FIGURE 143-1. Diagram showing the zonal anatomy of the human prostate gland. Benign disease most commonly occurs in the transition zone (TZ), whereas malignant nodules are most prevalent in the peripheral zone (PZ).
(Reproduced courtesy of Ellem SJ, Risbridger GP: Treating prostate cancer: a rationale for targeting local oestrogens, Nat Rev Cancer 8:621–627, 2007.)
Benign Prostatic Hyperplasia
CLINICAL DEFINITION AND PRESENTATION
Benign prostatic hyperplasia may be defined histologically as a hyperplasia of the epithelium, stroma, or both, in the periurethral transition zone of the prostate. The complex of symptoms that is often attributable to men with BPH are collectively referred to as lower urinary tract symptoms (LUTS). LUTS include frequency of urination, urgency, urinary incontinence, waking up multiple times at night to void (nocturia), weakened urinary stream, straining to void, and a sense of incomplete emptying of the bladder. It should be noted that unlike BPH, LUTS occurs in both men and women, and therefore these two disease entities are not technically interchangeable. Men may have histologic evidence for BPH without any symptoms, and there are many other potential causes of LUTS beside BPH, such as stricture disease, neurologic bladder dysfunction, and malignancies (including advanced prostate cancer), among others. In the short term, BPH can lead to a variety of problems, including gross hematuria, bladder calculi, urinary retention, and severe/bothersome LUTS. Left untreated, BPH can ultimately lead to permanent bladder dysfunction, acontractility and urinary retention, severe renal dysfunction, end-stage renal disease and the need for dialysis, and in the rarest of cases, death. The incidence of these end-stage consequences in developed countries such as the United States is very low, and in most cases men are evaluated and treated prior to this becoming manifest.
Histologic BPH is the most common symptomatic benign neoplastic condition in humans.33 BPH is rarely identifiable histologically in men under 40 years of age. However, the incidence of the disease increases rapidly with age to around 88% of men by the age of 80. Estimates vary, but the number who will require treatment of any sort for LUTS is 16/1000 person years overall, increasing from 3.3/1000 for men aged 40 to 49 up to 30/1000 person years for those over age 70.34 The disease appears to be linked to aging and the presence of testicular function. Cigarette smokers have historically been found to have a lower incidence of surgery for BPH than nonsmokers, possibly owing to decreased serum testosterone and increased estrogens in the smoking population.35,36 However, it should be noted that in more recent studies, no consistent link between smoking history and LUTS could be established.37
BPH occurs naturally in humans, dogs, and in some nonhuman primates including chimpanzees.38 Canine BPH, like its human counterpart, arises with increasing frequency with age and only occurs in animals with intact testicular function. Histologic and anatomic differences distinguish canine from human BPH. Human BPH is strongly focal with distinct nodules of hyperplasia within the gland, whereas the canine disease is usually diffuse and occurs throughout the gland.39–41 In the dog, a generalized expansion of the gland is observed which compresses the rectum, producing constipation. In humans, focal growth of transition-zone nodules compresses the urethra, resulting in urinary retention—widely believed to be the fundamental cause of the profile of LUTS due to BPH.
STROMAL-EPITHELIAL INTERACTIONS, FETAL REAWAKENING/MCNEAL HYPOTHESIS
The exclusive site of human BPH origin is the preprostatic region of the prostate.39 This is the region immediately distal to the urinary bladder and surrounding the preprostatic sphincter. The preprostatic region is further subdivided into the periurethral and transition zones. The periurethral zone surrounds the urethra within the sleeve of the preprostatic sphincter, whereas the transition zone in turn surrounds the sphincter. This focalized location of BPH pathogenesis and glandular expansion is directly related to the principle clinical manifestation of human BPH, which is LUTS.
Small early BPH nodules usually form within the transition zone.39 They occur in a clearly defined area either within or adjacent to the preprostatic sphincter, directly lateral or somewhat ventral to the urethral lumen. This represents a sharp focusing of the sites of nodule origin within the prostate into a region comprising about 2% of the total mass of the gland. In prostates with larger, more numerous BPH nodules (generally older age groups), anatomic focusing is the same but is not as restricted.
BPH nodules are of several different types, with fibrous and/or muscular stroma, either with or without an epithelial component. Periurethral nodules are often stromal in character or show only a few small glands penetrating from the periphery. The stroma is described as reminiscent of the embryonic mesenchyme.39 Transition-zone nodules are composed of glandular tissue derived from newly formed small duct branches. They bud off from preexisting ducts, grow into the adjacent stroma, and repeatedly branch, creating a new architectural system within the nodule. Individual patients usually have a number of nodes which appear to grow in a coordinated manner.
An important concept that has been identified with the origins of BPH is the reawakening of embryonic inductive potential in BPH stromal cells.39,40,42,43
This concept is based on the idea that growth of the prostate results from local interplay of growth factors between the epithelial and stromal elements of the organ under the influence of testicular androgens. The pubertal growth spurt is thought to be mediated by growth-promoting factors under androgenic control. In adulthood, growth-promoting factors are presumably either down-regulated or balanced by growth inhibiting factors. However, a breakdown of this homeostatic balance could lead to localized growth that is characteristic of BPH (Fig. 143-2). This idea enjoys considerable experimental support. For example, it is known that adult prostatic epithelium from rats, mice, and humans can respond to prostatic inductive mesenchyma with new growth and development,44–47 but these experimental data do not address the underlying, and at present unknown, cause of such a stromal-to-mesenchymal switch. In addition, the specific factors that mediate hyperplastic enlargement of the prostate remain to be determined, although it is likely that these will prove to be the same factors involved in normal prostatic growth.
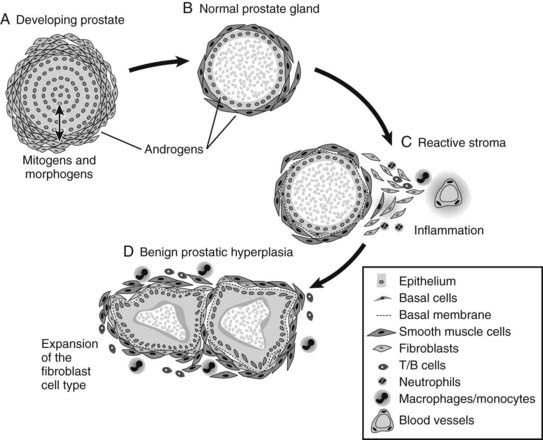
FIGURE 143-2. Schematic representation of stromal and epithelial cell interaction in developing, normal, and benign prostatic hyperplasia (BPH) tissues. In development, stromal–epithelial cell interactions lead to normal prostate gland development. Reactivation of the stroma in adulthood (e.g., due to inflammation) leads to expansion of the fibroblast cell type and occurrence of BPH.
INFLAMMATION AS A CAUSATIVE AGENT FOR BPH
Prostatic inflammation is a common histopathologic observation. Its association with chronic prostatitis/chronic pelvic pain syndrome has not yet been completely defined. Even less is known about how asymptomatic inflammatory prostatitis is associated with benign prostatic hyperplasia. The complexity of acute and chronic inflammatory processes may play a role in the development of benign prostatic hyperplasia, as shown in various studies to date.48–51 Increased inflammation is closely associated with the severity of BPH. Data from the Medical Therapy of Prostatic Symptoms (MTOPS) study suggest the risk of BPH progression and acute urinary retention is greater in men with prostatic inflammation.52,53 Inflammation has also been linked to the development of hyperplasia; in a mouse model of chronic prostatitis, multiple regions of epithelial hyperplasia and dysplasia were found next to areas of inflammation.54 In BPH patients, stromal nodules were found to contain increased numbers of T and B lymphocytes.55 Elevated levels of inflammatory cells were also detected in the interstitium and surrounding epithelial glands of human BPH tissues.56 In this study, the majority of inflammatory cells (60%) were CD4 helper T cells, with the remainder consisting of CD8 cytotoxic T cells (30%) and B cells (10%). Additional studies revealed that T cells present in human BPH samples were chronically activated.57 In BPH, infiltration of inflammatory cells is accompanied by increased expression of proinflammatory cytokines. Elevated levels of the interleukins, IL-2, IL-8, IL-17, and IFN-γ have been detected in BPH samples.58–60 In addition, expression of IL-15, a cytokine that promotes T-cell proliferation, and its receptor IL-15R were higher in BPH epithelial cells compared to normal prostate.61 Increased cytokine expression influences the proliferation of noninflammatory cells in the prostate, and the cytokines IL-2 and IFN-γ have been shown to increase proliferation of human BPH stromal cell lines.58 Increased inflammation can result in disruption of epithelial glands, resulting in increased serum levels of prostate-specific antigen (PSA). It is possible that agents that reduce inflammation could also be effective at reducing hyperplasia, thus reducing the symptoms associated with this disease.
MANAGEMENT OF BPH
Links between BPH and testicular function have long been suspected, based on the observation that men castrated before the age of 40 years do not develop the condition. The Skoptzys, a Russian sect in which the males underwent ritual castration at 35 years, did not suffer from prostatic enlargement.62,63 Furthermore, it has been shown that the absence of testicular function from a young age, either from castration or hypopituitarism, prevents the occurrence of BPH in elderly men (<55 years). Postmortem examination of 28 such patients showed no histologic evidence of BPH as compared to an age-matched control group where BPH was found in 50% of the patients.64
Other steroids have been implicated in BPH. Work on canine BPH showed that this condition can be induced with androstanediol and with combinations of androstanediol and estradiol. A combined dose of DHT and estradiol was also found to induce canine BPH.41,65
It is known that in the cynomolgus monkey, the periurethral zone of the prostate is the most sensitive to estrogenic stimulation and further that the result of such stimulation is stromal proliferation,66 suggesting a possible role for estrogens in the genesis of human BPH. Blockade of local estrogen production in the prostate by using aromatase inhibitors has been tested as therapy for BPH, the rationale for this being that estrogens produced in the prostatic stroma may play a role in prostatic hyperplasia.67,68 These compounds have proven to be clinically useful but are not part of routine medical practice.69,70
Attempts to inhibit androgens in BPH patients in the late 19th century, and in a very limited trial in 1940, were based on castration, a cheap and simple but understandably unpopular treatment.71,72 This idea has lived on, predominantly in the therapy of advanced prostate cancer, as a medical rather than surgical approach, with the use of both antiandrogens and LHRH agonists to blockade the effects and production, respectively, of androgens. While producing measurable increases in urine flow in around 50% of patients, this form of treatment does have significant associated morbidities, many of which become progressively worse the longer the patient is on therapy. These include gynecomastia, osteoporosis, loss of libido, erectile dysfunction, altered serum lipid profiles, decreased insulin sensitivity, and perhaps cognitive decline. 5α-Reductase inhibitors (such as finasteride and dutasteride) have also been used therapeutically for the management of BPH.52,73–75 This class of drugs acts to block the conversion of testosterone to DHT, the form of androgen that is preferentially utilized in the prostate.
A second medical approach to the treatment of BPH has been the use of α-adrenergic receptor antagonists to relax the muscle of the prostate and thus improve clinical symptoms.76 This treatment regimen does not actually slow the growth of the prostate but does delay the necessity for surgical intervention.77 The mechanism of action is believed to be primarily due the blockade of α-adrenergic receptors in the region of the bladder neck and prostate, though there may also be an effect attributable to centrally acting adrenergic receptors as well. The first drugs to be widely used clinically included relatively nonspecific α-adrenergic receptor blockers that were also utilized for the management of hypertension, such as terazosin and doxazosin. More recently, however, compounds that are relatively more specific for the α1-adrenergic receptor (such as tamsulosin) that is more prevalent in the urinary tract have been more generally used.
The MTOPS trial examined the ability of the 5α-reductase inhibitor finasteride and the α-adrenergic receptor antagonist doxazosin, either singly or in combination, to affect clinical progression of BPH.77 The findings of the clinical trial were that both drugs given singly were similarly effective in slowing clinical disease progression, but the combination of the two reduced the clinical progression risk more than either drug alone. Both combination therapy and finasteride alone significantly reduced the long-term risk of acute urinary retention and the need for surgical intervention.
Historically, the most common method of surgically treating BPH was by open prostatectomy. This procedure has a high morbidity and some mortality associated with it and requires a long postoperative recovery period. It was therefore largely replaced by transurethral resection of the prostate (TURP), which involves physically cutting the obstructing material away from the inside of the urethra using a resectoscope. This remains the predominant approach to the surgical management of BPH, although a number of ablative approaches to destroying prostatic tissue have been recently introduced. These include various means of local tissue destruction such as hyperthermia, microwave thermotherapy, and laser-based tissue coagulation. Of these, only laser vaporization or resection has established wide popularity.
CLINICAL ISSUES: PROGRESSION TO SURGERY VERSUS MEDICAL MANAGEMENT
Although the most definitive therapeutic approach to managing BPH would be elimination of prostatic tissue, whether by TURP or some other form of local tissue ablation, the advent of medical-based therapies, specifically the α-adrenergic receptor antagonists and the 5α-reductase inhibitors, dramatically changed the paradigm for the clinical management of BPH (Fig. 143-3). The widespread use of inexpensive and effective medical treatments such as α-adrenergic receptor antagonists as a first line of treatment has moved surgical intervention for BPH to a second-line therapy and has significantly reduced the numbers of surgical procedures for BPH as compared to the situation in the early to mid-1990s. With an expanding menu of options available, it has become increasingly important to tailor therapy to the needs of the individual patient. This process will often begin with the primary care physician, who is frequently the initial gatekeeper for urology-related complaints. Factors that may prompt referral for more detailed urologic evaluation in a man with complaints of LUTS consistent with BPH include evidence or suspicion of urinary retention by either history or physical exam, a history of hematuria or evidence for this on urinalysis, nodules or irregularities on digital rectal examination that are concerning for malignancy, elevated serum PSA, or LUTS that are bothersome and refractory to medical management.
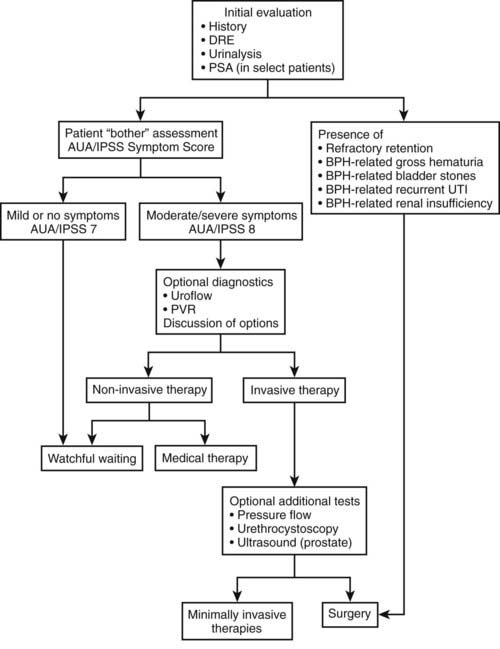
FIGURE 143-3. Flow diagram describing diagnosis and treatment of benign prostatic hyperplasia.
(Redrawn and modified from American Urological Association Practice Guidelines Committee: AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations, J Urol 170(2 Part 1):530–547, 2003.)
The choice of therapy and diagnostic evaluation of the man with LUTS attributable to BPH depends on the interplay of a variety of factors. Thus, a man with relatively mild symptoms (e.g., no evidence for hematuria, bladder stones, recurrent urinary tract infections, renal insufficiency, or urinary retention) who is sufficiently bothered by his symptoms that he desires therapy can generally start with a trial of medical therapy. The choice between starting with an α-adrenergic agonist versus a 5α-reductase inhibitor is driven by several considerations. The α-adrenergic agonists act relatively quickly, with results generally seen in 1 to 2 weeks. These medications work reasonably well on any size prostate gland but will not significantly change the prostate gland’s volume or the serum PSA. 5α-Reductase inhibitors act predominantly through reducing the size of the prostate gland. This mechanism of action takes longer, and so typically, symptomatic changes will not be seen for 3 to 6 months. It is generally felt that 5α-reductase inhibitors are more effective in larger glands. In the MTOPS trial referenced previously, 5α-reductase inhibitors were shown to reduce the long-term risk of urinary retention and the need for invasive therapy.77 Therefore, this may be the preferred medication in men with larger glands on examination or with an elevated PSA due to BPH and a higher risk of disease progression. 5α-Reductase inhibitors have also been shown to reduce the incidence of hematuria that is attributable to the prostate and therefore may be considered the preferred initial therapy in men who have undergone evaluation for gross hematuria that has been determined to be due to bleeding from the prostate. It should be remembered that the use of 5α-reductase inhibitors will shrink the size of the prostate over time, reducing volume by up to 30%. In a similar vein, because of their effects on the volume of prostate tissue and on AR function (i.e., by blocking conversion of testosterone to DHT), 5α-reductase inhibitors will generally reduce serum PSA levels over time. Although the exact degree of reduction in serum PSA will vary from individual to individual, on average the serum PSA will be reduced by 50%. It has been suggested that a failure of the serum PSA to be reduced by 50% may be a harbinger of occult prostate cancer.78
If one medical approach fails to have sufficient symptom relief, based on the results of the MTOPS trial, it is reasonable to attempt combination therapy with both an α-adrenergic antagonist and a 5α-reductase inhibitor. In some cases, particularly in men with more severe symptoms or in whom there is a modestly elevated postvoid residual, it is also reasonable to start with combination therapy. The results of the MTOPS trial would suggest that combination therapy will reduce the risk of clinical disease progression to a greater degree than with either drug alone. In some patients, it can become prohibitively expensive to indefinitely continue both medications, or some men may experience side effects due to the α-adrenergic antagonist. In these situations, if there has been sufficient symptom relief after 9 to 12 months, the α-adrenergic antagonist may be stopped and maintenance therapy continued with a 5α-reductase inhibitor alone, particularly patients with less severe baseline symptoms. The majority of these men (77% to 87%) will have ongoing adequate symptom relief with the 5α-reductase inhibitor alone.79,80
In a subset of patients, an early invasive approach to therapy (such as surgery) is indicated. These include patients who have refractory urinary retention due to BPH, those with persistent gross hematuria due to BPH, bladder calculi, recurrent urinary tract infections, or renal insufficiency due to urinary retention. In some cases, those who are at high risk of progression while on medical therapy may be considered for early surgical intervention. Factors that have been shown to increase the probability of disease progression with regard to BPH and require surgical intervention include an elevated PSA, prostate size, and elevated postvoid residual.81
For men with symptoms that are refractory to medical therapy and very bothersome, or for those who have urinary retention or high postvoid residual urine, an intervention to ablate and/or remove prostate tissue is reasonable. A large variety of options are currently available to accomplish this aim, and these may be performed in the operating room or in some cases undertaken using sedation in a urologist’s office without necessarily a need for anesthesia. Examples of some of the technologies available include thermal ablation of the prostate using a variety of different heat sources such as microwave energy (transurethral microwave therapy [TUMT]) and radiofrequency energy (transurethral needle ablation [TUNA]). Intraoperatively, prostate tissue can be ablated or removed/excised using electrocautery (such as in a TURP), transurethral incision of the prostate (for smaller glands), and transurethral electrovaporization. There are also several approaches based on the use of laser energy, such as holmium laser resection/enucleation of the prostate (HoLEP) and transurethral laser vaporization or coagulation. A full discussion of these options and their individual roles in the management of BPH is beyond the scope of this chapter. The interested reader is directed to several in-depth reviews on this topic.82 In general, although many of these procedures have gained acceptance and are used with increasing frequency in the United States and elsewhere, TURP remains the gold standard against which these modalities are measured. In selective cases, typically those with particularly large glands and especially if there are concomitant large bladder stones, open simple prostatectomy has a role, although its use is steadily declining.
COMORBIDITIES CONCURRENT WITH BPH
The subject of comorbidities concurrent with BPH and the management of patients with such conditions was recently reviewed, and the reader is referred to other texts on the subject for a more comprehensive overview in this area.83–87 Here we will briefly discuss the most common comorbidities seen in patients with BPH/LUTS, these being obesity, diabetes and associated “metabolic syndrome,” and erectile dysfunction (ED). To some extent, this is an unsurprising collection of comorbidities in that there are independent associations between the various individual conditions; intuitively, it would seem likely that these conditions would tend to coexist within the same population of aging patients. It has been strongly suggested that hypogonadism might underlie this pattern of comorbidity88 and should certainly be considered when treating patients with this pattern of ailments.
The epidemic of obesity in many developed countries is linked to a wide range of disease conditions and patterns of comorbidity. The increased rates of cardiovascular disease and increases in insulin resistance and type 2 diabetes are coupled together in “the metabolic syndrome” and considered in Chapter 44.89,90 Although causal links to BPH are unproven, there does appear to be a link between metabolic syndrome and LUTS,91 given that the two conditions are common comorbidities. There is a significant correlation between diabetes and increased BPH symptom severity even when other variables (such as age) which correlate with both conditions are factored out.92 This is particularly important given the differences in the incidence of type 2 diabetes between different ethnic groups.83
Like diabetes, ED is commonly seen in aging men and is particularly prevalent in the obese and diabetic populations. Men with moderate or severe LUTS have increased risk of ED, which may be improved by treating the symptoms of BPH.93 In treating patients with this pattern of comorbidities, it is clearly important for a physician to take into account the possibility that treatment of a given profile of symptoms might positively or negatively affect other symptoms that might at first sight be considered unrelated.
Prostate Cancer
HORMONE-RESPONSIVE PROSTATE CANCER
Role of Androgens
As discussed earlier, prostate cancer is an androgen-dependent disease.20 Initially, prostate cancer is dependent upon androgens for growth and differentiation, but ultimately the disease progresses and becomes insensitive or refractory to androgens. During the hormone-responsive phase of prostate cancer, there are significant changes to the way in which the tumor cells respond to androgens. As well as paracrine mechanisms of responding to androgens, prostate cancer cells acquire an autocrine mode of androgen action. The tumor cells respond directly to androgens and andromedins (yet to be fully defined but believed to include growth factors TGF or FGF superfamilies and transcription factors) that are produced by, and elicit a response from, the tumor cells themselves. In acquiring this mode of action, the tumor cells obtain a significant growth advantage. Furthermore, there is evidence that the AR on the tumor cells can be mutated, rendering it responsive to a range of steroid ligands, not only to androgens.94–96
Paradoxically, the incidence of prostate cancer increases with age, when the levels of serum testosterone are declining.97 Although testicular testosterone synthesis and serum testosterone levels fall in aging men, the levels of estradiol remain unchanged or increase.97–100 Levels of estrogen are thought to be maintained by increased aromatization of androgens, particularly in peripheral adipose tissue, which also tends to increase in the older male.98,101,102 Therefore, there is a significant change to the ratio of estradiol/testosterone that is temporally related to the onset of prostate disease, including prostate cancer.
Epidemiologic evidence provides some further support for this relationship. Serum levels of estrogens in African American men (who have the highest incidence of prostate cancer in the United States) are significantly higher compared to Caucasian American men.103,104 Conversely, serum levels of estrogens are lower in Japanese men (who are known to have a low risk of prostate cancer) compared to Caucasian Dutch men.105 The age-related shift in the balance of hormones in men as they become older also illustrates the gradual nature of the change and the long latency of the disease.
Dual Role of Estrogens in Prostate Cancer
It is difficult to directly demonstrate a change to the local hormonal milieu that results in a hormone imbalance within the prostate gland. Nevertheless, there is sufficient evidence that when cancer is present, the levels of tissue estrogens are altered, local synthesis of estrogen via aromatization is modified, alternate aromatase gene promoters are utilized, and there are changes in the expression of estrogen receptor subtypes. These adverse effects of estrogen are specifically mediated via activation of ER-α, resulting in aberrant proliferation, inflammation, and malignancy.106–109 Overall, these studies predict that antagonists of ER-α (such as toremifene) may be effective for prevention or treatment of prostate cancer. Subsequent clinical trials endorsed this prediction where efficacy of toremifene for the prevention of prostate cancer in men with PIN lesions was recently reported.110
In contrast, ER-β-mediated effects appear to be antiproliferative and beneficial. ER-β binds to a range of estrogens and estrogenic compounds, including dietary estrogens that are purported to have beneficial effects. This includes compounds such as phytoestrogens, lignans, flavonoids and lipoflavinoid, promoted as being beneficial to men’s health, specifically through the reduction and prevention of prostate disease.111 Epidemiologic studies suggest the consumption of dietary estrogens is linked to a lower incidence of prostate cancer.112 Significantly, these compounds preferentially bind to ER-β rather than ER-α and as a result, ligands that specifically activate ER-β have become of significant interest as possible therapeutic agents for prostate cancer (and BPH). Current evidence indicates that the activation of ER-β should be preserved rather than ablated, but proof that estrogens acting via ER-β are effective for prevention or treatment of prostate cancer remains unresolved.
Combined Role of Androgens and Estrogens in Hormone-Responsive Prostate Cancer
Altering the balance between androgens and estrogens in older men appears to be significant in the development of prostate cancer. Androgens are necessary, but androgens alone are insufficient to induce tumorigenesis. For example, the aromatase knockout (ArKO) mouse is an estrogen-deficient mouse model, and androgen levels are elevated throughout life, but the mice do not develop malignancies of the prostate gland.113
Instead, the mechanism of hormonal induction of prostate cancer involves both hormones, and there is some experimental evidence to support this postulate. It is well known that the Noble rat develops precancerous lesions and prostate adenocarcinoma when given estrogens in conjunction with androgens.114–116 Combined hormone treatment of grafted prostatic tissues promotes malignancy in Rb-deficient mouse tissue recombinants.117 Combined treatment of wild-type mice with high doses of testosterone and estradiol induces prostatic hyperplasia, dysplasia, and carcinoma in situ.118
The essential role for ER-α was deduced from the same treatment of αERKO and βERKO mice. ER-β knockout mice (that express ER-α) develop dysplasia and malignancy comparable to that observed in wild-type mice, but ER-α knockout mice did not.118 Hormonal induction of prostate cancer by combined hormone (T+E) treatment of αERKO and βERKO mice showed that signaling through ER-α (versus ER-β) was essential for this hormonal regime to initiate malignancy.118,119 Altogether, these data provide a basis for testing new-generation antiestrogens and selective estrogen-receptor modulators (SERMs), together with antiandrogens such as finasteride, as treatments for prostate cancer.120
STROMAL–EPITHELIAL CELL INTERACTIONS
In considering the imbalance of hormones and the adjustments that occur in hormone-responsive prostate cancer, it is clear that profound alterations to stromal–epithelial cell interactions accompany these changes. The perturbation of normal stromal–epithelial cell interactions is a key feature of prostate cancer, resulting in autocrine growth whereby the tumor cells escape from the regulatory influence of paracrine signals. Whether or not this is initiated by genetic damage to the epithelial cells or the stromal cells, a sequential disruption in the reciprocal relationships leads to a loss of the homeostatic interactions, and a vicious cycle of unregulated growth and differentiation ensues.121
The concept that the stroma may play a pivotal role in carcinogenesis has evolved over decades and is based on the observations that the pathology of tumor stroma differs from that of normal stroma.121,122 In the benign adult human prostate, the stroma consists of smooth muscle actin-positive cells that express ARs and maintain a highly differentiated secretory epithelium under the influence of androgens. In contrast, the stromal cells that surround tumor cells in the prostate are fibroblastic or myofibroblastic and are termed reactive stroma or cancer-associated fibroblasts (CAFs).12,123–126 Since the reciprocal interactions between the stroma and epithelia are critical for the maintenance of homeostasis in normal prostate, it was predicted that a change in epithelial and stromal pathology would interfere with or alter these interactions.
Not surprisingly then, studies by Thompson et al.127 using tissue recombination techniques to study the effects of inserting the myc and ras oncogenes in epithelial or stromal cells showed that carcinomas developed but only when both the epithelia and stroma were infected. Similarly, human CAFs promote carcinogenesis in combination with immortal, but nontumorigenic, human BPH-1 human prostate epithelial cells,126 whereas normal stroma does not. Although CAFs promote carcinogenesis in immortal epithelial cells, there is currently no evidence that CAFs initiate tumorigenesis in normal epithelial cells or that stromal alterations precede changes in the epithelia. Nevertheless, the stroma is regarded as an important therapeutic target in disease progression because it is part of the tumor microenvironment,128 and the molecular signatures of the normal versus tumor stroma have been interrogated by several groups of investigators.
Using Affymetrix microarrays, 31 genes encoding extracellular proteins as potential candidates of stromal-epithelial signaling were identified, together with a number of growth-factor (TGF-β and FGF) and signal-transduction pathways in reactive stroma.129,130 Experimental animal and tissue models have subsequently provided insight into the role of stroma in modulating the oncogenic potential of epithelia. Conditional inactivation of the receptor TGF-βRII in fibroblasts results in premalignant prostatic lesions in mice, and there is some further evidence that tumor progression is mediated by a paracrine TGF-β/Wnt3a signaling axis.131,132 Similarly, using cell reconstitution experiments, enhanced mesenchymal expression of FGF10 was shown to cause the formation of multifocal PIN or prostate cancer.133 Expression array analyses of reactive stroma in moderate-grade prostate cancer specimens compliments these experimental data and also implicates proteins or protein families such as TGF-β, wnt, and FGF that are of current interest because they are linked to stem cell maintenance. As well as FGF10 and TGF-β, transcription factor pathways (e.g., Sox9 and hedgehog Hh) have been assigned key roles in prostatic malignancy and may ultimately be shown to involve stromal signaling to malignant epithelia.134,135
USE OF PROSTATE-SPECIFIC ANTIGEN IN MONITORING HORMONE-REGULATED GROWTH IN PROSTATE CANCER
Secretory activity of the mammalian prostate gland is hormonally regulated; in particular, PSA (a proteolytic enzyme belonging to the kallikrein group of serine proteases) is produced by human prostate epithelium. Over the last decade, the assessment of serum PSA has allowed physicians to diagnose prostate cancer earlier in its biological evolution and led to a subsequent increase in local therapies with the aim of curing the disease.
PSA was initially identified in human seminal plasma,136 and in the late 1970s, a plasma protein was purified that was prostate specific and identical to that found in semen.137 Cancer is often a cause of elevation in serum PSA, but other causes include prostatic infarction, prostatitis, ejaculation, vigorous prostate massage but not routine digital rectal examination, and prostatic instrumentation.138–140 Therapy that alters hormonal status will also affect serum PSA, especially the use of the 5α-reductase inhibitor, finasteride, which reliably reduces serum PSA by 50%. The half-life of PSA is 2.2 to 3.2 days, and it can take several weeks for serum PSA to return to baseline after procedures such as prostate biopsy or resection.
In diagnosing cancer, the sensitivity ranges from 63% to 83% and specificity from 81% to 90% when using PSA of >4 µg/L as the cutoff value. Specificity is generally better in the younger age groups, because PSA production from the benign tissue of older men is a confounding variable. As such, recent studies suggested that lowering the cutoff point for younger men is appropriate to increase detection rates from 18% to 36% while reducing specificity from 98% to 94%.141 Whether or not this increases the detection of clinically insignificant cancer is uncertain. Nonetheless, combining digital rectal examination and serum PSA appears to give the best cancer-screening detection rates, with the combination detecting 27% more cancers than PSA alone and 34% more than with digital rectal exam alone.142,143 Randomized studies in progress may provide the answers as to whether or not these screening endeavors will reduce prostate cancer mortality. There is some evidence to suggest it may, such as the study from Tyrol, Austria, where men in that state were offered free PSA testing and were encouraged to participate. This study demonstrated a 33% reduction in mortality compared with the expected rate elsewhere in Austria.144 While many authors suggest this is evidence of the benefit of screening, others claim the causes of the decline in mortality may be due to different variables such as diet and lifestyle factors, attribution bias, and the earlier use of androgen-deprivation therapy. Other studies showed that despite vastly different rates for PSA screening and testing, the reduction in prostate cancer mortality was similar between the United Kingdom and the United States and between the U.S. regions of Seattle and Connecticut. The current status of screening remains uncertain145 but continues to be evaluated. Two large international studies are underway, designed to show whether mortality from prostate cancer is reduced by randomized screening (PSA and digital rectal exam testing) of participants compared to those men who are not being screened. The preliminary findings from the U.S.146 and European147 studies highlight a significant difficulty in conducting controlled clinical trials on prostate cancer screening because a greater awareness of prostate cancer has increased the rates of PSA testing that generally occurs in the population, making it difficult to provide an adequate control group for comparison. The findings to date suggest that population-wide screening for prostate cancer using the PSA test remains equivocal. It also demonstrates that significant overtreatment may be occurring, with only a small risk of prostate cancer death at 10 years, and a 40-fold increased risk of dying of other diseases within the first 5 years after initiation of testing. Nonetheless, the introduction of PSA into clinical practice has decreased the incidence of metastatic disease. Many cancers detected today are now potentially curable, and less than 20% have been described as pathologically insignificant,139 although what this constitutes remains debatable.
To improve the positive predictive value of PSA, several strategies were utilized. These include age-specific reference ranges, PSA velocity, PSA density, free PSA, and more recently serum proPSA or hK2. The use of age-specific ranges improves sensitivity in younger men and specificity in older men. The commonly used cutoffs are <2.5 µg/L for 40- to 49-year-old men and <3.5 µg/L, <4.5 µg/L, and <6.5 µg/L for men 50 to 59, 60 to 69, and 70 to 79 years old, respectively. Using these reference ranges, studies showed an 8% increase in positive biopsies in younger men and a 21% reduction in the number of biopsies, while missing only 4% of cancers, in older men.139 Serial PSA determination, namely PSA velocity, was thought to be valuable in predicting cancer from benign disease, and a PSA change of >0.75 µg/L/year was believed to indicate the presence of cancer. Unfortunately, this value is of limited benefit because of the day-to-day variation in serum PSA in any individual. This variability led many to conclude that an isolated elevation in PSA should be confirmed several weeks later before proceeding with biopsy.138 In addition, the velocity is not easy to calculate and varies depending upon the assays used. As such, the tendency is to not use it to determine the need for biopsy, although it may indicate the necessity to conduct the procedure. PSA density, namely the division of serum PSA by prostate volume, was thought to discriminate between cancer and benign tissue, based on the premise that cancer produced more PSA on a volume basis compared to benign tissue. There is marked variability in the amount of PSA produced by prostate tissue, both benign and malignant, owing to the variability in the stromal/epithelial ratios. This together with major interobserver variation in the measurement of prostate volume makes PSA density relatively unreliable. Similar problems emerged when using transition-zone PSA density.
More recently, attention focused on the various forms of PSA. PSA in the serum is often complexed to α1-antichymotrypsin (ACT) or α2-macroglobulin (A2M). PSA complexed to A2M is not detectable immunologically because the A2M molecule is large and blocks all epitopes on the surface of PSA. Thus it is possible to differentiate between free and PSA complexed to ACT and a % free PSA can be calculated and expressed. Using a % free PSA cutoff of >25%, specificity improves by 20% while maintaining 95% sensitivity.148 Other assays determine the ratios of complexed PSA that may be less sensitive to digital rectal exam and prostate manipulations and can be used as a single test that may be more stable after storage of the specimens. Even so, the % free PSA appears to be the assay in wider clinical use currently and appears particularly useful to assist with the detection of prostate cancer in the lower ranges of 2.5 to 4.0 µg/L.
Human glandular kallikrein belongs to the same family as PSA, shares an 80% homology with PSA, and is prostate specific and androgen dependent. Human kallikreinen 2 (HK2) cleaves PSA from its inactive precursor pro-PSA into active PSA. Studies suggested that this enzyme may be superior in the detection of organ-confined prostate cancer, especially in cancers of higher histologic grade, when serum PSA is less reliable.149 Early results examining the HK2/free PSA ratio appear promising but require substantial validation and testing before coming into regular use. Studies continue into the use of serum hK2, as well as pro-PSA, which appears to be secreted more by cancerous tissue than benign tissue. Recent studies suggested an improved specificity for cancer detection comparing % pro-PSA to either % free PSA or total PSA in the 2 to 10 µg/L range.150
PSA was found to be a useful tool in the assessment of tumor volume and clinical staging. Although some studies showed little correlation between PSA levels and cancer volume, PSA was utilized in many nomograms utilizing multivariate analyses, and increasing levels were associated with adverse prognostic factors such as extracapsular extension, seminal vesicle or lymph node involvement, and biochemical progression.151,152
PSA is particularly useful following treatment for prostate cancer. Those patients managed expectantly with watchful waiting normally undergo definitive therapy as a result of serial rises in serum PSA. Serial rises are also useful to determine the need for repeat biopsies to determine if pathologic disease progression has occurred.153 Following surgery, an undetectable serum PSA is usually obtained and based on the lowest limit of detection of the particular assay utilized. Those patients whose PSA did not fall to undetectable levels, who had a rapid PSA doubling time, and whose PSA was >1 µg/L were more likely to have micrometastatic disease and subsequently fail salvage radiotherapy.154 Following radiotherapy, PSA reaches a nadir over 17 to 32 months, occasionally with a bounce phenomenon where it may temporarily rise. Patients will usually demonstrate excellent long-term cancer specific survivals if PSA levels are <0.5 µg/L. The definition of radiation failure is based on three consecutive rises in PSA above a nadir (i.e., the American Society of Therapeutic Radiology and Oncology [ASTRO] criteria for failure) or a reading of 2 ng/mL above the nadir.155 Following androgen-deprivation therapy, PSA levels can often stratify patients into good and bad prognostic groups. Studies have shown that if PSA >4 µg/L after 24 to 32 weeks of treatment, median survival is 18 weeks, as compared to 40 weeks if PSA <4 µg/L.156
In summary, PSA has revolutionized the way prostate cancer is managed. It is useful in the diagnosis of the disease, is helpful in clinically staging disease once diagnosis has been made, helps predict survival posttreatment, and is invaluable in disease monitoring following definitive therapy and assisting in determining which adjuvant therapy should be utilized following failed curative treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








