FIGURE 131-1. Left panel, Cumulative conception rates during the first 12 months of unprotected intercourse. Right panel, Probability of a clinical pregnancy relative to the day of intercourse. Day 0 depicts the day of the serum luteinizing hormone (LH) surge. Individual lines depict 5th to 95th percentiles of the total population.
(Left panel data from Taylor A: ABC of subfertility: the extent of the problem, BMJ 327:434–436, 2003. Right panel data from Evers JL: Female subfertility. Lancet 360:151–159, 2002.)
Opposite to what most patients believe, a situation of (absolute) infertility rarely exists. No chance of conception (i.e., sterility) only occurs in cases of amenorrhea, bilateral tubal occlusion, or azoospermia. Therefore, the term subfertility seems more accurate in describing a condition of diminished fertility, when the three causes for absolute infertility have been ruled out. Decreased fertility of a given individual may be well compensated for by enhanced fertility of the partner. These couples may generate offspring without difficulty and therefore never visit a doctor. The clinical challenge is to assess chances for pregnancy, either spontaneously or after infertility treatment, of a given couple visiting an infertility clinic. The proposed management algorithm for a couple may include distinctly different options, ranging from expectant management without intervention, to causal therapy for those with sterility, to assisted reproduction as an empirical approach to increasing chances for offspring.
Primary (as opposed to secondary) subfertility involves individuals (or couples) with no previous pregnancy.
Although the debate continues as to whether subfertility should be considered a disease, the major impact of involuntary childlessness on psychosocial well-being and quality of life should not be underestimated. According to the World Health Organization (WHO), health should be defined as “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.” Subfertility, therefore, constitutes an unhealthy state, due to the threat of remaining childless for the rest of one’s life. For the benefit of the general public, the clinician should voice clearly and consistently to politicians and health care providers the message that subfertility should be considered a serious health issue. Most people worldwide still have insufficient access to infertility services.
History
Infertility care is a relatively young subspecialty that only started in the early 1960s. The first 2 decades were dominated by the possibilities of endocrine diagnosis and treatments and surgical procedures. Gonadotropin and steroid hormone assays were developed for widespread clinical use, and the synthetic antiestrogen clomiphene citrate and urinary gonadotropins (human menopausal gonadotropin [hMG], obtained from postmenopausal women excreting large quantities of pituitary follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) became available for stimulating gonadal function in both males and females.
Initial surgical approaches in the female involved wedge resection of polycystic ovaries, followed by microsurgery of endometriotic cysts and uterine myomas, adhesions, and tubal disease and, more recently, by endoscopic pelvic surgery and hysteroscopy. Surgical approaches in the male have been dominated by varicocele correction in attempts to improve sperm quality. Most of these initial surgical approaches have not proved efficacious when vigorous methods of assessment are applied.1,2
The past 2 decades have been dominated by the development of assisted reproductive techniques (ART), chiefly intrauterine insemination (IUI) and in vitro fertilization (IVF) in 1979,3 and intracytoplasmic sperm injection (ICSI) in 1992.4 These novel in vitro therapeutic strategies, combined with complex ovarian hyperstimulation protocols, have created new possibilities for offspring for thousands of couples with only limited chances for a child otherwise. These treatments have proved successful but have disadvantages in terms of patient discomfort, complications, and high cost.
The empirical approach to assisted reproduction technology has largely replaced conventional causal therapies, with consequent reduced interest in the mechanisms underlying decreased fertility, which may have a negative impact on the future development of novel causal treatment options. Because of changes in society and the commercial environment in which IVF is usually practiced, we have reached a paradoxical state with overconsumption of infertility therapies in some parts of the Western world and insufficient access to infertility care in many other societies.
Although studies have demonstrated that IVF should not replace a conventional simple treatment algorithm,5–7 recent reports claim a superior cost benefit for IVF as a primary therapeutic strategy for both unexplained and male factor subfertility.8
Recent developments in infertility care include the development of milder stimulation protocols, in vitro maturation and cryopreservation of oocytes obtained from unstimulated ovaries, and embryo selection by preimplantation genetic screening. Mild stimulation has proved beneficial in obtaining the best embryos available for that specific treatment cycle with reduction in burden to the patient.9,10 In vitro maturation with oocyte cryopreservation has yielded considerable numbers of healthy pregnancies but currently lacks sufficient levels of efficiency and safety to allow large-scale application.11 Finally, improvement in ART results detected by assessment of quality of the individual embryo through blastomere biopsy and subsequent numeric chromosome analysis has not been clearly established.12–14
Epidemiology
In the general population, the highest chance of pregnancy occurs in the first cycle of unprotected intercourse. The maximal probability for a clinical pregnancy is approximately 30% per cycle, and chances for pregnancy are highest when intercourse takes place 2 days before ovulation15 (see Fig. 131-1). The likelihood of pregnancy slowly decreases in subsequent cycles, generating a cumulative chance for pregnancy of 60% within three cycles, 70% within six cycles, and around 90% within a year16 (see Fig. 131-1). Very fertile couples are likely to achieve a pregnancy within the first few cycles, whereas others will conceive within 12 months (Table 131-1). Moreover, a considerable proportion of couples will still conceive spontaneously after 1 year or even later (Figs. 131-2 and 131-3). As was mentioned previously, a 1 year period has been chosen arbitrarily as the cutoff to define subfertility and to justify an infertility workup. Subfertility is described as occurring in around 10% to 15% of the population.
Table 131-1. Estimated Spontaneous Cumulative Pregnancy Rates in Normal Fertile and Infertile Couples*
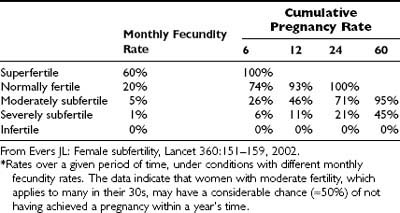
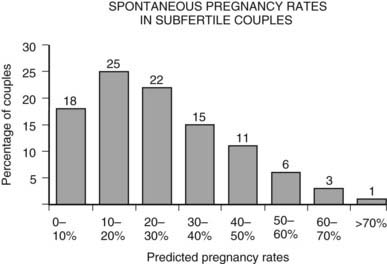
FIGURE 131-2. The distribution of the probability to become pregnant among untreated subfertile couples within the year after intake for subfertility workup (defined as having had at least 1 year of unprotected intercourse).
(Data from Eimers JM, te Velde ER, Gerritse R, et al: The prediction of the chance to conceive in subfertile couples, Fertil Steril 61:44–52, 1994.)
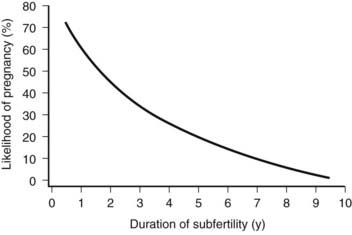
FIGURE 131-3. The likelihood of pregnancy in couples relative to the duration of their subfertility. Note the considerable chance to conceive within the first 3 years of unprotected intercourse. For instance, the probability for a couple to become pregnant in the course of the second year of trying is still 50%.
(Data from Evers JL: Female subfertility, Lancet 360:151–159, 2002.)
Multiple societal tendencies interfering with normal fertility have been described, especially (1) early sexarche and promiscuous sexual behavior (resulting in increased chances for tubal disease due to sexually transmitted disease), (2) postponement of pregnancy and consequently an increased proportion of women older than 30 years of age with already decreased natural fertility due to ovarian aging when offspring is desired, (3) female lifestyle factors (especially obesity and resulting ovarian dysfunction), and (4) environmental and toxic agents (e.g., cigarette smoking inducing direct effects upon the oocyte or leading to accelerated follicle pool depletion). Consequently, more couples will not conceive spontaneously within a year of unprotected intercourse and therefore will visit a doctor for subfertility care.
It appears that the prevalence of subfertility in the Western world has remained unaltered over the past 50 years or may even have been reduced,17–19 although more couples have been seeking access to infertility services.20
Pathogenesis and Clinical Features
According to textbooks, the female factor accounts for approximately 35% of subfertility, the male factor for 35%, and in about 30% of cases, both male and female factors are involved. In female subfertility, approximately 30% to 40% of cases involve ovulatory dysfunction, and 30% to 40% tubal and pelvic pathology; 30% of cases are attributed to other unexplained causes, of which reproductive age may be an important one.21,22 Clinical data supporting these figures are not robust, however, especially in light of uncertainties regarding causal factors, differences in population, and referral characteristics. Overall, a full workup of the subfertile couple will yield a cause leading to infertility in ≈23% of cases (bilateral tubal obstruction, amenorrhea, azoospermia); in the remainder, only factors that will reduce the probability of conception (i.e., subfertility) are identified.23
Couples who suffer from subfertility usually do not have associated complaints or symptoms. Exceptions include abnormal or absent menstrual bleeding, a history of pelvic inflammatory disease, a ruptured appendix, or maldescensus of one or both testes. The general rule that the more tests you do, the more likely it is that at least one test will be abnormal by chance certainly also holds true for subfertility. It may not be easy to identify the disease state that is causally related to reproductive failure in a given couple. Classic examples include conditions such as endometriosis or uterine leiomyomas, which were widely believed to induce infertility, until it was found that both these conditions are prevalent in the general fertile population.
Similar findings apply to the identification of male subfertility. Poor sperm quality (according to WHO criteria) was widely believed to be the cause of infertility in many cases, until more recent studies showed that diminished sperm quality can be readily observed in males with children.24,25 Although criteria for defining abnormal sperm have been revised repeatedly, the predictive power of sperm quality assessment should be viewed with caution in a subfertility population. A clear effect upon pregnancy rates has been demonstrated only in males with severely reduced sperm density.26–28
Several classes of disease conditions that may underlie female subfertility can be identified, notably ovulatory dysfunction, mechanical dysfunction, ovarian aging, and unexplained subfertility.
OVULATORY DYSFUNCTION
Changes in ovarian function usually are presented by extended bleeding intervals (oligomenorrhea) or absence of bleeding (amenorrhea). Amenorrhea is usually associated with a hypoestrogenic status, whereas bleeding in oligomenorrhea may represent occasional ovulation or breakthrough bleeding due to continued estrogen exposure. In primary amenorrhea, with or without disturbed sexual maturation, one should consider rare conditions involving abnormal gonadal or uterine development due to (inherited or spontaneous) gene mutations.
Ovulatory dysfunction can be classified in three categories based on FSH and estradiol (E2) hormone assays in peripheral blood.29,30 Low FSH and E2 indicate a central origin of the abnormality at the level of the hypothalamus or pituitary. Hence, ovaries fail to function normally owing to insufficient stimulation by pituitary gonadotropins (WHO group 1). In contrast, the combination of high FSH and low E2 concentrations indicates that the primary defect is at the ovarian level (WHO group 3, i.e., early menopause or premature ovarian failure [POF]), with the ovaries failing to produce estrogens despite maximal stimulation by endogenous FSH. The third group of patients (WHO group 2) is represented by FSH and E2 levels within the normal range. This heterogeneous group also includes polycystic ovary syndrome (PCOS) characterized by clinical or biochemical androgen excess and/or polycystic ovaries.31–33
Luteal phase insufficiency as an expression of ovulatory dysfunction has long been believed to be an important factor in subfertility, occurring in up to 14% of infertile patients. However, controlled studies conducted to establish its role in subfertility or the value of any treatment in improving pregnancy rates are lacking.34,35
ANATOMIC DYSFUNCTION
The normal functioning of the reproductive tract is important for undisturbed fertility. Many uterine abnormalities, including congenital uterine malformations, malformations due to fetal exposure to diethylstilbestrol, fibroids (in particular, the submucosal or cornual myomas), polyps, and adhesions related to previous intrauterine procedures, have been associated with infertility or early miscarriage.36
Normal fertility may also be affected by pelvic adhesions (especially involving the ovaries and fallopian tubes) caused by pelvic inflammatory disease, complicated appendicitis, or intra-abdominal surgery. Abnormal tubal function may be due to isthmic or terminal occlusion or phimosis of the fimbriae due to salpingitis. Peritubal adhesions may interfere with normal tubal motility and, hence, with gamete and zygote transport. Severely damaged tubes may fill with fluid, giving rise to hydrosalpinges.
Endometriosis is a topic of continued controversy related to disease conditions associated with subfertility.37,38 Endometriosis is the ectopic localization of endometrial tissue, most commonly in the pelvis; retrograde menstruation may be the important causal factor. Severe forms of endometriosis give rise to adhesions, cyst formation, and reduced fertility chances. In addition, abdominal discomfort and dyspareunia are frequent complaints. However, limited data suggest that minimal and mild levels of endometriosis are prevalent in the general population. Moreover, positive effects on subsequent fertility of intervention studies using medication or surgical procedures are questionable.39,40
Myomas reportedly occur in 1% to 2.5% of infertile patients without other evident cause of infertility,41 and removal has long been thought to improve subsequent pregnancy chances. A recent meta-analysis has shown that infertility patients with fibroids that distort the uterine cavity have poorer reproductive outcomes than those infertile patients without fibroids, and removal therefore is justified. Cases with intramural myomas may have a poorer prospect, but the lack of quality studies prevents advocating their removal. Subserosal fibroids are not associated with fertility issues.42
As with many other factors associated with infertility, comparative data on the frequency of similar abnormalities in a matched, fertile population are lacking. In addition, randomized follow-up studies of pregnancy rates comparing intervention versus expectant management are rare, so the efficacy of treatment is uncertain.
OVARIAN AGING
In many societies around the world, increased participation of females in extended education and careers has resulted in postponement of child-bearing to a later age. For instance, in The Netherlands, the average age of the mother who has her first child has risen steadily and is currently 29.6,43 An increased proportion of women make their first attempts to conceive when they are beyond 30 years of age. After age 30, natural fertility starts to decrease, with a major subsequent decline in fertility noted after 37 years of age44 (Fig. 131-4). Beyond age 41, chances of having a baby have decreased on average to almost zero.
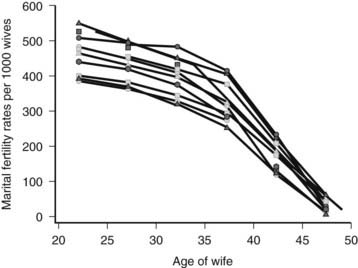
FIGURE 131-4. Natural marital fertility rates as a function of age of the woman, indicated for different populations from the sixteenth until the twentieth century (Hutterites, Geneva bourgeoisie, Canada, Normandy, Tunis, Norway, and Iran).
(Data from Menken J, Trussel J, Larsen U, et al: Age and infertility, Science 233:1389–1394, 1986; Schwartz D, Mayaux MJ: Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS, N Engl J Med 306:404–406, 1982.)
Around 20 weeks of female fetal life, initial germ cell mitosis is arrested, and the primordial follicle pool reaches its maximum size of around 8 million per ovary. Thereafter, this stock of resting primordial follicles is gradually depleted (by the onset of growth of follicles), falling to 2 million at birth and 0.5 million at menarche (first menstrual period), with final exhaustion of the pool with the last spontaneous menstrual period. Thus, only a small proportion (around 10% to 15%) of the initial primordial follicle pool is present at the time that many women consider conceiving their first child.
Menopause, which marks the definitive end of the reproductive lifespan, occurs at the average age of 51, with a range from 40 to 60 years of age. This indicates that exhaustion of the follicle pool takes 50% more time (60 rather than 40 years) in some women than in others. The association between chronologic age and ovarian aging therefore may be poor. Indirect evidence indicates that a fixed time interval exists between the transition from normal fertility into decreased and finally into absent fertility (sterility).45,46 Some women at the age of 35 may already have severely compromised fertility (these women will also present with an early, although normal, menopause), whereas, for other women of identical age, natural fertility is still completely normal. The latter group of women probably will experience menopause at a later age. The clinical challenge is to assess the reproductive aging status of a given woman, which will help to predict her pregnancy chances more accurately than will chance assessment based on chronologic age alone. This may help the clinician in counseling the patient regarding expectant management or therapeutic strategies.46
The mechanisms underlying reduced natural fertility in relation to ovarian aging have not yet been fully elucidated. It has been clearly established that the size of the cohort of growing follicles is diminished, and this coincides with an increased proportion of chromosomally abnormal oocytes. The latter is exemplified by increased rates of aneuploid (e.g., trisomy 21) pregnancy, increased rates of early pregnancy loss (most likely based on aneuploid conceptus), and increased numbers of aneuploid oocytes and embryos with increasing female age.47 Changes in follicle cohort size can be assessed by transvaginal ultrasound or by hormone estimates in peripheral blood, as will be discussed later. Unfortunately, assessment of average oocyte quality on an individual basis so far has not proven feasible.
UNEXPLAINED SUBFERTILITY
The indicated percentage of subfertility of unknown origin is usually around 30%. The reported incidence is very much dependent on the extent of the infertility evaluation, the characteristics of the population studied, female age, and interpretation of data, along with the reported incidence of other “known” factors related to subfertility. Again, a clearcut causal relationship between an abnormal condition as established in initial evaluation and subfertility is often absent. This implies that several factors are known that do not exclude the possibility of achieving a pregnancy (e.g., amenorrhea, tubal occlusion, azoospermia), but they only contribute to a reduction in monthly fecundity. Key factors are female age, previous pregnancy, and semen quality.48,49
On the other hand, many processes are involved in natural fertility that cannot be tested properly in a routine clinical setting. These processes involve oocyte maturation and fertilization, embryo transport through the tube and uterine cavity, chromosomal constitution of embryos, apposition and implantation of embryos, and, finally, endometrial receptivity, including regulation of endometrial maturation by steroids produced by the corpus luteum.50 Moreover, many pregnant women, under natural conditions or in a subfertile population, may miscarry very early, before the occurrence of regular menses.50
Evaluation of the Female in the Subfertile Couple
The extent to which basic infertility testing should be performed is extensively debated in the current era, which is dominated by assisted reproductive techniques. Consequently, marked differences exist with regard to the numbers and types of tests performed.51,52 Tests are time consuming and costly, and some are not performed without risks. According to some clinical investigators, results may have little bearing on assisted reproduction outcomes. In a commercial environment where patients have to pay for services, “customers” may prefer to spend their money on treatment with at least some chance for pregnancy, than on diagnostic procedures that will have no impact on their chances to conceive.
Many tests are not clearly standardized, and therefore reproducibility is limited. The usefulness of infertility tests should be based on the proper evaluation of (1) sensitivity (the capacity of a test to detect abnormalities), (2) specificity (the chance that an abnormality is actually present if the test yields an abnormal result), (3) invasiveness, complexity, and cost of the test, and, finally, (4) the predictive value of the test. Indeed, few studies have been undertaken to evaluate the predictive validity of tests, despite their widespread use in clinical practice.30
During the initial consultation, the physician should take a full medical history from both partners. Relevant information includes duration of subfertility, past sexual development, children from current or previous relationships, obstetric history, previous surgery, abdominal or menstrual discomfort, and sexually transmitted diseases. Moreover, the clinician should counsel the couple regarding timing of intercourse and fertility chances, effects of lifestyle factors (such as excessive smoking, alcohol intake, caffeine consumption, and exercise), food intake and obesity, and professional hazards. A general physical examination usually is advised only for women.20,53–55
Diagnostic tests that may be performed for the evaluation of subfertile couples include assessment of the normal menstrual cycle, assessment of anatomic factors, and assessment of ovarian aging.
ASSESSMENT OF THE NORMAL MENSTRUAL CYCLE
Regular menstrual periods strongly suggest normal ovulatory cycles. Ovulation and normal corpus luteum function can be assessed in different ways.35 However, the tests are not optimal in terms of accuracy and reproducibility, nor in the detection of a meaningful and treatable disease condition relevant to subfertility.56
Menstrual Period Chart
This represents the simplest way to document the menstrual cycle pattern. The days of onset and cessation of menses should be recorded on a calendar for several months. A normal menstrual cycle ranges between 25 and 35 days, with a median duration of 28 days.
Basal Body Temperature (BBT) Chart
Progesterone produced by the corpus luteum has many effects on the central nervous system, including changes in body temperature set point. Consequently, daily assessment of body temperature can be used to document a persistent increase in temperature (typically from 36.6°C to 37.1°C) and therefore can provide proof of ovulation. A rise in temperature indicates that ovulation and corpus luteum formation have taken place. The length of the second half of the cycle should be at least 10 days. Daily temperature assessments should be performed at the same time during the early morning before the activities are begun. Although performing these tests for several months can be quite stressful to women, it may be helpful for the couple to be aware of the day of ovulation, so they can time intercourse to achieve optimal pregnancy chances. However, the BBT is notoriously unreliable for the timing of ovulation.57
Urinary LH Surge
When LH concentrations are high in serum during the midcycle surge, sufficient quantities are excreted into urine to allow its detection. Kits are commercially available for use at home. On average, LH appears in the urine around 12 hours after onset of the LH surge in the blood, and this can accurately predict ovulation. This method, again, can help with optimal timing of intercourse, although evidence for a beneficial effect upon pregnancy rates has so far not been found.57,58
Transvaginal Ultrasonography
The preovulatory, Graafian follicle usually attains a diameter of around 20 mm (ranging from 16 to 24 mm) before rupture and subsequent release of the oocyte. Rupture of the follicle can be established by visualizing the disappearance or decrease in size of the dominant follicle by transvaginal ultrasound. This method usually is reserved for monitoring of ovarian stimulation during infertility therapy or for proper timing of a postcoital test or midluteal phase progesterone assay (see the following section).
Progesterone Assay
Appropriate timing of the assessment of progesterone serum concentrations is crucial and should always be checked in retrospect, especially in women whose cycle length may vary. A level above 2 ng/mL indicates ovulation, although levels between 6 and 25 ng/mL are considered normal and are associated with chances for pregnancy. Some investigators advocate that multiple hormone assays should be performed around the midluteal phase to document insufficient corpus luteum function. The luteal phase may be reduced in length, or insufficient quantities of progesterone may be produced during the luteal phase. However, the exact role of these variations in terms of female fecundity remains a matter of debate.59–61
ASSESSMENT OF ANATOMIC DYSFUNCTION
Some type of mechanical factor relevant to subfertility is reported to occur in 10% to 30% of subfertile couples. Several methods exist to establish abnormalities at the level of the uterus, fallopian tubes, or ovary/ampulla complex relevant for in vivo oocyte pickup. Until recently, diagnosis of mechanical abnormalities by hysterosalpingography (HSG) during the early infertility workup and by laparoscopy later in the evaluation has been standard procedure for each patient. Currently, the tendency is toward the use of screening tests to identify women at high or low risk for tubal disease and the performance of more invasive diagnostic procedures only in those women at increased risk for such abnormalities.62 The question remains as to what extent any mechanical deviation from normal truly contributes to the subfertility. The clinician should take this reservation seriously, especially in cases of minor abnormalities of the uterine cavity and of presumed unilateral tubal damage.
History Taking
The guidelines of the National Institute for Clinical Excellence (NICE) in the United Kingdom advise clinicians to use the past medical history in the woman to decide whether invasive diagnostics should be applied to assess the presence of tubal pathology. Especially, previous deliveries, previous pelvic inflammatory disease, or previous pelvic surgery enables the identification of such couples.63,64 The addition of Chlamydia testing will further enhance the correct identification of couples at such low risk for anatomic dysfunction that invasive diagnostics can be avoided.
Screening by Chlamydia Antibody Testing in Serum
Chlamydia trachomatis represents the most common sexually transmitted pathogen in the Western world, with a reported prevalence of infection of around 5% to 10%. Most of these infections remain without symptoms, but around 10% can cause pelvic inflammatory disease.65 Chlamydia acts as an immunogen that gives rise to antibody formation. Immunoglobulin G (IgG) antibodies remain for years in the circulation and therefore can be used as a marker for a past infection. IgG concentrations are reportedly elevated in 30% to 60% of women from subfertile couples. The capacity of IgG antibody levels to predict tuboperitoneal disease (as assessed by laparoscopy) has been evaluated prospectively, with acceptable receiver operator characteristic (ROC) curves and the use of titers between 16 and 3266,67 (Fig. 131-5). In subfertile couples with a relatively good fertility prognosis, a workup strategy starting with screening for Chlamydia is an effective approach,68,69 and for many women, performance of more invasive procedures can be prevented.
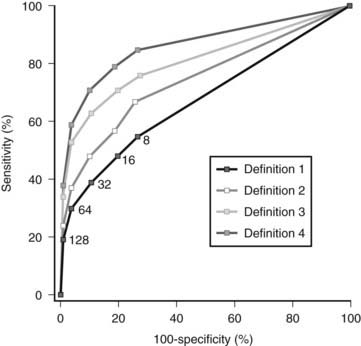
FIGURE 131-5. Receiver operating characteristic curves for Chlamydia antibody titers using different definitions for tubal factor subfertility. Definition 1: Any periadnexal adhesions and/or proximal or distal occlusion of at least one tube. Definition 2: Extensive periadnexal adhesions and/or proximal or distal occlusion of at least one tube. Definition 3: Extensive periadnexal adhesions and/or distal occlusion of at least one tube. Definition 4: Extensive periadnexal adhesions and/or distal occlusion of both tubes.
(From Land JA, Evers JL, Goossens VJ: How to use Chlamydial antibody testing in subfertility patients, Human Reprod 13:1094–1098, 1998.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








