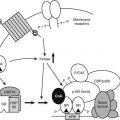
FIGURE 154-1. Dysdifferentiation results in aberrant gene expression. The malignant transformation of a progenitor cell results in a clonal population of cells that have undergone atypical differentiation. These cells share some features with their progenitor cells (shaded areas) and may continue to express genes associated with these immature, incompletely differentiated cells. Malignant transformation of a cell can lead to persistent, or enhanced, hormone gene expression in the clone of proliferating cells (heavily shaded area).
Extrapituitary expression of the ACTH gene (pro-opiomelanocortin [POMC]) is described in detail in the following. Ectopic ACTH production shares many features of other ectopic hormone syndromes and may provide a paradigm for understanding general mechanisms of ectopic hormone production.
HORMONES IN SMALL CELL LUNG CARCINOMA
Small cell lung carcinoma (SCLC) is the most common and aggressive neuroendocrine tumor. Patients with SCLC seldom present to the endocrinologist, but because there is evidence of hormone expression, it is a useful model from which to extrapolate to other less common syndromes.
The normal adult lung contains a diffuse population of cells that synthesize very low levels of peptide hormones, including ACTH, GHRH, and gastrin-releasing hormone. In fetal life, these cells are more densely represented and produce higher concentrations of hormones, suggesting a possible role in differentiation. These scattered endocrine cells have APUD properties and comprise part of a diffuse neuroendocrine system.
SCLC, a bronchogenic tumor, produces most of the hormones found in normal bronchial epithelium. ACTH, vasopressin, and calcitonin secretion have been studied most extensively because of the clinical syndromes they cause and because they produce potential disease markers that can be used for diagnosis or response to treatment. In a study of 157 primary lung tumor extracts, 83% expressed at least one peptide hormone. Multiple hormone production is common and includes ectopic ACTH syndrome in 20% of cases, although production of one hormone tends to dominate the clinical picture.16,17 However, only about 1% of patients with SCLC have clinical features of cortisol excess, possibly because of the short duration of disease.18 Peptide hormones often undergo incomplete processing. In particular, POMC is not efficiently cleaved to ACTH, resulting in high-molecular-weight forms of ACTH in tumor extracts and in the circulation.16,19 It is now clear that these alternate forms represent the prohormones POMC and pro-ACTH. The abnormal protein processing may result from a lack of specific cleavage enzymes, prohormone convertases 1 and 2, which are expressed only in specialized endocrine tissue or from a switch from the regulated secretory pathway to the constitutive one.20
Ectopic Adrenocorticotropic Hormone Syndrome
THE POMC GENE
Pituitary corticotroph cells are the only cells that express the POMC gene at a high level. The human POMC gene is encoded in three exons on chromosome 2. The first exon is noncoding. The second exon contains the signal peptide, which targets the protein product to the regulated secretion pathway. The third exon encodes the majority of the mature protein, including ACTH.21 The mature mRNA from the POMC gene is 1200 nucleotides. In addition, a short form of the mRNA has been found at low levels in most tissues analyzed. This arises from a transcription start site 5′ to exon 3 and thus only includes the coding sequence for exon 3.22 Therefore, this transcript lacks a signal peptide and does not give rise to the mature POMC molecule. There is no evidence that this transcript produces a peptide product, and its physiologic role is unclear. A third POMC transcript has also been described that is longer than the pituitary form (∼1500 nucleotides). It arises from a site (or multiple sites) within the 5′ flanking region of the human POMC promoter.23,24 This mRNA species includes the entire coding region of the peptide and does give rise to a secreted peptide product. This “long” form of the POMC mRNA is particularly found in extrapituitary tissues and tumors.
REGULATION OF POMC GENE EXPRESSION
Expression of the POMC gene appears to be predominantly controlled at the level of gene transcription.25 The rat POMC gene has been most extensively studied, and pituitary expression is conferred by the 5′ flanking region of the gene. It has recently been found that pituitary corticotroph expression of POMC requires the action of a tightly restricted transcription factor, a member of the T-box family, termed Tpit.26 This factor acts with the homeodomain protein PitX1 and promotes recruitment of SRC-family coactivators to the POMC promoter, leading to enhanced gene transcription.27
Corticotropin-releasing hormone (CRH) acts on pituitary corticotroph cells to increase cyclic adenosine monophosphate (cAMP) accumulation and activates mitogen-activated protein kinases. There is also evidence of activation of the orphan nuclear receptor nerve growth factor–induced clone B (or Nur 77),28–30 leading to enhanced POMC transcription through the recruitment of SRC coactivators to nerve growth factor–induced clone B.27,31 As nerve growth factor–induced clone B and Tpit act synergistically, this suggests the formation of a regulatory complex on the POMC promoter with Tpit, NGFI-B, and SRC coactivators.27 It is important that expression of Tpit promotes corticotroph cell differentiation and that its expression is more limited than that of POMC. Therefore, there is no Tpit expression in hypothalamic POMC-expressing neurons, suggesting that Tpit is specific for corticotroph-specific expression of POMC; other mechanisms are responsible for expression elsewhere. Tpit expression has been found specifically in human pituitary corticotroph adenomas.26
Glucocorticoids repress transcription of the POMC gene by binding to two DNA elements in the 5′ flanking region of the promoter. The more proximal element, an imperfect palindrome 63 nucleotides upstream from the transcription start site, is thought to bind three glucocorticoid receptor molecules in an unusual trimer formation.32–34 This conformation of receptors on DNA directs repression of transcription rather than enhancement. Further upstream, between −480 and −320, there is another glucocorticoid-regulated element, suggesting that these two DNA elements interact to achieve the full effect of glucocorticoid repression.35 It is interesting that Tpit expression, essential to the corticotroph cell type and to POMC expression, is not affected by glucocorticoids, in contrast to POMC, which is repressed.36 However, it now appears clear that the activated glucocorticoid receptor can antagonize the actions of Nur 77 and, by removing a positive transactivator, lead to POMC gene repression.37 The mechanism of glucocorticoid receptor interaction with Nur 77 requires the chromatin remodeling protein Brg1, which stabilizes their binding. Furthermore, Brg1 assists recruitment of a further chromatin remodeling enzyme, HDAC2, to the glucocorticoid receptor bound to the POMC gene. The pathophysiologic importance of Brg1 and HDAC2 are illustrated by finding deficient expression of one or both in 50% of glucocorticoid-resistant pituitary corticotroph adenomas.38 The expression of Brg1 and HDAC2 in ectopic ACTH syndrome has not been explored.
A number of other hypothalamic factors act on the pituitary corticotroph to influence POMC expression. However, their modes of action are not well defined. In particular, arginine vasopressin stimulates POMC expression rather weakly but augments CRH action. The intracellular pathways activated by arginine vasopressin appear to be protein kinase C dependent, but arginine vasopressin also potentiates the action of CRH on cAMP generation.25,39
However, many other peptide growth factors and cytokines are capable of activating cAMP, mitogen-activated protein kinase (MAPK), and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling cascades and thus are potentially capable of regulating POMC expression in nonpituitary tissue. Although extrapituitary tissues lack expression of corticotroph-specific transcription factors, activation of common signaling cascades might be expected to result in POMC gene expression. In extrapituitary tissues, the POMC gene may be modified to render it transcriptionally silent. One such irreversible modification is DNA methylation. The loss of methylation in tumor tissue may allow transcription of the gene to be activated by the common signaling pathways described previously. There is some evidence that such changes in DNA methylation occur in cell-line models of ectopic ACTH syndrome.40,41 It seems likely that POMC expression per cell is lower in most extrapituitary tumors compared with the pituitary corticotroph, but this relative inefficiency of expression is compensated for by the greater number of cells expressing the gene in extrapituitary tumors.
ECTOPIC ADRENOCORTICOTROPIC HORMONE SYNDROME
ACTH immunoreactivity has been recognized to show size heterogeneity for many years, with the presence of high-molecular-weight forms detected in human plasma.42,43 Ectopic ACTH syndrome was the first of the ectopic hormone syndromes to be recognized. In its most florid form, it is rare, in one study affecting 4.5% of patients with SCLC, although there is evidence of derangement in the hypothalamic-pituitary-adrenal axis in the majority of patients with SCLC42,44 (Table 154-1). Analysis of tumor tissue surprisingly suggests the presence of immunoreactive ACTH, even in the absence of clinical features of hormone excess. ACTH is present predominantly in a high-molecular-weight form of approximately 20 kD, but this purified material can be cleaved to mature ACTH (4.5 kD) by the action of trypsin. Further work identified the presence of immunoreactive ACTH-like peptide in a variety of normal tissues, suggesting that extrapituitary ACTH expression is less ectopic than it is inappropriately regulated. The ACTH immunoreactivity was found to have no biological activity and was assumed to be “big” ACTH. However, identification of predominantly high-molecular-weight forms of ACTH in the circulation of patients with clinically apparent Cushing’s syndrome suggests that the precursors of ACTH may have some activity at the ACTH receptor.
Table 154-1. Types of Tumors Causing Ectopic Adrenocorticotropic Hormone Secretion
PRO-OPIOMELANOCORTIN PROCESSING
The POMC gene leads to the generation of a pre-prohormone, POMC. This protein undergoes a series of proteolytic cleavages at dibasic amino acid residues to give rise to a series of small molecules, including ACTH, melanocyte-stimulating hormone, and β-endorphin.45–47 In the anterior pituitary, ACTH is cleaved by the action of a specific protease, termed PC1 (for prohormone convertase type 1).48 In the rodent intermediate lobe melanotroph, the POMC molecule undergoes more comprehensive digestion to give smaller fragments, melanocyte-stimulating hormone, β-endorphin, and corticotropin-like intermediate lobe peptide as a result of cleavage by prohormone convertase 2. Expression of prohormone convertase 2 and thus detection of circulating ACTH fragments have been described in ectopic ACTH-syndrome tumors.49
In the majority of extrapituitary tumors that cause ectopic ACTH syndrome, processing of the preprohormone is incomplete. Therefore, ectopic ACTH syndrome is characterized by high-molecular-weight forms of ACTH in the circulation.17,19 It is likely that the extent of processing correlates with the degree of neuroendocrine differentiation of the tumor, and hormonal manifestations are probably only seen in tumors with significant hormone-processing capacity. A number of small, highly differentiated, slow-growing tumors (typically bronchial carcinoid) have been shown to process POMC in the neurointermediate lobe manner, giving rise to small fragments in the circulation, such as corticotropin-like intermediate lobe peptide and α-melanocyte-stimulating hormone. These have been used to aid diagnosis in some cases of Cushing’s syndrome, although the series are small.8,50
DYSREGULATION OF POMC GENE EXPRESSION IN EXTRAPITUITARY TUMORS
In contrast to POMC gene expression in pituitary corticotroph cells, expression in extrapituitary tumors is characteristically resistant to glucocorticoid.7,51 This is the basis of the high-dose glucocorticoid suppression test used to distinguish eutopic from ectopic sources of ACTH in Cushing’s syndrome. Because the test has approximately 10% false-positive and 10% false-negative rates, it has largely been superseded by sophisticated imaging and inferior petrosal sinus sampling for differential diagnosis.52 With the availability of recombinant CRH, responses of extrapituitary tumors to this peptide have been measured. In general, only pituitary corticotrophs stimulate POMC expression in response to CRH, but exceptions are increasingly being identified.53
Pituitary expression of POMC was defined with the aid of a cell-line model, so a cell-line model was sought for extrapituitary expression. To this end, a panel of human SCLC cell lines was established. These cell lines express the POMC gene, and expression is resistant to glucocorticoid suppression.16,54–58 Further, the cell lines secrete predominantly unprocessed POMC and partially processed forms, again reflecting the pattern of activity characterized in vivo.54
It is intriguing that the majority of extrapituitary tumors are resistant to glucocorticoid inhibition of POMC expression. Receptors for glucocorticoids are present in most cells, including malignant cells, so exploring the mechanisms of glucocorticoid resistance was important. Using the panel of cell lines, expression of glucocorticoid receptor was identified using both Western blot with a polyclonal anti–glucocorticoid receptor antibody, and ligand-binding assays using tritiated dexamethasone.56–58. To determine whether the receptors were sufficient for glucocorticoid signaling, a synthetic, glucocorticoid-responsive gene was used. This gene was transfected into the cells, and the effects of glucocorticoid incubation on expression of the reporter measured. In contrast to the brisk induction of expression seen in control pituitary cells, none of the human SCLC cells responded to either natural or synthetic glucocorticoids.56,58 Thus, resistance of the POMC gene to glucocorticoid is only part of a global resistance of malignant cells to glucocorticoid action. High concentrations of wild-type receptor in the cells was found to be sufficient to restore glucocorticoid signaling, thereby suggesting that resistance resides at the level of the endogenous receptor.56 Because one of the actions of glucocorticoids on pituitary corticotrophs is to inhibit proliferation, and in the developing lung, glucocorticoids act to promote differentiation, it is possible that evasion of glucocorticoid signaling confers a survival advantage on the malignant cells. Indeed, it has recently been shown that overexpressing the wild-type glucocorticoid receptor in human small cell long cancer cells powerfully induces apoptosis. Intriguingly, this effect occurs even in the absence of added glucocorticoid.59 As yet, a single, unifying molecular mechanism of glucocorticoid resistance in the human SCLC cell lines remains to be defined.
It is interesting that well-differentiated carcinoid tumors causing ectopic ACTH syndrome sometimes show appropriate POMC suppression to supraphysiologic glucocorticoid levels, as in pituitary-dependent Cushing’s disease, but these tumors express high levels of the glucocorticoid receptor.60
DIAGNOSIS
The diagnosis of Cushing’s syndrome and the differential diagnosis of ACTH-dependent Cushing’s syndrome are described elsewhere (see Chapter 15). Dynamic endocrine testing is required to diagnose Cushing’s syndrome, and detection of ACTH using a sensitive two-site immunoradiometric assay is useful for making the diagnosis of ACTH-dependent Cushing’s syndrome. A variety of dynamic endocrine and imaging protocols may be used to identify a pituitary or extrapituitary source of the ACTH excess (Tables 154-2 and 154-3). These all have variable sensitivity and specificity, but combined tests afford nearly 100% diagnostic accuracy.61
Table 154-2. Laboratory Investigation of Ectopic Adrenocorticotropic Hormone Syndrome
| Investigation | Test Results |
|---|---|
| ACTH | Higher in ectopic disease; partially processed forms more common in ectopic disease |
| Cortisol | Higher in ectopic disease |
| Hypokalemia | Nearly 100% in ectopic ACTH secretion; ∼10% (<3.2 mmol/L) in Cushing’s disease and alkalosis |
| High-dose dexamethasone testing (8 mg) | No suppression in 89% of ectopic disease; suppression in 78% of pituitary-dependent disease |
| CRH test | Absent response in ectopic disease; exaggerated response in pituitary-dependent disease |
| Tumor markers | Presence of elevated calcitonin, hCG, α-fetoprotein, 5-HIAA suggests ectopic disease |
ACTH, Adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; hCG, human chorionic gonadotropin, 5-HIAA, 5-hydroxyindoleacetic acid.
Table 154-3. Features of Different Causes of Ectopic Cushing’s Syndrome
| Small Cell Lung Carcinoma | Carcinoid | |
|---|---|---|
| ACTH | Very high | Similar to pituitary-dependent disease |
| Cortisol | Very high | Similar to pituitary-dependent disease |
| Features | Not cushingoid | Cushingoid |
| Potassium | Marked hypokalemia | Potassium < 3.2 mmol/L |
ACTH, Adrenocorticotropic hormone.
Most occult tumors are carcinoid, pheochromocytoma, or medullary thyroid carcinoma and originate in the neck, chest, or abdomen. Computed tomography or magnetic resonance imaging can be used to detect chest tumors in patients with a normal chest radiograph. There has been some success in using indium-labeled octreotide scanning to identify occult neuroendocrine tumors, although its diagnostic performance is poor, tending only to confirm the presence of tumors found with conventional imaging.61
TREATMENT
Treatment is based on two objectives: controlling endocrine manifestations and managing the underlying tumor. Individual patients will present with different priorities. The ideal treatment is curative resection of the primary tumor, achieving both objectives. If this is not possible, patients with small, occult primary tumors may be managed by chemical or surgical adrenalectomy; in most cases, the primary tumor is not life threatening. Patients with extensive carcinoma—for example, small cell carcinoma where ACTH excess coexists—may be best managed by chemotherapy, which indirectly reduces ACTH expression. Chemotherapy should be tailored for the cell type and tumor stage, regardless of hormone excess.
In some patients with florid Cushing’s syndrome, control of cortisol production may help in preparation for surgery. In these cases, treatment with metyrapone, mitotane, or ketoconazole, in combination or individually, is helpful.62 Side effects are common with these agents in the doses required, and so combinations (e.g., ketoconazole and metyrapone) may allow more effective control. Rapid control of hypercortisolemia may require intravenous etomidate.61 There are case reports of good responses to long-acting somatostatin analogs63 (Table 154-4).
Table 154-4. Medical Therapy of Cushing’s Syndrome Caused by Ectopic Adrenocorticotropic Hormone
| Drug | Mechanism of Action |
|---|---|
| Metyrapone | Inhibition of 11β-hydroxylase |
| Ketoconazole | Inhibition of several steps of cortisol synthesis |
| Aminoglutethimide | Inhibition of cholesterol conversion to pregnenolone |
| Octreotide | Inhibition of adrenocorticotropic hormone secretion |
| Etomidate | Adrenolytic agent |
| RU486 | Glucocorticoid receptor antagonist |
| Mitotane | Adrenolytic agent |
Ectopic Corticotropin-Releasing Hormone Secretion
It has been more than 30 years since the original description of ectopic CRH secretion, and it is now clear that true, isolated secretion of CRH is very rare (Table 154-5). In tumors secreting ACTH peptides, there are frequent reports of CRH immunoreactivity, which may play a paracrine role in the development of the hormone syndrome, but such a role has not been defined.64,65 CRH is expressed outside the central nervous system, particularly in sites of inflammation, and may subserve other roles, including vasodilatation. CRH is seldom measured in the peripheral blood during the workup of Cushing’s syndrome or in inflammatory disease, so evidence of a true endocrine role of this hormone in the peripheral circulation is lacking.
Table 154-5. Tumors Associated With Ectopic Corticotropin-Releasing Hormone Secretion
The clinical features are typical of Cushing’s syndrome, and the hormonal features may resemble either pituitary secretion of ACTH (if the ectopic source secretes purely CRH) or ectopic ACTH syndrome (if the tumor co-secretes ACTH-related peptides). Measurement of CRH is probably best left to those cases with definitive pituitary ACTH production and confirmed corticotroph hyperplasia by histology.
Syndrome of Inappropriate Antidiuretic Hormone Secretion
SIADH is the most common cause of hyponatremia and one of the most frequent hormone syndromes associated with malignant disease (Table 154-6). It may be caused by a wide range of underlying disorders that can be categorized into several broad groups: malignancies, neurologic disorders, lung disease, and drugs. These conditions cause hyponatremia as a result of abnormal hypothalamic vasopressin, when secretion comes under aberrant control from either neuronal inputs or circulating humoral factors.
Table 154-6. Diagnostic Criteria for the Syndrome of Inappropriate Antidiuretic Hormone Secretion
The vasopressin gene is expressed in a number of separate neuronal nuclei and peripheral tissues. Regulation of vasopressin expression is dependent on the site. For example, hyperosmolality increases vasopressin expression in the supraoptic nucleus and the magnocellular division of the paraventricular nucleus, but vasopressin mRNA in other sites is unaltered. For example, vasopressin expression in the suprachiasmatic nucleus is under diurnal regulation. Androgens up-regulate expression of vasopressin in the striae terminalis, and glucocorticoids suppress expression in the parvocellular division of the paraventricular nucleus. Differential regulation, even within anatomically related sites, likely results from differential expression of hormone receptors in the cells and different neuronal afferents. Vasopressin gene transcription is under positive regulation by cAMP and protein kinase C pathways. Little is known about regulation of vasopressin outside the central nervous system, but glucocorticoids have been shown to suppress its expression in an SCLC cell line.
Ectopic secretion of vasopressin occurs in squamous cell carcinoma; small cell carcinoma; neuroblastoma; pancreatic, duodenal, prostatic, and urothelial tumors; and undifferentiated carcinoma65–68 (Table 154-7). In one series, 16% of patients with SCLC had hyponatremia (<130 mmol/L) at diagnosis, compared with 0% of patients with non-SCLC. Hyponatremia was found to be an independent predictor of poor prognosis in extensive-stage disease. In vitro studies found that 7 of 11 tumors in culture produced vasopressin, 9 of 11 tumors produced atrial natriuretic factor, and 5 of 11 tumors produced both hormones. All the cells studied from patients with hyponatremia produced one of the two hormones.65
Table 154-7. Tumors Associated With Syndrome of Inappropriate Antidiuretic Hormone Secretion
The active hormone, vasopressin, is the product of a precursor peptide cleavage, which also gives rise to neurophysin II, and a C-terminal glycopeptide. Similar to the identification of partially processed forms of ACTH in the circulation in ectopic ACTH syndrome, the vasopressin-neurophysin precursor has been found in plasma from patients with SIADH due to SCLC.69 This is in contrast to patients with SIADH caused by central nervous system disease. Differential hormone processing may provide an additional diagnostic test for the underlying cause of SIADH.
DIAGNOSIS
Hyponatremia presents with features of neuropsychiatric dysfunction in most cases (Table 154-8). The elderly and the young are more likely than others to be symptomatic. The absolute sodium concentration is less reliable as a predictor of symptoms than the rate of decrease in sodium concentration, although almost all symptomatic patients will have plasma sodium concentration of less than 120 mmol/L. Clinical features include lethargy, fatigue, impaired conscious level, coma, seizures, and psychosis. Hyponatremia may cause death as a result of cerebral edema, uncontrolled seizures, and the consequences of coma. Although mild hyponatremia (>125 mmol/L) is usually regarded as a straightforward condition that may not require specific treatment, hyponatremia should not be regarded as benign.
Table 154-8. Clinical Features of Hyponatremia
A set of diagnostic criteria must be fulfilled before a secure diagnosis is reached (see Table 154-8). The underlying cause is then sought. Neurologic, lung-related, drug-related, and miscellaneous causes can result in dysregulation of vasopressin regulation in the hypothalamus and should not, therefore, be regarded as ectopic hormone secretion states. In contrast, a variety of tumors (see Table 154-7) has been shown to aberrantly secrete vasopressin and express the vasopressin gene inappropriately. There is evidence from T1-weighted magnetic resonance imaging scans of the pituitary that such ectopic vasopressin secretion results in central suppression of vasopressin synthesis.
MANAGEMENT
The management of this disorder falls into two parts. The first is to diagnose and treat the underlying cause, and the second is to remove excess free body water. Discussion of specific therapy on the variety of underlying tumors is beyond the scope of this chapter, but surgical cure or debulking, chemotherapy, and radiotherapy have all been applied. In general, the circulating vasopressin concentration bears a direct relationship to tumor bulk in an individual patient, but there is a low correlation across a patient cohort, presumably reflecting intertumoral differences in cellular differentiation and hormone production. Decisions about the acute correction of hyponatremia are complicated by the occurrence of both pontine and extrapontine myelinolysis as consequences of therapy. The risk of myelinolysis is linked to the rate of change in sodium concentration. Therefore, a prudent approach is always justified, with an increase of between 0.5 and 1.0 mmol/L sodium per hour and a maximum of 8 mmol/L over 24 hours. This requires close monitoring every 2 to 3 hours.70 In symptomatic patients, treat with furosemide and hypertonic saline until convulsions cease and the level of consciousness improves. This is usually achieved by an increase in sodium concentration of 10% (∼10 mmol/L) and subsequent water restriction. In asymptomatic patients, the condition is almost always chronic; these patients should be treated by water deprivation initially.
Specific approaches to antagonize the action of vasopressin usually rely on demeclocycline, in divided doses up to 1200 mg daily. These agents induce a state of nephrogenic diabetes insipidus. Alternatively, oral sodium supplementation, up to 3 g daily, with furosemide, 40 to 80 mg, results in net loss of free water. More recently, a vasopressin receptor antagonist (Vaprisol, Astellar Pharma) has been approved. The indications for intravenous use are currently limited to moderate to severe hyponatremia that would warrant use of hypertonic saline.70 In the future, chronic treatment with orally available agents seems likely, although the precise indications remain undefined.
Humoral Hypercalcemia of Malignancy
Hypercalcemia is a common complication of malignancy (Table 154-9). It may result from the direct lytic effect of bony metastases or the action of tumor-derived humoral factors, although it is now clear that a spectrum of disorders lies between these two extremes, and in most cases, there is a humoral component. Calcitonin has been detected in peripheral blood in a number of patients with malignancy and appears to be incompletely processed, in a manner similar to ACTH, with higher-molecular-weight variants.71 However, there is no clinical syndrome ascribed to such ectopic production. Calcitonin secretion appears to be most commonly associated with a multihormonal secretory phenotype, with other peptide hormones including ACTH and gastrin55,71–74 (Table 154-10).
Table 154-9. Mechanisms of Malignancy-Associated Hypercalcemia
| Mechanism | Agent | Tumor Type |
|---|---|---|
| Lytic metastases | TGF-β | Squamous cell carcinoma of the lung |
| IL-1 | Breast | |
| TNF | Kidney | |
| Lymphotoxin | Myeloma | |
| PTHrP | ||
| Humoral effects | PTHrP | Solid tumors, particularly squamous cell carcinoma of the skin, lung, kidney, and head and neck |
| PGE | Solid tumors | |
| TNF | Multiple myeloma | |
| TGF-β | ||
| IL-1 | ||
| Lymphotoxin | ||
| 1,25-Dihydroxy-vitamin D | T-cell lymphoma | |
| Non-Hodgkin’s lymphoma | ||
| Hodgkin’s lymphoma | ||
| Melanoma | ||
| Small cell lung carcinoma | ||
| Ectopic PTH | Small cell lung carcinoma (very rare) | |
| Coexistent other causes of hypercalcemia | Primary hyper-parathyroidism | Ovarian cancer |
| Sarcoidosis | ||
| Vitamin D mediated |
1,25-DHCC, 1,25-Dihydroxycholecalciferol; IL-1, interleukin 1; PGE, prostaglandin E; PTH, parathyroid hormone; PTHrP, parathyroid hormone–related protein; TGF-β, transforming growth factor β; TNF, tumor necrosis factor.
Table 154-10. Tumors That Produce Ectopic Calcitonin
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|