FIGURE 125-1. Photomicrograph of an adult primate ovary showing the follicular and luteal units in the cortex and large blood vessels and nerves in the medulla. Gf, Graafian follicle; pf, primary follicle; se, serous or surface epithelium; sf, secondary follicle; ta, tunica albuginea; tf, tertiary follicle.
(Modified from Bloom W, Fawcett DW [eds]: A textbook of histology, Philadelphia, 1975, WB Saunders, p 860.)
The cortex is a dynamic structure with follicles and corpora lutea (see Fig. 125-1). These histologic units exhibit a specialized architecture that reflects their stage of growth and development. The pool of follicles can be divided into two major classes: growing and nongrowing. Most (90% to 95%) of the follicles are nongrowing or primordial follicles. Once a primordial follicle has been recruited to grow, its size, structure, and position in the cortex begin to change dramatically. During this process, the follicle passes through three developmental stages: primary, secondary, and tertiary or graafian stages (see Fig. 125-1). Selection of a dominant follicle occurs during the graafian stage, and those follicles that are not selected degenerate by a process called atresia. After ovulation of the large dominant follicle, the follicle wall transforms into a corpus luteum through a process called luteinization. The corpus luteum of the cycle ultimately degenerates by a process called luteolysis.
Folliculogenesis
The ability of a primordial follicle to undergo folliculogenesis is at the very foundation of the expression of a menstrual cycle. As a follicle grows and develops, important changes occur within the three major cell populations: oocytes, granulosa cells, and theca cells. These cellular changes include proliferation, differentiation, growth, and apoptosis. Two major classes of regulatory molecules control these cellular activities, namely, hormones and growth factors. Folliculogenesis can be divided into two phases: preantral and antral growth. That interval from recruitment of a primordial follicle to the end of the secondary stage is referred to as the preantral or gonadotropin-independent phase. In the antral or gonadotropin-dependent phase, a fluid-filled cavity or antrum develops within the follicle, which is now termed a tertiary or graafian follicle. The graafian follicle grows progressively larger, predominantly through the accumulation of increasing amounts of fluid in the antrum. The fully developed graafian follicle is termed a preovulatory follicle.
PREANTRAL FOLLICLES
The development of a preantral follicle is characterized by three major developmental events: (1) entry of a primordial follicle into the pool of growing follicles; (2) growth and differentiation of the oocyte; and (3) acquisition of FSH and LH receptors in the granulosa and theca cells, respectively.
Primordial Follicle
The primordial follicles comprise a pool of nongrowing follicles from which all preovulatory follicles are ultimately derived. A primordial follicle consists of a small (≈15 µm in diameter) oocyte arrested in the dictyotene stage of meiosis, a single layer of squamous granulosa cells, and a thin basal lamina that encloses both cell types (Fig. 125-2). No blood vascular system is directly associated with the primordial follicles2; however, evidence in the rhesus monkey suggests that they may be innervated, most notably by vasoactive intestinal peptide (VIP) nerves.3
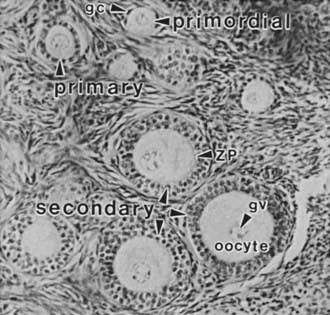
FIGURE 125-2. Photomicrograph of a portion of the cortex of the ovary. Primordial (nongrowing) follicles consisting of a small oocyte arrested in diplotene of meiosis I surrounded by a single layer of squamous granulosa cells (gc). Primary follicle containing a growing oocyte and a single layer of cuboidal granulosa cells. Secondary follicles containing a nearly full-grown diplotene oocyte with an intact germinal vesicle (gv) or nucleus, a thin zona pellucida (ZP), and two or more layers of cuboidal granulosa cells.
In humans, the primordial follicles are formed in the fetal ovaries between the sixth and ninth months of gestation.4 Because all viable female germ cells have committed to meiosis, no unspecialized stem cells are capable of producing new oocytes by the end of gestation. Consequently, all the oocytes in a woman’s ovaries are present at birth. Once the primordial follicles are formed, some are recruited to grow. As a woman ages, the process of recruitment continues on a regular basis until the total primordial follicle compartment is exhausted. This event, the menopause, occurs in most women at about 51 years of age.5 An important concept is that the loss of primordial follicles or ovary reserve (OR) is not constant during aging. For example, a significant accelerated decrease in OR occurs at about 37 years in most women (Fig. 125-3). Clinically, this age-related acceleration of primordial follicle depletion is of great importance because it is associated with a significant decrease in fecundity.5
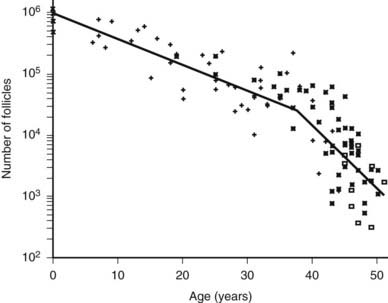
FIGURE 125-3. Morphometric analysis of normal human ovaries showing the age-related decrease in the total number of primordial follicles (PF) within both ovaries from birth to the menopause. As a consequence of recruitment, the number of PFs decreases progressively from approximately 1 million at birth to about 25,000 at age 37. Note that the rate of loss of PF accelerates approximately twofold at 37.5 ± 1.2 years, with the number being reduced to about 1000 at approximately 51 years of age.
(From Faddy MJ, Gosden RG, Gougeon A, et al: Accelerated disappearance of ovarian follicles in midlife: implications for forecasting menopause, Hum Reprod 7:1342–1346, 1992.)
Certainly, one of the foremost questions in reproductive biology concerns the basis of initial primordial follicle growth and maturation. Structurally, the process of primordial-to-primary follicle transition begins with a change in shape in the granulosa cells from a squamous to a cuboidal shape, which, in turn, is accompanied by or associated with a commitment of the granulosa to divide, albeit very slowly.6,7 During or immediately following this sequence of events, the oocyte genome becomes activated and the oocyte begins to grow.8,9 That the first visible signs of primordial follicle growth occur in the granulosa cells fits the prediction that granulosa cells may play a key role in the process. Initiation of follicle growth appears to be regulated by negative and postive growth factors.10,11 For example, evidence in laboratory animals suggests that follicle maturation is inhibited by epidermal growth factor12 and anti-Müllerian hormone (AMH), otherwise known as Müllerian inhibiting substance.13 In AMH-null mice, the numbers of preantral and small antral follicles were greater in the ovaries of 25-day-old and 4-month-old females compared with wild-type females. Moreover, in 4-month-old AMH-null females, smaller numbers of primordial follicles were present, and in 13-month-old animals, essentially no primordial follicles were observed.14 These findings reflect a generalized progression of follicle growth in the absence of AMH.
Conversely, several factors, including kit ligand,15 insulin,16 testosterone,17 bone morphogenetic protein-7 and -15,18,19 growth and differentiation factor-9,20 and restricted food intake, have been shown to stimulate follicle development.12 For example, in the rat ovary, BMP-7 has been shown to activate advancement of primordial folicle growth.18 In sheep but not mice, BMP-15 has an essential role in promoting the primary to secondary transition of follicles during early folliculogenesis, while restraining the transition of follicles to the dominant preovulatory stage.19,21 The absence of this oocyte-derived peptide as in homozygous carriers of the ovine Inverdale and Hanna BMP-15 mutations results in infertility with streak ovaries and a block at the primary stage of folliculogenesis.19 Similarly, another specific factor secreted by oocytes was shown to have a role in follicle development when it reported that the targeted deletion of GDF-9, the closest homologue of BMP-15, precludes the development of ovarian follicles past the primary stage in mice.20 These findings are compelling, as several reports have associated mutational defects in BMP-15 and GDF-9 in women with ovarian failure, although corroboration of these findings is warranted.22–24
Primary Follicle
A primary follicle consists of a growing oocyte, a single layer of one or more cuboidal granulosa cells, and a basal lamina (see Figs. 125-2 and 125-4). In the primary follicle, several fundamental and important activities are expressed, including FSH receptors in the granulosa cells, gap junctions between the oocyte and granulosa cells, dramatic oocyte growth, and zona pellucida deposition.
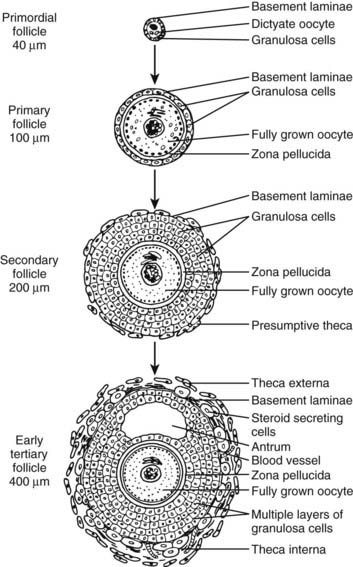
FIGURE 125-4. Diagram of developing preantral follicles showing steps in the gonadotropin-independent stages of folliculogenesis from recruitment of a primordial follicle into the growing pool through its development to the early antrum or tertiary (cavitation) stage.
(From Erickson GF: The ovary: basic principles and concepts. In Felig P, Baxter JD, Frohman LA [eds]: Endocrinology and metabolism, ed 3, New York, 1995, McGraw-Hill, pp 973–1015.)
During evolution of the primary follicle, the gene that encodes the FSH receptor is turned on in the granulosa cells25; this, in turn, is followed by the acquisition of cell-surface FSH receptors that can be identified using 125I FSH autoradiography.26 This is an important event because it allows the potential of the follicle to be stimulated by FSH-signaling pathways. Thus, although preantral follicle growth is gonadotropin independent, granulosa cells of this imature structure are gonadotropin responsive. Although the process of FSH receptor expression is poorly understood in women, evidence in rodents indicates that activin produced by the granulosa cells can increase FSH receptor expression by autocrine mechanisms.5,27
A second key event that occurs in the primary follicle (Fig. 125-5) is the development of gap junctions between the granulosa cells and the oocyte.28 Gap junctions are formed by a family of proteins termed connexins (Cx).29 Functionally, they allow for nonspecific transfer between cells of low-molecular-weight molecules, including the second messengers cyclic adenosine monophosphate (cAMP) and calcium.28–30 Gap junctions between granulosa cells consist of Cx43 and are expressed at the earliest stages of primary follicle development.28 Gap junctions between the oocyte and surrounding granulosa cells comprise Cx37.31 It is important to note that Cx37-deficient female mice (Cx37 knockouts) lack mature (graafian) follicles, fail to ovulate, and develop numerous inappropriate corpora lutea.31 Thus, physiologically important interactions between oocytes and granulosa cells via Cx37 channels are obligatory for normal folliculogenesis and fertility in mice.
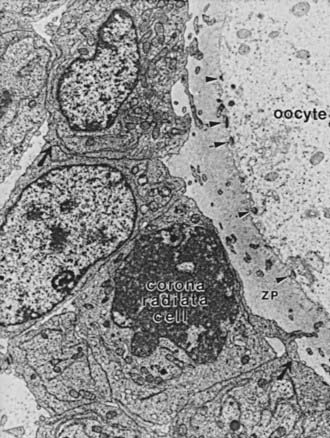
FIGURE 125-5. Electron micrograph of a portion of an oocyte/cumulus complex. Gap junctions (comprising connexin 37) between the processes of corona radiata cells traversing the zona pellucida (ZP) and the oolemma are illustrated by arrowheads. Gap junctions between the corona granulosa cells are illustrated by black arrows.
(From Gilula NB, Epstein ML, Beers WH: Cell-to-cell communication and ovulation: a study of the cumulus-oocyte complex, J Cell Biol 78:58–75, 1978.)
A third event is the growth and differentiation of the oocyte in the primary follicle (see Figs. 125-2 and 125-4). Studies in rodents have demonstrated that the granulosa cells are essential to oocyte growth and development.32,33 It seems likely that Cx37 connections between the granulosa and the oocyte allow for the exchange of nutrient and regulatory molecules that are required for proper oocyte function. Specifically, they evoke changes in oocyte metabolism that influence gene expression. One example is a marked increase in zona pellucida gene expression. As the oocyte grows, it synthesizes and secretes an extracellular matrix, the zona pellucida (ZP), which in time encapsulates the egg (see Figs. 125-2, 125-4, and 125-5). The importance of the ZP is demonstrated by the fact that it contains the species-specific receptor for capacitated sperm and provides a block to polyspermy.34 The ZP consists of three glycoproteins designated ZP-1, ZP-2, and ZP-3.35 ZP-3 is particularly important in sperm binding through its carbohydrate moiety.36 Clinically, considerable interest has been noted in the development of immunocontraceptive vaccines based on the ZP-3 antigen.37,38
Secondary Follicle
The secondary follicle consists of a fully grown oocyte surrounded by a complete zona pellucida, two to eight layers of cuboidal or columnar granulosa cells, and a presumptive theca layer immediately peripheral to the basal lamina (see Fig. 125-4). The acquisition of a theca layer is a major new feature of developing secondary follicles. The presumptive theca consists of several layers of elongated fibroblast-like cells that run radially around the entire follicle (see Fig. 125-2). Theca development is accompanied by angiogenesis. Consequently, the secondary follicle is exposed to important blood hormones such as FSH, LH, and insulin. The mechanisms of theca formation and angiogenesis during secondary follicle development are not clear.1 However, because theca formation requires the presence of follicles, it is believed that granulosa cells, or possibly the oocytes, produce factors that direct this process. Among those peptides under consideration are Kit ligand (KL), GDF-9, insulin, insulin-like growth factor-1 (IGF-1) and/or IGF-2, and activin.39–41 In addition to these growth factors, ample evidence indicates that LH is responsible for early theca cell (TC) differentiation.1,42,43 The degree to which LH influences TC growth is uncertain.
ANTRAL OR GRAAFIAN FOLLICLES
During the late stages of growth in the secondary follicle, a clear fluid begins to accumulate between some granulosa cells. When the follicle reaches approximately 400 µm in diameter, the fluid coalesces into a crescentic space called the antrum.44 By definition, the follicle is now a tertiary or graafian follicle (see Figs. 125-4 and 125-6). This process, termed cavitation or beginning antrum formation, results in remodeling of the granulosa cells to form a miniature graafian follicle (see Fig. 125-6). Cavitation is gonadotropin independent.44 Precisely how the antrum forms is unknown, but direct evidence of a role for activin has been provided.45
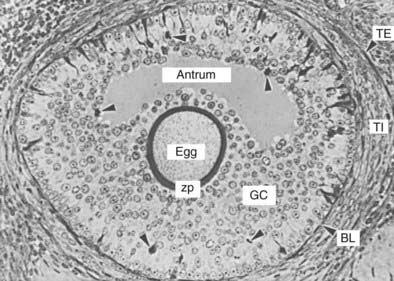
FIGURE 125-6. Photomicrograph of a tertiary follicle approximately 400 µm in diameter at the time of cavitation or early antrum formation. It comprises a fully growing dictyate oocyte (egg) surrounded by a thick zona pellucida (zp); an antrum containing follicular fluid; multiple layers of granulosa cells (GC) organized morphologically into recognizable zones; a basal lamina (BL); a theca interna (TI); and a theca externa (TE). Note the presence of dividing GCs (arrowheads) and of light and dark GCs, the physiologic significance of which is unknown.
(From Bloom W, Fawcett DW [eds]: A textbook of histology, Philadelphia, 1975, WB Saunders, p 869.)
Theca cell differentiation is an important step that occurs at cavitation. It is characterized by a change in which a subpopulation of cells that surround the growing follicle becomes epithelial-like and acquires the ultrastructural and functional characteristics typical of active steroid-secreting cells.1 This cellular layer of tissue is called the theca interna. As these changes occur, the cells, now called theca interstitial cells, express LH receptors.1 At the periphery of the theca interna is a layer of concentrically arranged fusiform cells that have the ultrastructural and functional characteristics of smooth muscle cells.44 They constitute the theca externa of the tertiary follicle (see Fig. 125-6).
By the time of cavitation, the oocyte has attained its full size (≈120 µm in diameter) and appears fully developed structurally (see Fig. 125-6). Therefore, the oocyte does not grow further, despite the fact that the graafian follicle as a whole might continue to enlarge to a diameter of 2 cm or more.46
Classification of Graafian Follicles
Graafian follicles can be defined structurally as a heterogeneous family of relatively large follicles (400 µm to >2 cm at ovulation) that display an antrum containing follicular fluid, or liquor folliculi. The antrum is a characteristic structural feature of all graafian follicles. As such, the term antral follicle is a synonym for graafian follicle.
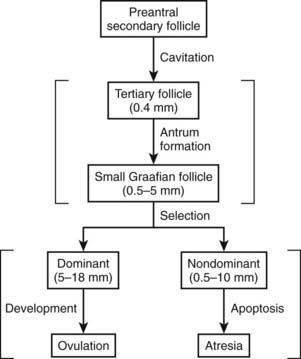
In a broad sense, graafian follicles can be divided into two major groups: healthy and atretic (Fig. 125-7). The main difference between these two groups is whether or not apoptosis, or programmed cell death, is occurring in the granulosa cells. All graafian follicles (healthy or atretic) follow a progressive course during their development over time. The healthy graafian follicle becomes progressively larger and more differentiated with increasing time until it reaches the preovulatory stage (see Fig. 125-7). The mechanism by which a healthy graafian follicle is formed depends on pituitary gonadotropins and involves the differential and temporal pattern of expression of specific genes that lead to cytodifferentiation and proliferation of follicle cells.47 In a similar manner, atretic or nondominant graafian follicles undergo a temporal pattern of expression of specific genes, which, in turn, results in cessation of growth and activation of apoptosis (see Fig. 125-7).
Structure of the Graafian Follicle
A healthy graafian follicle is a complex tissue that comprises multiple layers of precisely positioned cells (Fig. 125-8). The theca externa is innervated by autonomic nerves that seem to play an active role in their contraction.44,48 The full significance of the theca externa is not known, but the regulated contraction of these cells has been implicated in the processes of both ovulation and atresia.44 In the theca interna, the number of differentiated theca interstitial cells increases progressively, and their endocrine function is reflected in the large capillary plexus. It is not uncommon to have five to eight layers of theca interstitial cells in a graafian follicle.1
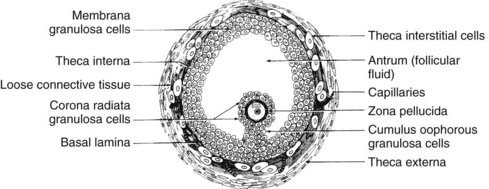
FIGURE 125-8. Diagram of the cross-sectional appearance of a typical healthy graafian follicle showing the organization of its various cell types.
(From Erickson GF: Primary cultures of ovarian cells in serum-free medium as models of hormone-dependent differentiation, Mol Cell Endocrinol 29:21–49, 1983.)
Internal to the theca is a basal lamina that functions as a barrier to vascular tissue, as well as multiple layers of granulosa cells that appear heterogeneous by virtue of their position within the follicle wall (Fig. 125-9). The position of the granulosa cells creates at least four different domains: (1) an outer or membrana domain that comprises a pseudostratified epithelium, which makes contact with the basal lamina; (2) an inner or periantral domain, which makes contact with the membrana cells; (3) the cumulus domain, which makes contact with the periantral cells; and (4) a corona radiata domain, which makes contact with the cumulus cells, as well as with the ZP and oocyte (see Fig. 125-9). By virtue of their position, the granulosa cells are directed to express different cellular functions in response to FSH stimulation.49,50 For example, in the central domains (corona radiata, cumulus, periantral), the granulosa cells continue to divide throughout graafian follicle development, suggesting that the cells might be stem cells. By contrast, the cells in the membrana domain become postmitotic and respond to FSH with expression of their fully differentiated state (see Fig. 125-9). How such a heterogeneity of granulosa cells is generated and precisely what is accomplished by it are important questions, the answers to which remain largely unknown. It is interesting to note, however, that the concept is emerging that morphogens produced by the oocyte function as novel determinants of granulosa proliferation and differentiation and are responsive to FSH stimulation.49,50
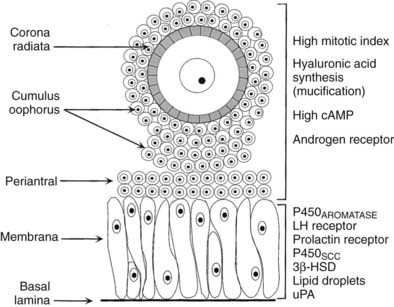
FIGURE 125-9. Diagram of the structure-function heterogeneity of the granulosa cells in a healthy graafian follicle. By virtue of their position in the follicle wall, the granulosa cells become different from one another, and this is reflected in different patterns of proliferation and differentiation.
(From Erickson GF: The graafian follicle: a functional definition. In Adashi EY [ed]: Ovulation: evolving scientific and clinical concepts. New York, 2000, Springer-Verlag.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree