FIGURE 147-1. Serum thyroxine (T4), triiodothyronine (T3), and thyroxine-binding globulin (TBG) as a function of gestational age. Each point gives the mean value (±1 standard deviation [SD]) of determinations performed at the initial evaluation, pooled for 3 weeks, between 5 and 28 weeks (n = 510), and again for samples obtained between 28 and 39 weeks (n = 355). The latter samples include both late initial evaluations and the second series of determinations at 30 to 33 weeks. Each point represents an average of 72 individual determinations. The dashed lines illustrate the theoretical curves of T3 and T4 concentrations required to yield the average molar ratios of T4/TBG and T3/TBG that correspond to nonpregnant reference subjects (0.37 for T4/TBG and 0.0089 for T3/TBG with a molecular weight of 57 kDa for TBG).
(Data from Glinoer D, De Nayer P, Bourdoux P, et al: Regulation of maternal thyroid during pregnancy, J Clin Endocrinol Metab 71:276.
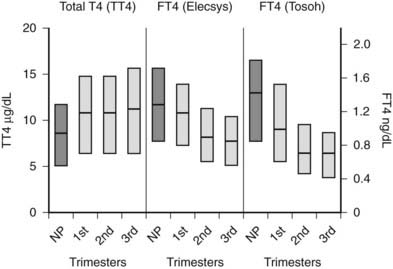
FIGURE 147-2. Serum total thyroxine (TT4) and free thyroxine (FT4) levels by trimester. Interquartile ranges are shown by the shaded boxes, with the median value indicated by the line. Serum TT4 levels rise to approximately 1.5 times the normal nonpregnant reference range. Although serum FT4 ranges were method dependent, as shown by differences in measurement by the Elecsys system, Roche Diagnostic and Tosoh, Tosoh Corporation methods, both methods show a consistent decrease in FT4 as pregnancy progresses. (NP, Nonpregnant [n = 62]; 1st, first trimester [n = 105]; 2nd, second trimester [n = 39]; 3rd, third trimester [n = 64].)
(Data courtesy of Carole Spencer, PhD.) (From Chan GW, Mandel SJ: Therapy insight: management of Graves’ disease during pregnancy, Nat Clin Pract Endocrinol Metab 3:470.
In pregnant women, the T3/T4 ratio is increased in the third trimester. Increased binding of T4 and T3 to monocyte nuclear receptors has also been reported in human pregnancy.77 Unfortunately, except for the equilibrium dialysis FT4 assay, none of the other commercial assays has reported trimester-specific and method-specific FT4 reference ranges during pregnancy. The commercially available automated FT4 assays that use two-step or labeled antibody methods are protein sensitive and therefore are affected by pregnancy-induced changes in serum albumin or TBG.78,79 Consequently, no universal absolute FT4 value can be used to define a low serum FT4 level across methods. It has been suggested that the normal range for the serum total T4 level during pregnancy is 1.5 times the nonpregnant reference range.68 Until validated pregnancy reference ranges are available for serum FT4 assays, the serum total T4 level (adjusted for protein binding) may be more reliable for use during pregnancy.
Because serum free thyroid hormone measurements are difficult to assess during pregnancy, serum TSH measurements remain the best assessment of a pregnant woman’s thyroid status. However, population-specific normal ranges for serum TSH are derived primarily from healthy, nonpregnant individuals. Recently, some have advocated “trimester-specific” reference ranges for TSH.80,81,117 These data derive from analysis of serum TSH in healthy euthyroid women who then are assessed during pregnancy (Fig. 147-3). Serum TSH values in the first trimester range much lower than would be expected in a nonpregnant individual, generally between 0.03 mIU/L and 2.5 mIU/L. In the second and third trimesters, greater variance is seen, although the lower limit of “normal” remains below what would be expected for nonpregnant individuals.80 Debate on how these data should be translated into clinical practice is ongoing. Regardless, these data suggest that, compared with nonpregnant reference ranges, mildly suppressed TSH in a pregnant woman should be viewed as safe, and perhaps physiologically normal.
FIGURE 147-3. Serum thyroid-stimulating hormone (TSH) and human chorionic gonadotropin (hCG) as a function of gestational age.
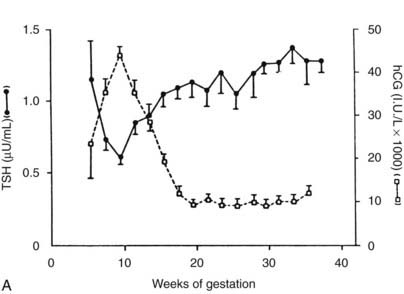
A, Serum hCG was determined at the initial evaluation and TSH at the initial evaluation and during late gestation. The symbols give the mean value (±standard error [SE]) for samples pooled for 2 weeks’ gestation. Each point corresponds to an average of 33 determinations for hCG and 49 for TSH.
(From Glinoer D, De Nayer P, Bourdoux P, et al: Regulation of maternal thyroid during pregnancy, J Clin Endocrinol Metab 71:276.
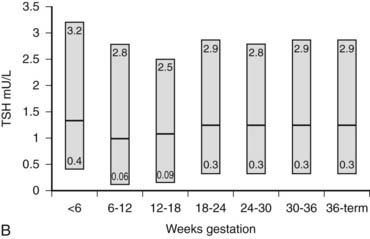
B, Gestational age–specific serum TSH concentrations in women without thyroid autoimmunity. The shaded areas represent the 2.5th to the 97.5th percentile values, with the median value indicated by the line.
(Data graphed from Stricker R, Echenard M, Eberhart R, et al: Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals, Eur J Endocrinol 157:509, 2007.)
Thyroid Stimulation and Regulation
The histologic picture of the thyroid gland during pregnancy is one of active stimulation. Columnar epithelium can be seen lining hyperplastic follicles.82 The increase in maternal T4 production that occurs during normal gestation is most evident from clinical observations of thyroxine-replaced hypothyroid women who require a 25% to 40% dosage increase to maintain normal serum TSH levels in pregnancy.71,83 Furthermore, findings of relative hypothyroxinemia and slightly increased serum TSH levels during pregnancy in women from areas of borderline iodine sufficiency (<100 mcg/day) support the view that pregnancy constitutes a stress for the maternal thyroid by stimulating thyroid hormone production.84
Several factors are known to tax gravid thyroid economy, and each may have relative importance at a different time in gestation. In early pregnancy, the serum concentration of TBG increases rapidly and more thyroid hormone may be needed to saturate binding sites. Glomerular filtration rate increases, resulting in increased iodide clearance. Later, with placental growth, metabolism of T4 to its inactive metabolite reverse T3 is increased by the high levels of placental type III deiodinase.85 In addition, transplacental passage of maternal T4 may occur.86 Finally, alterations in the volume of distribution of thyroid hormone may occur because of both gravid physiology and the fetal/placental unit.
Human Chorionic Gonadotropin
Serum hCG has thyromimetic effects and is responsible for the hyperthyroidism associated with trophoblastic disease.87–94 In normal pregnancy, hCG is a physiologic regulator of thyroid function early in gestation.73,74,95–100 Clinically, hCG levels peak in pregnancy at 50 to 100 times 100,000-200,000 IU/L at between 9 and 14 weeks; this peak correlates with reduced TSH levels in the first trimester73,98 (see Fig. 147-3). Levels decline thereafter and are undetectable by a few weeks postpartum. An overall increase in thyromimetic activity in the sera of women during early pregnancy may be due to hCG, as determined by immunoadsorption studies using hCGmonoclonal antibodies.99,100
Ekins and colleagues have effectively argued that an alternative control system such as hCG may regulate maternal thyroid activity in early pregnancy, when the most important changes in TBG and T4 secretion occur, to ensure an adequate supply of thyroid hormones to the placenta and embryo (Fig. 147-4).98,101,102 Experimentally, in vitro studies show that hCG binds to the TSH receptor (TSHR), as assessed by radioreceptor assays using porcine and human thyroid membranes incubated with 125I-TSH,103–106 stimulates adenylate cyclase activity and cyclic adenosine monophosphate (cAMP) generation, and enhances T3 secretion in human and porcine thyroid slices.107 More recently, hCG has been shown to stimulate growth, iodide uptake, and cAMP generation in the rat thyroid cell line FRTL5.99,100,108–110 Species differences111,112 and microheterogeneity of hCG molecules through pregnancy and in gestational trophoblastic diseases may account for the variable thyrotropic activities reported.98–100,106–108,113–115 However, with reported TSH bioactivity of up to 0.7 µU/U hCG,106,108 the hCG levels obtained in early pregnancy could produce a noticeable thyrotropic effect, and it has been reported that up to 9% of pregnant women may have an isolated subnormal serum TSH level in the first trimester.116 A recent study reported greater susceptibility to hCG-associated suppression of serum TSH in pregnant women whose serum TSH levels were in the lowest 25th percentile in early pregnancy.72 Overall, maternal serum TSH concentrations decrease in the first half of pregnancy compared with the nonpregnant state and remain lower until term (see Fig. 147-3B).117 Consequently, in healthy pregnant women at between 6 and 18 weeks’ gestation, the lower limit of the 95% confidence interval for serum TSH levels is between 0.03 and 0.09 mIU/L, which then rises to 0.3 mIU/L as pregnancy progresses.80,117
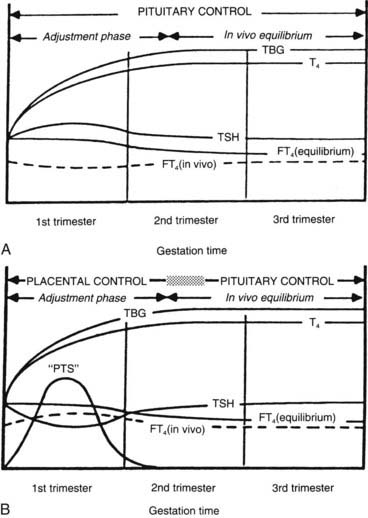
FIGURE 147-4. A, Conventional model of maternal thyroid gland control throughout pregnancy if based on the traditional hypothalamic-pituitary feedback mechanism. B, Hypothetical model of maternal thyroid gland control throughout pregnancy if a putative “placental thyroid stimulator” (PTS), possibly human chorionic gonadotropin, assumes regulatory control over maternal thyroid secretion. FT4, Free T4; T4, thyroxine; TBG, thyroxine-binding globulin; TSH, thyroid-stimulating hormone.
(From Ballabio M, Poshyachinda M, Ekins RP: Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as a putative regulator of maternal thyroid, J Clin Endocrinol Metab 73:824.
FETAL THYROID FUNCTION
The fetal hypothalamic-pituitary-thyroid axis develops autonomously and has been extensively studied in the human, sheep, and rat.52,118–120 A number of agents and maternal factors may affect fetal thyroid function, depending on whether they cross the placenta (Table 147-2).
Table 147-2. Placental Transfer and Fetal Thyroid Function
| Without Difficulty | Some Transfer | Little or No Transfer |
|---|---|---|
| Iodides | T4 | TSH |
| Thionamides | T3 | |
| Thyroid autoantibodies | ||
| TRH |
T3, Triiodothyronine; T4, thyroxine; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone.
Modified from Burrow GN: Thyroid diseases in pregnancy. In Burrow GN, Oppenheimer JH, Volp JR (eds): Thyroid function and disease, Philadelphia, 1989, WB Saunders, p 292.
Placental Transfer
The placenta is impermeable to TSH but permeable to TRH, although endogenous maternal levels are probably too low to influence fetal thyroid function.121 Pituitary and serum TSH in the fetus may be under the control of pancreatic TRH before the maturation of hypothalamic TRH after 20 weeks’ gestation.122–126 Injection of TRH in the mother is accompanied by increased cord serum TSH, T4, and T3 levels, thus indicating that endogenous TSH stimulates the fetal thyroid.127
Before the onset of human fetal thyroid gland function at 10 to 12 weeks’ gestation,128–132 any requirement for thyroid hormone would be met by the maternal supply. The presence of human fetal tissue thyroid hormones and receptors before 12 to 18 weeks, when fetal serum T4 production increases, is consistent with an early requirement for thyroid hormones from the mother.133 The placenta has generally been viewed as a substantial barrier to thyroid hormone transfer, in part because of preferential 5′-monodeiodination of T4 to reverse T3.119,121 Studies in rats have provided good evidence for transfer of maternal thyroid hormones to the fetus in early and late pregnancy, which may be important for early brain development and later brain growth and neuronal differentiation.134–139 In the rat, maternal T4 is the principal source of intracellular T3 in the early developing brain.139 The local intracellular generation of T3 from T4 of maternal origin protects the fetal brain from T3 deficiency in cases of fetal thyroid failure, because cerebral 5′ type 2 deiodinase activity increases markedly in response to a minor decrease in T4.140 In humans and sheep, maternal thyroid hormones have more limited access to the fetal circulation.119 Recent human studies have confirmed the presence of T4 and T3 in coelomic and amniotic fluid in the first trimester. Although the concentrations of total T4 and T3 are 100-fold lower than those in maternal serum, because of the lack of binding protein in these fetal compartment fluids, the concentration of FT4 is biologically relevant.141
The apparent normality of sporadic congenitally hypothyroid infants at birth indicates the role of maternal thyroid hormones. The devastating effects of maternal and fetal/neonatal thyroid hormone deficiency in endemic cretinism in humans underscore the overall importance of thyroid hormones to the fetus.54,142,143 Fortunately, this problem does not seem to occur in areas where iodine intake is just marginal,55 but concern remains with respect to any effect of maternal hypothyroxinemia on early fetal brain development and its effects on progeny.101,102,133,144–146 Early studies suggested limited transfer of T4 and T3 across the placenta in humans in the later part of pregnancy and at term,147–153 with T3 crossing more readily than T4. Vulsma and colleagues have convincingly demonstrated maternal-to-fetal T4 transfer in neonates born with a complete organification defect. These infants have subnormal fetal T4 concentrations when compared with normal newborns, but their levels are approximately 40% of the maternal concentration.86 Because of their absolute inability to produce thyroid hormone, this T4 must be maternal in origin.
Iodide is actively transported to the fetus.121 The fetal and neonatal thyroid is susceptible to iodine-induced hypothyroidism and goiter with excessive exposure.119,154,155 This complication can occur after intravenous, oral, mucosal, or topical exposure and absorption in the mother,156 after amniography,157 and as a result of postnatal topical absorption,158 as well as through breast milk.159 A number of pregnant women have been treated with amiodarone, an antiarrhythmic drug containing 75 mg of iodine per 200-mg dose that partially crosses the placenta and increases maternal and fetal iodide levels.160 Although thyroid function may remain normal, case reports have described fetal or neonatal goiter, hypothyroidism, or hyperthyroxinemia in association with maternal amiodarone therapy.161–163
Diagnosis of Fetal Thyroid Disorders In Utero
The possibility of thyroid disease in the fetus is usually considered because of maternal thyroid disease. Fetal hyperthyroidism is generally encountered in the setting of maternal active or previously ablated Graves’ disease via the transplacental passage of maternal TSH receptor–stimulating antibodies. Fetal hypothyroidism is associated with fetal thyroid maldevelopment, iodine deficiency disorders, thyroid autoimmunity, and excessive maternal antithyroid drug therapy.164–166 In fetal hyperthyroidism, ultrasound usually demonstrates a fetal goiter with increased vascularity, and fetal bone maturation is accelerated.167 In hypothyroidism, a fetal goiter may be visible on ultrasound,167,168 or the radiographic appearance of distal femoral or proximal tibial epiphyses may be delayed.169,170 The latter has limited clinical application. Although the fetus incurs serious risk, measurement of T4 and TSH in serum collected by percutaneous umbilical cord sampling (cordocentesis)120 is currently the most reliable means of diagnosing hypothyroidism167,171 or hyperthyroidism in utero.167,172 This technique has advantages over measurement of thyroid hormones or TSH in amniotic fluid, which has not been shown to reliably predict fetal thyroid status.173,174 Fortunately, the diagnosis of fetal thyroid dysfunction can usually be inferred from the clinical scenario presented by the maternal thyroid disease status (see “Fetal and Neonatal Thyrotoxicosis”). The fetus can absorb thyroid hormones injected into amniotic fluid, and such therapy has been used successfully in the treatment of hypothyroidism and goiter in utero.171,175
GOITER AND PREGNANCY
Goiter has historically been associated with pregnancy, but its incidence and prevalence vary with the geographic area and iodine status of the general population. Up to 70% of pregnant women in Scotland and Ireland were considered on clinical grounds (visible and palpable thyroid gland) to have a goiter versus 38% of nonpregnant controls.176 No cumulative influence of successive pregnancies was observed, inasmuch as goiters were seen in 39% of nulliparous women and 35% of nonpregnant parous women. These studies were conducted in areas of relatively low iodine intake. A comparative study in Iceland, an area of iodine sufficiency, showed a lower basal prevalence of goiter (20%) and no increase in the incidence of goiter in pregnancy.177 Similar results have been reported in studies from North America,178 which has led some authors to suggest that goiter in pregnancy is a myth.179 Most goiters during pregnancy in North America are related to autoimmune thyroid disease, colloid nodular disease, or thyroiditis.
Ultrasonography has added a quantitative perspective to the assessment of goiter in pregnancy. In Denmark, an area of marginal iodine intake, a 30% increase in thyroid volume has been documented at between 18 and 36 weeks’ gestation.180 Volume returned to baseline postpartum, and no evidence of thyroid dysfunction or thyroid autoimmunity was apparent. Only 25% of the women actually had a goiter on clinical grounds. Serum thyroglobulin levels were also increased during pregnancy.181 Only a 13% increase in thyroid volume was reported in a North American study.182 The largest longitudinal and cross-sectional study of thyroid volume in pregnancy involved more than 600 women from Belgium, another area of marginal iodine intake.73 Seventy percent of the women had a 20% or greater increase in thyroid volume during pregnancy, although only 9% had a significant goiter as defined by thyroid volume in excess of 23 mL. Thyroid volume showed positive correlations with higher serum thyroglobulin levels and an increased serum T3/T4 ratio. No correlation was seen with urine iodide excretion, and a negative correlation with serum TSH levels was noted. The latter may have been due to the influence of hCG during pregnancy. The same authors from Belgium prospectively studied preexisting mild thyroid abnormalities through pregnancy and noted a significant goitrogenic effect as well as an increase in the incidence and prevalence of thyroid nodules.50 Many of these nodules were subclinical and were detected only on thyroid ultrasound. Serum thyroglobulin levels were disproportionately increased in women with goiters and nodules when compared with controls and pregnant women with autoimmune thyroid disease or a history of previous thyroid abnormalities. The authors further suggested that previous pregnancies were a significant risk factor for goiter and thyroid nodules. This same risk was also suggested in a study from the Netherlands.183 It should be noted that an increase in thyroid volume during pregnancy does not necessarily denote increased mitotic activity because increased colloid volume, cell hypertrophy, inflammation, or increased thyroid blood flow could account for some of the enlargement.
No evidence of adverse effects on fetal development or neonatal thyroid function has been seen in these studies from areas of marginal iodine uptake.50,55,73 Nor does there appear to be an increase in risk of neonatal thyroid dysfunction in goitrous, iodine-sufficient areas.184 This finding contrasts with results from areas with endemic iodine deficiency.54,185 Maternal smoking has been shown to be a risk factor for neonatal thyroid enlargement, as determined ultrasonographically.186 Neonatal thyroid volume correlated with cord serum thyroglobulin and thiocyanate levels, but no evidence of neonatal thyroid dysfunction was found.
Pathology of Thyrotoxicosis in Pregnancy
INCIDENCE AND ETIOLOGY
All forms of thyroid disease are more common in women than in men, and thyrotoxicosis is not a rare event during pregnancy. It occurs in about 2 of every 1000 pregnancies. Autoimmune thyrotoxicosis, or Graves’ disease, the most common cause of thyrotoxicosis in pregnant women, accounts for about 90% of cases. Toxic adenomas or nodular goiters are much less common in this age group. Other causes of thyrotoxicosis in pregnancy include gestational trophoblastic neoplasia93 and hyperemesis gravidarum (see Table 147-1).
Gestational Thyrotoxicosis
A spectrum of hCG-induced hyperthyroidism occurs during pregnancy, and this entity has been referred to recently as “gestational thyrotoxicosis.”187–189 This disorder differs from Graves’ disease in several ways: (1) nonautoimmune origin, with negative antithyroid and anti–TSH receptor antibodies (TRAbs); (2) absence of large goiter; and (3) resolution in almost all patients after 20 weeks.187
Findings range from an isolated subnormal serum TSH concentration (approximately 9% of pregnancies116) to elevations of free thyroid hormone levels in the clinical setting of hyperemesis gravidarum. Systematic screening of 1900 consecutive pregnant women in Belgium demonstrated low TSH and elevated FT4 in 2.4%, half of whom had weight loss, lack of weight gain, or unexplained tachycardia.189
Hyperemesis gravidarum, or pernicious nausea and vomiting in pregnancy, is usually associated with weight loss and fluid and electrolyte disturbances. Its manifestation and diagnosis can be complicated because other causes of severe nausea and vomiting in pregnancy must be excluded. Suppressed TSH levels may occur in 60% of women with hyperemesis gravidarum, along with elevated FT4 levels in almost 50%.188–192 Serum hCG concentrations correlate with FT4 levels and inversely with TSH determinations. The magnitude of the deviation from normal values increases with the severity of nausea and vomiting.116,193 Only 12% of such women have an elevated free T3 index, which may help to differentiate this entity from Graves’ disease.116 Furthermore, thyroid-stimulating activity, as measured by adenylate cyclase activity per international unit of hCG, is reported to be greater in women with hyperemesis gravidarum than in those with occasional or no vomiting.187
Similar thyroid hormone changes and emetic symptoms may be present in multiple gestations, which are associated with higher peak and more sustained hCG levels.194 In addition, a recent case report further supports the concept of hCG-induced thyrotoxicosis. A woman with recurrent gestational thyrotoxicosis and her mother with the same obstetric history were found to have a missense mutation in the extracellular domain of the TSH receptor. When this receptor was studied in transfected COS-7 cells, it caused a twofold to threefold increase in cAMP generation when exposed to hCG as compared with wild-type receptor.192 This genetic mutation induced hyperresponsiveness to hCG as well as thyrotoxicosis.
Gestational thyrotoxicosis is transient and usually resolves within 10 weeks of diagnosis.195 Treatment with antithyroid drugs is not recommended61 but may be needed if there is concomitant Graves’ disease. The clinician may consider antithyroid drug therapy for patients with hyperemesis who remain symptomatic after 20 weeks’ gestation and continue to have elevated thyroid hormone concentrations and suppressed TSH levels, or who have evidence of significant clinical thyrotoxicosis.
DIAGNOSIS
The clinical diagnosis of mild to moderate hyperthyroidism is not easy and may be much more difficult to confirm during pregnancy. Hyperdynamic symptoms and signs, which are common in normal pregnant women, include anxiety, heat intolerance, tachycardia, and warm, moist skin. Laboratory tests may support a suspicion of thyrotoxicosis, but confirmation may be difficult. The ocular changes of thyroid ophthalmopathy or pretibial myxedema do not indicate whether Graves’ thyrotoxicosis is active. A resting pulse above 100 that is not decreased by Valsalva’s maneuver and a goiter with a palpable thrill are strongly suggestive of thyrotoxicosis.
Despite the difficulty associated with interpretation of thyroid function tests because of the elevated TBG concentration during pregnancy, the diagnosis of hyperthyroidism in pregnant women depends on laboratory testing. A sensitive TSH determination with a value less than 0.01 mU/L196,197 and an elevated serum FT4 (or total T4 concentration above pregnancy reference values) is generally diagnostic. This illustrates the need for the manufacturers of commercial FT4 assays to report trimester-specific and method-specific reference ranges during pregnancy. As noted, during the first trimester, the serum TSH may be below the nonpregnant reference range in response to an increase in serum hCG concentration.73 The delay in TRAb measurement renders it generally impractical for routine diagnostic use in this clinical scenario. If the diagnosis is not clearcut, one can usually wait 3 to 4 weeks and then can repeat the thyroid function tests because most pregnant women tolerate mild to moderate thyrotoxicosis without difficulty.224
TREATMENT
Once the diagnosis of thyrotoxicosis has been established in a pregnant woman, therapy should be instituted. Treatment of a pregnant woman with thyrotoxicosis is limited to antithyroid drug therapy or surgery because radioactive iodine is absolutely contraindicated.198–200 After the 10th to the 12th week of gestation, once the fetal thyroid has the ability to concentrate iodine, congenital hypothyroidism may be induced by 131I treatment. In one study, in which 182 fetuses were inadvertently exposed to 131I therapy during the first trimester, pregnancy resulted in two (1.1%) spontaneous abortions, two (1.1%) intrauterine deaths, six (3.3%) hypothyroid children, and four (2.2%) mentally retarded children. If 131I treatment is inadvertently administered to a woman in early pregnancy, the effects on the thyroid could be blocked by iodide administration, but the optimal dosing of iodide has not been studied.
Thionamide Therapy
The thionamides propylthiouracil, methimazole, and carbimazole have all been prescribed for the treatment of thyrotoxicosis during pregnancy. Carbimazole, which is metabolized to methimazole, is used mainly in Europe. All these agents cross the placenta and are also secreted in breast milk.201 The serum half-lives of propylthiouracil and methimazole are 1 hour and 5 hours, respectively.202–206 These two antithyroid drugs have been used interchangeably. Thionamides block the synthesis but not the release of thyroid hormone. Propylthiouracil does have the potential additional advantage of partially blocking the conversion of T4 to T3. With propylthiouracil or methimazole, the typical patient will note some improvement after 1 or 2 weeks and may approach euthyroidism after 6 to 8 weeks of treatment, with no difference in the median time to lowering the FT4 index to appropriate pregnant levels.207
If minor drug reactions occur, the thionamides may be interchanged, but cross-sensitivity is seen in about half of patients.208 The most common reactions include fever, nausea, skin rash, pruritus, and metallic taste.209 Transient leukopenia, not an uncommon reaction to thionamide therapy, occurs in about 12% of adults.209,210 This association may be complicated because mild leukopenia is not uncommon in untreated Graves’ disease.202 This mild leukopenia is not a sign of agranulocytosis, which occurs in about 0.5% of patients, usually within 12 weeks of initiation of therapy, and may be an autoimmune phenomenon.211–214 Hepatitis and vasculitis have also been reported as rare side effects of thionamide therapy, specifically with propylthiouracil215–217; these complications have not been reported to affect the fetus, although they have occurred in the pregnant mother.
Additionally, the possibility has been raised that methimazole is associated with the development of aplasia cutis of the scalp in the treated mother’s offspring.218–220 Some endocrinologists nonetheless recommend propylthiouracil as initial therapy during pregnancy because no cases of aplasia cutis have been reported in babies born to propylthiouracil-treated mothers.61,220 In addition, perhaps of greater concern than aplasia cutis are recent descriptions of a methimazole embryopathy, which may include findings of choanal atresia, tracheal-esophageal fistulas, and hypoplastic nipples.126 A recent case-control study raised the possibility that maternal hyperthyroidism itself, rather than methimazole treatment, might be the causal factor for this embryopathy, specifically choanal atresisa.221 When the significance of all these reports is considered, it must be emphasized that no case reports of aplasia cutis or other congenital anomalies have been associated with propylthiouracil exposure. This generally remains the preferred drug therapy of maternal hyperthyroidism, in pregnancy. However, some have recently advocated propylthiouracil use only during the first trimester. Thereafter, methimazole may be substituted for propylthiouracil and continued until delivery.
The goal of antithyroid drug therapy is to gain control of the maternal thyrotoxicosis to ensure favorable gestational outcomes and to minimize the impact on the fetus.61,222 Studies show a strong correlation between maternal and neonatal levels of free T4, indicating that maternal thyroid status is the most clinically practical index of fetal thyroid status.199,223 To optimize neonatal thyroid function and minimize the incidence of transient newborn hypothyroidism, maternal serum FT4 should be maintained at or slightly higher than (<10%) the nonpregnant reference range.61,223 An alternative approach would be to keep the total T4 concentration in the high normal range for pregnancy (1.5 times the nonpregnant reference range).61,68 When detectable, serum TSH concentrations at or just below the trimester-specific 95% confidence interval are acceptable.
Therefore, the therapeutic target for maternal thyroid hormone levels is actually very mild hyperthyroidism as compared with true “normal” levels for gestational physiology. Fortunately, subclinical and mild hyperthyroidism during pregnancy is not associated with adverse maternal gestational outcomes.224
Propylthiouracil (PTU) usually is started at doses sufficient to control the hyperthyroidism; the dose may be increased after 4 weeks if necessary to gain control of the thyrotoxicosis. Some women may require high doses (up to 450 mg/day of PTU) for this purpose and must be carefully monitored. Doses of this magnitude may cause fetal hypothyroidism and may justify a change in therapy to surgery. The need for larger doses may correlate with low serum concentrations of PTU225 and could be caused by documented individual variability in serum propylthiouracil levels after an oral dose226 or poor compliance with the medication. Methimazole should be used if PTU is not available, the side effect profile of PTU is deemed unacceptable, or if there is difficulty with adherence to a multidose, multi-pill PTU regimen. Free or total T4 and sensitive TSH measurements should be performed monthly during pregnancy and the dose of thionamide decreased to maintain the therapeutic targets for FT4, total T4, and TSH indicated above. If a requirement for higher doses of PTU (>450 mg/day) or methimazole (>30 mg/day) continues and effects on the fetus are a concern, the clinician should consider thyroid surgery.
β-Adrenergic Blockers
If it is necessary to give drugs to a pregnant woman, they should be the least toxic agents possible. For this reason, the use of β-adrenergic blocking drugs has been advocated for the treatment of pregnant women with hyperthyroidism.227 β-Blockers have been used in large numbers of pregnant women to treat hypertension without apparent significant side effects.228 However, intrauterine growth retardation with a small placenta, impaired response to anoxic stress, postnatal bradycardia, and hypoglycemia have been reported in the offspring of mothers receiving these agents, indicating the need for caution in their use.229 β-Blockers are particularly useful for rapid control of the β-adrenergic manifestations of thyrotoxicosis such as tremor and tachycardia. Propranolol, 20 to 40 mg three or four times a day, or atenolol, 50 to 100 mg/day, is usually adequate to control the maternal heart rate at 80 to 90 beats per minute. Esmolol, an ultra–short-acting cardioselective β-blocker given intravenously, controlled the heart rate in a pregnant woman with hyperthyroidism who required emergency surgery and was unresponsive to large doses of propranolol.230 Current practice is to control hyperthyroidism with antithyroid drugs and to add β-blockers only in exceptional cases.
Surgery
In pregnant thyrotoxic women with poor compliance or for whom maternal or fetal toxicity from antithyroid drug therapy is a concern, subtotal thyroidectomy may be advised.61 Thyrotoxicosis needs to be controlled medically before subtotal thyroidectomy can be performed. This treatment includes antithyroid drugs, β-blockers, and, rarely, possible short-term use of oral iodides.231 A useful clinical parameter of control is a resting heart rate of 80 to 90 beats per minute. Because of concern about spontaneous abortion, surgery is often delayed until after the first trimester. The small but real anesthetic and surgical risk is probably greater than the risks associated with thionamide therapy.232,233
Iodide
Iodide has not been recommended in the routine treatment of hyperthyroidism during pregnancy because of its association with neonatal goiter and hypothyroidism when given in conjunction with thionamides. One study in which gravidas with mild Grave’s disease (GD) were treated with low-dose iodine alone (6 to 40 mg daily) showed that 6% of neonates had elevated serum TSH levels, but none had a goiter.231 With such little evidence, iodide should not be considered as primary therapy but may be used short term for control of thyrotoxicosis prior to thyroidectomy, or in the management of thyroid storm.
PREGNANCY OUTCOME
Severe maternal thyrotoxicosis significantly increases morbidity in both the fetus and the mother. The prevalence of low birth weight offspring is higher, with a trend toward increased neonatal mortality.26,234 Whether fetal wastage is increased in established pregnancy is not clear. In one study of 57 thyrotoxic pregnancies, the fetal wastage rate was 8.4%, which compares favorably with the estimated overall fetal wastage rate of 17% in normal women, including those undergoing spontaneous abortion.44 Very early spontaneous abortions could easily be missed in thyrotoxic women. A recent study reported a higher miscarriage rate in mothers affected by resistance to thyroid hormone. The inference is that these miscarriages may have predominantly involved genetically unaffected fetuses who therefore were exposed to high maternal thyroid hormone levels with resultant fetal thyrotoxicosis that proved toxic. This study provides valuable insight about the effects of in utero thyrotoxicosis without accompanying maternal gestational hyperthyroid physiology.235 A higher incidence of minor congenital malformations has been suggested to occur in the children of thyrotoxic women who were untreated during the first trimester of pregnancy,236 but this finding has not been confirmed by others.207 Only one study has reported Down syndrome to occur more frequently in children born to women with hyperthyroidism.237 Preterm delivery, perinatal mortality, and maternal congestive heart failure were markedly increased in untreated and inadequately treated thyrotoxic patients in a retrospective study of 60 pregnant women with hyperthyroidism admitted to an inner-city hospital over a 12-year period.238 Women with newly diagnosed thyrotoxicosis during pregnancy had a higher incidence of morbidity and mortality than did women in whom the condition was diagnosed and treated before conception. Socioeconomic conditions might have played a role in the severity of hyperthyroidism or poor outcomes, but treatment of thyrotoxicosis is indicated nonetheless. These patients are at risk for congestive heart failure because the hyperdynamic state of thyrotoxicosis is superimposed on the increased cardiac output of normal pregnancy.239 Management of diseases such as diabetes mellitus is also complicated in a pregnant woman with thyrotoxicosis, causing erratic glycemic control and a need for increased insulin.240 Fortunately, these adverse outcomes are observed only with untreated overt or poorly controlled hyperthyroidism, not with subclinical hyperthyroidism.224 In addition, successful treatment of overt hyperthyroidism with antithyroid drugs by the third trimester reduces the risk of low birth weight newborns to that of a control euthyroid populaton.241
Fetal and Neonatal Thyrotoxicosis
Fetal hyperthyroidism complicates pregnancy in 1% of women with active or treated Graves’ disease, including those who have become hypothyroid after radioactive iodine therapy. These women have thyroid-stimulating immunoglobulins that, similar to other immunoglobulins such as IgG, cross the placenta.242 In high enough concentrations, they may cause fetal or neonatal thyrotoxicosis. Maternal IgG antibodies, particularly subclasses 1 and 3, and thyroid autoantibodies are able to cross the placenta after 20 weeks’ gestation242,243 by micropinocytosis after IgG binding to Fc receptors on the syncytiotrophoblast. Maternal levels are indicative of the degree of fetal exposure and the potential for fetal thyroid stimulation.244
Measurement of maternal TSH receptor antibody (TRAb) levels may provide prognostic information about the development of fetal Graves’ disease.245–247 At present, TSH receptor antibodies can be measured by two techniques: an immunoassay (TSH binding inhibitory immunoglobulin [TBII]) or a functional assay of biological stimulation (thyroid-stimulating immunoglobulin [TSI]).119,248 These antibodies may exhibit stimulating or blocking activity, resulting in fetal/neonatal hyperthyroidism or hypothyroidism.166,245,249,250 Alternating neonatal hyperthyroidism and hypothyroidism have been described in infants born to the same mother.251
TSH receptor antibodies should be measured by the end of the second trimester in mothers with current Graves’ disease, a history of Graves’ disease and treatment with 131I or thyroidectomy, or birth of a previous neonate with Graves’ disease.61 Women who have a negative TRAb and do not require antithyroid drug therapy have a very low risk of fetal or neonatal thyroid dysfunction.
In a woman with active or previously ablated Graves’ disease, the diagnosis of fetal hyperthyroidism should be considered if persistent fetal tachycardia (>180 beats per minute) without beat-to-beat variation and advanced bone maturation are present, sometimes with fetal growth restriction.167 The diagnosis is strengthened by the ultrasound documentation of a goiter with diffusely increased vascularity.167 Diagnosis can be confirmed by cordocentesis if considered necessary after discussion of the potential risks, but this usually is not needed.252 The pathology of fetal thyrotoxicosis includes goiter, visceromegaly, adenopathy, and pulmonary hypertension.253 If fetal thyrotoxicosis occurs in the setting of active maternal Graves’ disease, usually the mother is overtly hyperthyroid. Appropriate maternal antithyroid therapy will treat both the mother and the fetus because, as in the mother, fetal thyroid hormone synthesis represents the balance between the transplacental passage of inhibitory maternal antithyroid drug and stimulating maternal TRAb concentrations.
Levothyroxine replacement in women with a history of Graves’ disease and prior 131I ablation or thyroidectomy antedating pregnancy may still produce TRAb, causing fetal thyrotoxicosis. In this scenario, maternal therapy with propylthiouracil, 150 mg/day, may decrease the fetal heart rate to the normal range (140 to 160 beats per minute) within 2 weeks.244,254 Maternal thyroid hormone levels should be monitored regularly, with thyroxine supplementation given if hypothyroxinemia occurs.
TSH receptor antibody levels usually decline progressively toward term,13,251,255 and the PTU drug dose can be lowered to 50 to 75 mg/day or even discontinued with titration to the fetal heart rate and growth. In women with persistent elevations of TSH receptor antibodies at term (more than threefold elevated above reference values),167,256,257 neonatal hyperthyroidism may occur, requiring continued antithyroid drug therapy after birth. In addition, the presence of cord blood TSH receptor antibodies is highly predictive of the development of neonatal hyperthyroidism.256–259 If the mother has been treated with antithyroid drugs during pregnancy, the manifestations of neonatal thyrotoxicosis may be delayed until 5 to 10 days of life, when the pharmacologic effect of the transplacentally acquired antithyroid drug has cleared.258 The neonate may become irritable and have feeding problems. Other manifestations include proptosis, goiter, and failure to thrive. In severe cases, congestive heart failure, jaundice, and thrombocytopenia may occur and can cause significant mortality.260 Usually, the disease runs a self-limited course over a period of several months.119 Temporary treatment with iodides, β-blockers, and antithyroid drugs is indicated. As maternal antibody levels decrease over the first 3 months of life, treatment generally can be discontinued.259
In addition, it has been reported that infants with central hypothyroidism have been born to mothers with uncontrolled Graves’ disease. The hypothesis is that these infants are exposed to high levels of thyroid hormone in utero, which results in impaired development of the fetal hypothalamic-pituitary-thyroid axis and eventual central hypothyroidism.258,261 The incidence of such central congenital hypothyroidism is estimated to be 1 in 35,000 live births.262
LACTATION
Until the last decade, lactation was strongly discouraged in women receiving thionamide therapy. The doctrine against lactation in women receiving antithyroid drug therapy originated in a 1948 report, which stated that the concentration of thiouracil measured 2 hours after ingestion was three times higher in breast milk than in serum.263 Over the last 20 years, several studies have examined the extent of secretion of propylthiouracil and methimazole in breast milk and have evaluated the thyroid function of infants nursed by mothers taking these drugs. Propylthiouracil is more tightly protein bound than methimazole; therefore, the mean milk-to-serum concentration ratio is lower for propylthiouracil, at approximately 0.67,264 than for methimazole, for which the ratio is 1.0.265,266 Therefore, the mean total amount of methimazole excreted in breast milk is greater than that of propylthiouracil (0.14% versus 0.025%264,265).
Several studies have prospectively evaluated whether maternal antithyroid therapy affects the thyroid function of breastfed infants. Thyroid function remained unaffected in 171 infants whose mothers received daily doses of 50 to 300 mg of propylthiouracil, 5 to 20 mg of methimazole, or 5 to 15 mg of carbimazole for periods ranging from 3 weeks to 8 months.264,267–269 In fact, serum TSH and T4 levels remained normal even in six women in whom elevated serum TSH levels (19 mU/L and 120 mU/L) had developed while they were receiving propylthiouracil.269 Therefore, although the number of infants monitored is small, breastfeeding with continued antithyroid drugs may be contemplated as long as the doses are less than 300 mg daily for propylthiouracil and 20 mg daily for methimazole. It is important to remember that these antithyroid drugs have other nonthyroidal effects such as agranulocytosis; no data are available on the possible occurrence of such effects in infants nursed by mothers taking these drugs.
Pathology of Hypothyroidism in Pregnancy
MATERNAL HYPOTHYROIDISM
Incidence and Etiology
Hypothyroidism is encountered more often in pregnancy than is hyperthyroidism. The most recent epidemiologic data have been obtained through the analysis of banked serum obtained at approximately 16 weeks’ gestation in healthy pregnant women. In these women (without prior thyroid disease), two independent studies documented a 2% to 3% incidence of an elevated serum TSH concentration in midpregnancy.26,28 In industrialized societies, the main causes of maternal hypothyroidism in pregnancy are related to thyroid autoimmunity: Hashimoto’s thyroiditis and postablative hypothyroidism in Graves’ disease (see Table 147-1, Fig. 147-5). A third cause that has been noted increasingly is primary hypothyroidism due to surgical thyroidectomy. In most women, the hypothyroidism has already been diagnosed and treated before pregnancy. Worldwide, the main cause of maternal hypothyroidism is iodine deficiency, which has been estimated to affect more than one billion individuals. Population screening programs have documented that a substantial proportion of women of child-bearing age may also have mild TSH elevations. Furthermore, even those hypothyroid women treated to a goal TSH within the normal range, often are found to have abnormal hormone levels.270,271 The former point is notable in that the average maternal age at pregnancy has been increasing in the United States over the past three decades. In summary, it is estimated that between 4% and 7% of women of child-bearing age in the United States are hypothyroid and unaware of it, or are at risk for hypothyroidism during pregnancy if their levothyroxine dose is not adjusted upon conception. This impressive number draws attention to the importance of this medical problem.
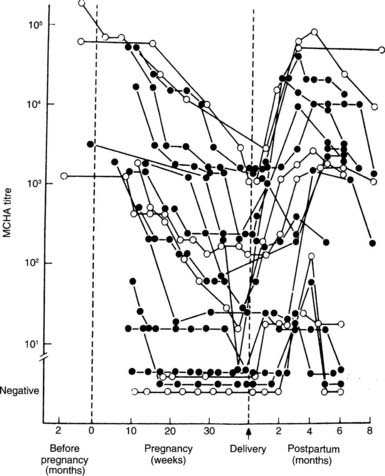
FIGURE 147-5. Sequential changes in serum antithyroid antibodies (MCHA) during pregnancy and after delivery in patients with Graves’ disease (black dots) and autoimmune thyroiditis (open dots).
(From Amino N, Kuro R, Tanizawa O, et al: Changes in serum antithyroid antibodies during and after pregnancy in autoimmune thyroid diseases, Clin Exp Immunol 31:30, 1978.)
Evidence of thyroid autoimmunity is seen in other autoimmune endocrine disorders such as insulin-dependent (type 1) diabetes mellitus,272 including that occurring during pregnancy.273 Up to 40% of diabetic women are thyroid antibody positive, and up to 10% are mildly hypothyroid with elevated TSH levels. Hypothyroidism does not appear to progress in diabetic pregnancy unless proteinuria develops, in which case overt hypothyroidism may ensue.274 Hypothyroidism may result from increased urinary loss of thyroid hormones as occurs with proteinuria, combined with the preexisting impaired thyroid reserve. Thyroxine therapy in this situation is appropriate but may result in an increase in insulin requirements.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








