microarray analysis performed in cells selectively expressing GRβ, the isoform was found to alter the expression of over 5000 genes.63 Less than 20% of the genes were commonly regulated by ligand-activated GRα, indicating that GRβ possesses its own unique gene-regulatory profile. GRβ was also shown to bind the glucocorticoid antagonist mifepristone (RU486), and binding of this ligand abolished most of the GRβ-mediated changes in gene expression.63 As a bona fide transcription factor, GRβ may contribute to alterations in glucocorticoid responsiveness in healthy and diseased tissues by genomic effects independent of its dominant negative activity on GRα.
Alternative translation initiation of the GR mRNA produces an additional cohort of receptor subtypes (see Fig. 98-2).10,64,65 Eight GR isoforms with progressively shorter NTDs are generated from one GRα mRNA transcript via different AUG start codons: GRα-A, GRα-B, GRα-C1, GRα-C2, GRα-C3, GRα-D1, GRα-D2, and GRα-D3. GRα-A is the classic, full-length 777 amino acid protein that is generated from the first initiator AUG codon. The GRβ mRNA also contains the identical start codons and would be expected to give rise to a similar complement of subtypes. The GRα translational isoforms show a widespread tissue distribution; however, the relative levels of the subtypes differ both between and within tissues.64,66 Functionally, the isoforms bind glucocorticoids with similar affinity and bind GREs with similar capacity.66 Additionally, all eight isoforms occupy the nucleus of cells following glucocorticoid treatment. In the absence of hormone, however, the subcellular distribution of the subtypes differ, with the GRα-D isoforms residing predominantly in the nucleus and the others predominantly in the cytoplasm.64
Marked differences have also been reported in the transcriptional properties of the GRα translational isoforms.64,66 On GRE-containing reporter and endogenous genes, the GRα-C3 isoform was the most active stimulating gene expression, whereas the GRα-D subtypes were the most deficient. These isoform-selective effects have been attributed to differences among the subtypes in their ability to recruit various transcriptional factors and coregulators, such as CBP and RNA polymerase II, to the promoter. In contrast to these divergent effects on gene induction, no significant differences have been observed so far in the ability of the GRα isoforms to repress NF-κB.66 In a whole-genome microarray analysis performed on U2OS osteosarcoma cells selectively expressing the individual receptor isoforms, each subtype was found to regulate both a common and a unique set of genes.66 Of the approximately 6500 genes regulated by at least one GRα isoform, less than 500 were regulated commonly by all the receptor isoforms. Thus the majority of glucocorticoid-responsive genes were selectively regulated by different GRα subtypes. These isoform-unique gene regulatory profiles were further shown to produce functional differences in cellular responsiveness to glucocorticoids; the GRα translational isoforms exhibited distinct capabilities to induce apoptosis.66 Cells expressing GRα-C3 were the most sensitive to the apoptosis-inducing actions of glucocorticoids, whereas cells expressing GRα-D3 were the most resistant. Isoform-selective differences in the induction of the proapoptotic enzymes granzyme A and caspase-6 may account for the observed phenotype.
GR Control of Inflammation and Immune Response
There are two general mechanisms by which ligand-activated GR controls inflammation and the immune response. First, GR protects cells at sites of injury or inflammation from undergoing inflammation-induced apoptosis. This is accomplished by both positive and negative regulation of gene transcription and protein expression. GR stimulates production of antiinflammatory proteins such as secretory leukocyte protease inhibitor, IL-1 receptor antagonist, IL-10, and neutral endopeptidase.2 In addition, by regulating the activity of NF-κB, AP-1, and STATs, GR inhibits the expression of a variety genes important to the control of inflammation and the immune response, including proinflammatory cytokines (e.g., IL-2, IL-3, IL-6, and TNF-α), chemokines that attract inflammatory cells to sites of inflammation, nitric oxide synthase (NOS), and cyclooxygenase 2 (COX-2), among others.2 The second mechanism by which GR controls inflammation and the immune response is by inducing programmed cell death in immune cells that underlie inflammation. Glucocorticoids reduce the survival of eosinophils, T lymphocytes, mast cells, and dendritic cells.2 Although the mechanisms and target proteins responsible for GR-induced apoptosis are not well understood, GR-dependent control of transcription has been reported to be involved in the initiation of programmed cell death in lymphocytes.67
The proper modulation of immune system activity and inflammation is critical to normal human function. A blunted immune response leaves the door open for potentially fatal infections, whereas an overstimulated immune response can result in autoimmune activity that damages organs. Glucocorticoids are potent modulators of the immune response and use a variety of GR-dependent mechanisms to accomplish this regulation on target genes. The traditional view that glucocorticoids exert these effects through one receptor isoform has changed dramatically in recent years with the discovery of a family of receptor isoforms arising from alternative processing of the single GR gene. These receptor isoforms possess unique expression, functional, and gene-regulatory profiles. Moreover, the potential for these isoforms to undergo posttranslational modifications and to function as monomers, homodimers, and/or heterodimers on both common and unique sets of genes provides cells with a wealth of possibilities for controlling the immune response with fine-tuned precision. Important goals of future research will be to determine the contribution each isoform makes to the specificity and sensitivity of glucocorticoid responsiveness and to assess whether changes in the cellular expression of these isoforms contribute to the etiology of various inflammatory and immune diseases.
GLUCOCORTICOID RECEPTOR RESISTANCE*
Generalized glucocorticoid resistance (GGR) is a rare disorder characterized by ACTH-dependent hypercortisolism and the absence of the clinical features of glucocorticoid excess. Adrenocorticotropin (ACTH)-mediated overproduction of adrenal androgens and mineralocorticoids produces a clinical syndrome in some individuals. The disorder is often receptor-mediated, and 10 distinct human glucocorticoid receptor alpha (hGR) mutations have been described. Therapy is reserved for those patients with significant clinical abnormalities.
History
Vingerhoeds et al. first described generalized glucocorticoid resistance, also known as primary cortisol resistance, in 1976.68 It is quite rare; fewer than 30 separate probands have been reported.68–79 It is sometimes caused by functionally abnormal hGRs. The clinical presentation of glucocorticoid resistance varies from asymptomatic hypercortisolism to clinical syndromes of mineralocorticoid or adrenal androgen excess. Since the first description of a pathogenetic hGR mutation in 1990,80,81 nine other pathogenetic hGR mutations have been identified.69,77–79,82–85
Pathogenesis
ENDOCRINE PATHOPHYSIOLOGY
GGR may be caused by abnormal hGR. This was first demonstrated in assays of hGR function.70–76 More recent studies have validated this expectation by identifying hGR mutations69,77–85 that decrease hGR-mediated gene transcription.
The clinical findings are a consequence of impaired hGR function. The HPA axis and its negative feedback regulation of cortisol production are described elsewhere. The glucocorticoid sensitivity of all tissues is decreased in GGR. The entire HPA axis is reset (Fig. 98-3). At the pituitary and hypothalamus, serum cortisol concentrations, which otherwise would be considered normal, are insufficient to suppress corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) secretion. Consequently, ACTH secretion is increased. ACTH stimulates the adrenal glands to produce greater-than-normal amounts of cortisol, adrenal androgens, and mineralocorticoids. In the peripheral tissues, the glucocorticoid resistance is equal to that of the pituitary and hypothalamus; however, sensitivity to androgens and mineralocorticoids is normal. Hence, the clinical findings are not those of glucocorticoid excess but rather those of mineralocorticoid or androgen excess. The glucocorticoid circadian rhythm and response to stress are maintained.
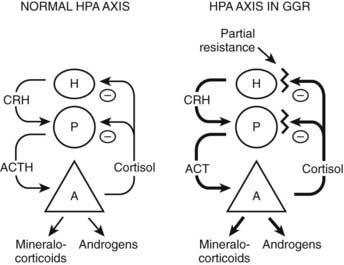
FIGURE 98-3. Hypothalamic-pituitary-adrenal (HPA) axis in normal and in generalized glucocorticoid resistance subjects. Normally, corticotropin-releasing hormone (CRH) from the hypothalamus (H) stimulates the pituitary (P) to produce adrenocorticotropic hormone (ACTH). ACTH stimulates the adrenal (A) to produce of mineralocorticoids, cortisol, and adrenal androgens. Cortisol inhibits (−) secretion of CRH and ACTH from the hypothalamus and pituitary, respectively. In GGR, there is partial blockade of the negative feedback at the pituitary and hypothalamus. This causes increased secretion of CRH and ACTH. ACTH stimulates the adrenal gland to make excess glucocorticoids, mineralocorticoids, and androgens. The HPA axis is qualitatively normal but quantitatively reset at higher hormone concentrations than normal.
(Adapted from Javier EC, Reardon GE, Malchoff CD: Glucocorticoid resistance and its clinical presentations. Endocrinologist 1:141–148, 1991.)
The resistance to cortisol is partial, and plasma ACTH concentrations can be suppressed by high doses of exogenous glucocorticoids. Complete glucocorticoid resistance may be incompatible with life, as suggested by animal models.86
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree