FIGURE 146-1. Schematic representation of changing insulin requirements over the course of pregnancy and after delivery in pregestational diabetes mellitus.
(Data from Phelps RL, Metzger BE, Freinkel N: Medical management of diabetes in pregnancy. In Sciarra J (ed): Gynecology and obstetrics, vol 3, Philadelphia, 1988, Harper & Row, pp 1–16.)
In early pregnancy, there is little if any increase in insulin secretion in response to glucose. Conversely, insulin secretion in response to oral or intravenous glucose in the last trimester of pregnancy is approximately 1.5 to 2.5 times greater than that seen in nongravid conditions15 and is accompanied by islet cell hyperplasia. The product of β-cell secretion is primarily insulin and not a disproportionate amount of proinsulin or intermediates, which have substantially less activity than insulin. Insulin does not cross the placenta. Although the human placenta is small in proportion to total maternal mass, it actively degrades insulin and moderately increases insulin clearance in normal pregnancy and GDM.19,21
These changes occur temporally in parallel with increasing size of the placenta and growth of the fetus. However, the specific mediators of increased insulin secretion and insulin resistance are not entirely clear. Table 146-1 lists a number of the many factors potentially implicated in these changes. Numerous studies suggest that progesterone, acting either separately or in concert with estrogens, has direct β-cell cytotropic actions. When the two sex steroids are administered to nonpregnant animals in appropriate molar concentration ratios, effects on plasma insulin and fuel storage in liver and adipose tissue similar to those seen in normal pregnancy are observed without significantly affecting skeletal muscle sensitivity to insulin.22 Higher circulating concentrations of maternal leptin, potentially of placental origin,23 may reflect the change in insulin sensitivity rather than directly contributing to it. During the latter half of pregnancy, circulating levels of human chorionic somatomammotropin (hCS) or placental lactogen, estrogen, and progesterone reach maximal plasma concentrations with increasing placental mass.15 The concentration of pituitary growth hormone decreases,24 but the increasing level of the growth hormone variant (hGH-V) of placental origin may offset the decline.24 Prolactin also increases throughout gestation and may contribute to the insulin resistance. Free cortisol levels increase, but the diurnal variations are maintained25 despite the presence of placental corticotropin and corticotropin-releasing factor. In recent years, several other factors derived from the placenta and/or adipose tissue have been identified as potentially important contributors to insulin resistance in normal pregnancy and GDM. These include increases in tumor necrosis factor α (TNF-α)26 and decreases in adiponectin.27 Several other factors that potentially contribute to insulin resistance in type 2 DM have not been fully evaluated in normal pregnancy or GDM.28
Table 146-1. Factors of Placental Origin That May Influence Maternal Insulin Sensitivity
| Estrogens and progesterone |
| Human chorionic somatomammotropin (hCS) or placental lactogen (HPL) |
| Prolactin |
| Placental growth hormone variant (hGH-V) |
| Corticotropin-releasing factor (CRF) and corticotropin |
| Leptin |
| Tumor necrosis factor α (TNF-α) |
| Adiponectin* |
| Resistin |
| Ghrelin |
| Interleukin 6 (IL-6) |
* There is controversy about whether the placenta is or is not a source of adiponectin.
In a recent review, Friedman and colleagues29 concluded that at the molecular level, the insulin resistance of normal pregnancy is multifactorial, involving reduced ability of insulin to phosphorylate the insulin receptor, decreased expression of insulin receptor substrate 1 (IRS-1), and increased levels of a specific kinase. Further changes occur in GDM that inhibit signaling and lead to substantially reduced GLUT4 translocation. The net effect of these combined hormonal and metabolic changes is to oppose insulin action at peripheral (muscle and adipose tissue) and hepatic sites.
Utilization of Maternal Fuels by the Conceptus
The placenta is the conduit through which the conceptus continuously draws maternal fuel for its metabolic and biosynthetic needs, and glucose is the major source of its metabolic energy. In addition, glucose or three-carbon intermediates derived from glucose (lactate) are precursors for glycogen, glycoproteins, and the glyceride-glycerol in triglycerides and phospholipids of the conceptus. Glucose utilization rates as high as 6 mg/kg/min have been estimated in the human fetus at term,30 in contrast to glucose turnover of 2 to 3 mg/kg/min in normal adults. Glucose delivery across the placenta occurs by facilitated diffusion, and maternal glucose usually exceeds fetal glucose concentration by 10 to 20 mg/dL (0.6 to 1.1 mmol/L).
In the third trimester, growth of the human fetus requires the net placental transfer of approximately 54 mmol of nitrogen per day.31 Furthermore, amino acids may be used in the conceptus for oxidative energy. Although quantitative measurements of nitrogen requirement for fetal growth in humans are not available, it is clear that the fetus exerts an unremitting drain on maternal nitrogen reserves.
Although maternal triglyceride represents the largest reserve fuel depot, it can directly support the metabolic needs of the conceptus only to a limited extent. Triglycerides cross the placental barrier poorly, and the net transfer of free fatty acids (FFAs) to the fetus may be limited. Glycerol can cross the placenta readily, but its contribution in nonruminant mammalian species is probably small. Ketones readily cross the placenta, are present in the fetal circulation in concentrations approaching those in maternal blood,32 and the enzymes necessary for ketone oxidation are present in the human fetus. When fetal tissues, including the brain, are incubated in vitro with concentrations of ketones similar to those present during fasting, substantial oxidation of ketones is seen, even in the presence of alternative fuels (i.e., fasting concentrations of glucose, lactate, and amino acids).32 Oxidation of ketones lessens that of the other fuels and may spare them for biosynthetic disposition or other pathways in the fetus.33 However, such diversion to the metabolism of ketones may have adverse consequences. Ketones inhibit pyrimidine and purine synthesis in developing brain cells in the rat fetus33 and at high concentrations disrupt organogenesis in rodent embryos in culture. Controversial epidemiologic evidence suggested that maternal ketonuria during human pregnancy may be associated with reduction in the intelligence quotient (IQ) of the offspring in childhood.34 Rizzo and co-workers35 reported an inverse association between increased plasma FFAs and β-hydroxybutyrate concentrations in the second and third trimesters of pregnancy and intellectual development of offspring at age 2 to 5 years.
Circulating Concentrations of Nutrient Fuels
In Normal Pregnancy
Normal women have a decrease in the concentration of fasting plasma glucose (FPG) during pregnancy. The greatest decline in FPG (10- to 12-hour fast) occurs early in gestation,36 well before the rate of glucose utilization by the fetus is sufficient to increase total maternal glucose turnover. It has been reported that severely obese women do not show a decline of FPG during pregnancy.36 A lower FPG persists during late gestation despite relatively higher postmeal glucose levels. However, reports of diurnal glucose profiles of ambulatory pregnant women obtained by capillary blood glucose monitoring or continuous monitoring of subcutaneous fluid confirm that glycemic excursions vary within a narrow range in normal subjects, even during late gestation.37,38 Basal concentrations of plasma glycerol and FFAs do not change until late gestation, at which time significant elevations occur, and transition to the metabolic profile characteristic of the fasting state is accelerated in association with mounting lipolysis and insulin resistance.39 Progressive increases occur in all major lipid fractions, including triglycerides, cholesterol, and phospholipids.22 Total plasma amino acid concentrations also decline in early pregnancy and persist throughout gestation.40 The reasons for these changes are not clear. The suppressive effects of insulin on plasma amino acids are well known and could account for this finding. However, in later gestation, release of amino acids from skeletal muscle is less restrained by insulin, at least in pregnant rats. This suggests that during this time of insulin resistance, increased fetal removal, as opposed to impaired muscle release, may play a primary role in sustaining maternal hypoaminoacidemia.
In Gestational Diabetes Mellitus
Basal and postprandial levels of glucose, FFAs, triglycerides, and amino acids tend to exceed those of normal pregnant control subjects,41 and the changes tend to persist during dietary intervention, with the extent of the abnormalities paralleling the severity of the GDM.41 Branched-chain amino acids are sensitive to insulin, are often altered in obesity and other insulin-resistant states, and are the most consistently disturbed.41 The propensity to “accelerated starvation” (e.g., a more rapid decline in circulating glucose concentration in association with a greater increase in FFAs and ketones) in women with GDM is similar to that found in women with normal glucose homeostasis.42 Diurnal glucose profiles of ambulatory women with diet-treated GDM obtained by continuous monitoring of subcutaneous fluid show greater glycemic excursions and delay in reaching postprandial peak values than seen in normal subjects.38
In Women With Preexisting Diabetes Mellitus
In pregnant women in whom type 1 DM is well controlled, few disturbances in plasma lipids (FFAs, cholesterol, and triglycerides) have been found, and individual lipoprotein fractions have little change in their lipid content.43 The greatest departures from the norm during pregnancy occur in plasma glucose profiles; plasma amino acid concentrations also may be markedly disturbed. Changes in amino acids and indices of glycemic control (blood glucose self-monitoring records and hemoglobin A1c levels) are poorly correlated, especially in late pregnancy.44 Lipids tend to be altered more extensively in pregnant women with type 2 DM, with higher total plasma triglycerides and an increased triglyceride content of very-low-density lipoproteins.43 The cholesterol content of high-density lipoproteins may be decreased when compared with levels in normal pregnancy or in pregnant women with type 1 DM.43 The relative roles of obesity and diabetes in the development of these lipid aberrations remain to be defined. Studies of amino acid metabolism in type 2 DM in pregnancy have not been reported.
MATERNAL METABOLISM AND PREGNANCY OUTCOME
The pioneering hypothesis advanced by Pedersen45 a half century ago stated that maternal hyperglycemia leads to fetal hyperinsulinism, which is responsible for macrosomia and neonatal morbidity. Extensive experimental and clinical evidence indicates that metabolic disturbances in the mother contribute to virtually all the adverse effects of DM on the offspring.15,46,47 The importance of alterations in other metabolic fuels, in addition to glucose, was recognized later.41 Results of the HAPO Study5 indicate that the associations between maternal glycemia, fetal insulin, and parameters of fetal growth extend through the full range from “normal” to those that reflect overt diabetes. Freinkel15 emphasized the temporal relations between a metabolic insult and the adverse outcome expected (“fuel-mediated teratogenesis”) and postulated that the altered intrauterine environment of diabetes can have lifelong as well as perinatal consequences.15,46 The key features of the hypotheses of Pedersen and Freinkel are schematically integrated in Fig. 146-2.
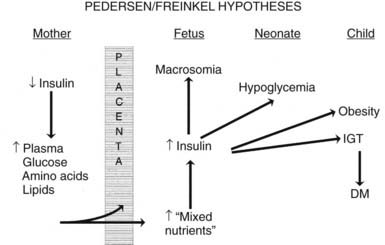
FIGURE 146-2. Effect of maternal fuels on fetal development. The classic hyperglycemia-hyperinsulinemia hypothesis of Pedersen45 has been modified to show the contribution of other insulin-responsive maternal fuels besides glucose. All of these fuels can influence the growth of the fetus and the maturation of its insulin secretion. As indicated here, altered fetal nutrients and enhanced insulin secretion are associated with consequences that extend well beyond the neonatal period.
(Data from Silverman BL, Purdy LP, Metzger BE: The intrauterine environment: Implications for the offspring of diabetic mothers, Diabetes Rev 4:21–35, 1996.)
Congenital Malformations and Early Fetal Loss
Increased risks of congenital malformations and spontaneous abortions in diabetic pregnancies are linked to metabolic control at conception.15,47 Good control during the period of organogenesis may reduce the prevalence of these adverse outcomes.47 Risk of spontaneous abortion increases in direct proportion to hemoglobin A1c concentration measured shortly before or after conception.48,49 The specific relation between metabolic control and risk of congenital malformations has been more difficult to define. Greene and associates49 found a prevalence of congenital malformation of about 5% until initial hemoglobin A1c concentrations were in excess of 10 to 12 SD of the mean control value. If put in the context of current analytical methods and reference range for pregnancy,50 this would represent hemoglobin A1c in the range of 9.5 to 10%. Beyond that, the risk of malformations increased steeply. Several groups reported that improving control of DM before conception51 reduces rates of major congenital malformations to those expected in the general obstetric population. In populations in which most pregnancies in women with diabetes are planned, congenital malformations have declined to rates similar to those of the general population.52 However, data from general population–based sources indicate that the overall risk of birth defects remains nearly 10% in pregnancies in women with preexisting diabetes.53,54
In vivo and in vitro animal models suggest that diabetic embryopathy is multifactorial.55 Oxidative stress, increased generation of free radicals, disruption of signaling pathways, including the expression and action of the Pax3 gene, or a combination of these have been implicated.53,54,56 When tight metabolic control is restored, levels of circulating serum factors that may mediate embryopathy decline more slowly than hyperglycemia and hyperketonemia.55 Hypoglycemia also is potentially teratogenic,57 so measurements of blood glucose or hemoglobin A1c may not fully reflect the “toxicity” of the maternal environment for the fetus. This lack of specificity is reflected in the fact that 60% to 70% of offspring of mothers with first-trimester hemoglobin A1c levels indicative of poor metabolic control are normally developed at birth.49 Consequently, neither the precise degree of glycemic control nor the interval over which good control must be maintained to achieve optimal outcome is known.47
Disturbances of Fetal Growth
Development of macrosomia (traditionally defined as birth weight > 4000 g or above the 90th percentile for gestational age) is the quintessential fulfillment of the Pedersen hypothesis and a frequent complication of pregnancies complicated by DM and GDM. Increased adiposity is the primary component of the macrosomia. Infants of diabetic mothers may have up to twice the body-fat content of infants of normal mothers. Increased fat content was reported in infants of mothers with GDM, even with total body weight identical to that of controls,58 and data from the HAPO Study5 showed that the risk of high infant percent body fat increased in association with higher maternal glucose concentration across the entire range of subdiabetic glucose levels. Adiposity tends to be prominent in the shoulder region, enhancing risks for cesarean delivery, shoulder dystocia, and birth trauma.59 Skin-fold measurements may be used to document adiposity at birth and reflect maternal metabolic regulation.60 However, skin-fold measurements are difficult to standardize and are seldom used in routine clinical assessment.60
Asymmetric growth is one hallmark of diabetic fetopathy. In addition to hypertrophy of subcutaneous fat, other organs responsive to insulin (e.g., the heart and liver) may be larger, whereas insulin-insensitive tissues such as the brain are of normal size. Thickness of fetal humeral soft tissue61 or cheek-to-cheek dimensions62 can be used to detect asymmetric growth caused by maternal diabetes. Some investigators use ultrasound-measured abdominal circumference that is greater than the 75th percentile to identify pregnancies at higher risk for macrosomia and target them for intensive therapy with insulin.63
Fetal hyperinsulinemia may develop early in gestation, well before adipose tissue develops.64 Morphometric studies of the pancreas from fetuses of mothers with diabetes demonstrated islet hypertrophy and hyperplasia during the second trimester. We observed a stronger association between fetal islet function near term or at birth and metabolic control in the second trimester (hemoglobin A1c concentration) than in the third trimester.65 Once initiated, β-cell overactivity may promote development of macrosomia, even without sustained elevations in nutrient fuels. Visceromegaly and fat accumulation also resulted from insulin administration to normal fetal monkeys (via implanted insulin pumps), without concurrent infusion of additional nutrients.66 The results of the HAPO Study indicate that the risk of fetal hyperinsulinemia increases in a linear fashion across the range of maternal glucose concentrations below those characteristic of overt diabetes.5 Alterations of multiple nutrient fuels65 may contribute to premature activation of fetal islet function and increases in insulin-like growth factors (IGFs).67
Historically, intrauterine growth restriction (IUGR) was a common finding in offspring of type 1 diabetic mothers. This was thought to be secondary to maternal vascular disease, resulting in uteroplacental insufficiency.45 However, in early pregnancy very poor metabolic control (in the absence of vasculopathy) may retard growth irreparably, even without associated birth defects.1 At the present time, growth restriction is rarely seen with diabetes except in pregnancies complicated by hypertension or nephropathy.
Anthropometric and Metabolic Development in Childhood
Pettitt and colleagues68 found a correlation in Pima Indian mothers between the 2-hour response to oral glucose during pregnancy and the occurrence of obesity in their offspring. Moreover, the risk of obesity was not limited to those whose birth weight was increased.69,70 Greater relative weight for height was reported at age 4 years in the offspring of diabetic mothers whose control was “poor” rather than “good” during the index pregnancy.69 In a prospective long-term follow-up study of offspring of diabetic mothers, our group at Northwestern University found that macrosomia of offspring disappeared by age 1 year. However, by age 8 years, obesity was highly prevalent; nearly half had a weight greater than the 90th percentile.69,71 Recently, Hillier and colleagues reported weight of children (ages 5 to 7 years) from a large multiethnic group cohort whose mothers had glucose challenge tests and/or glucose tolerance tests during pregnancy.72 Risk of obesity in the children increased progressively across the range of subdiabetic maternal glucose values.
In Pima Indians, by age 20 to 24 years, type 2 DM is present in 45.5% of offspring of “diabetic” mothers, 8.6% in offspring of “prediabetic” mothers, and 1.4% of offspring of “nondiabetic” mothers.73 The differences remain after adjustment for diabetes in the father, age at onset of diabetes in either parent, and obesity in the offspring (Fig. 146-3). The authors concluded, “The findings suggest that the intrauterine environment is an important determinant of the development of diabetes and that its effect is in addition to effects of genetic factors.”73 The offspring of diabetic mothers enrolled in the Northwestern University long-term follow-up had a high prevalence of impaired glucose tolerance (IGT),74 particularly during puberty. IGT developed at similar rates in the offspring of mothers with GDM and preexisting DM. Excessive insulin secretion in utero was a strong predictor of both IGT and obesity in childhood, independent of degree of obesity.74 Together, these observations indicate that in offspring of diabetic mothers, nature (genetic factors) and nurture (intrauterine metabolic environment) may interact in predisposing to obesity and type 2 DM.
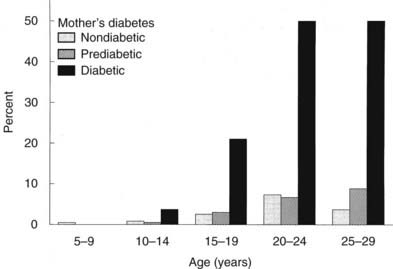
FIGURE 146-3. Age-specific prevalence of non–insulin-dependent diabetes mellitus (plasma glucose > 200 mg/dL 2 hours after oral glucose) in offspring of Pima Indian women without diabetes mellitus (open bars), those developing diabetes only subsequent to pregnancy (hatched bars), or those with diabetes during pregnancy (solid bars).
(Data from Pettitt DJ, Aleck KA, Baird HR et al: Congenital susceptibility to NIDDM: Role of intrauterine environment, Diabetes 37:622–628, 1988.)
Diagnosis and Classification
CLASSIFICATION
We advocate the scheme outlined in Table 146-2 for the classification of carbohydrate intolerance during pregnancy. It incorporates many of the recommendations of an American Diabetes Association expert committee12 and those of the Fourth11 and Fifth75 International Workshop Conferences on GDM.
Table 146-2. Classification of Glucose Metabolism
| Class | Classification Criteria* |
|---|---|
| Gestational diabetes mellitus (GDM) | Carbohydrate intolerance of varying degrees of severity that is recognized for the first time during the present pregnancy |
| GDM class A1 | Fasting glucose normal for pregnancy: venous plasma < 95 mg/dL (5.3 mmol/L) |
| GDM class A2 | Fasting glucose exceeds normal for pregnancy: venous plasma ≥ 95 mg/dL (5.3 mm/L) on at least two occasions |
| Postpartum reclassification: Evaluate as per recommendations of the Fifth International Workshop Conference on GDM (Table 146-6) | |
| Previous GDM | Abnormality of glucose tolerance in a previous pregnancy, without diabetes mellitus (DM) having been diagnosed postpartum (postpartum glucose tolerance test normal, impaired, or not performed) |
| Class A1 previous GDM | (See above for fasting plasma glucose parameters.) |
| Class A2 previous GDM | |
| Preexisting DM | DM diagnosed according to criteria of ADA Expert Committee12 when not pregnant |
| DM type 1 | |
| Uncomplicated | Absence of retinopathy, nephropathy, neuropathy, coronary artery disease, or hypertension |
| Complicated | Presence of one or more of the above (see below for designations and definitions)† |
| DM type 2 | |
| Uncomplicated | As for type 1 DM |
| Complicated | As for type 1 DM |
* Classification is based on prevailing practice in the authors’ center and the recommendations of the American Diabetes Association’s Expert Committee and the Fourth and Fifth International Workshop Conferences on Gestational Diabetes.11,12,75
† Designations and definitions of DM types 1 and 2 (designations are appended to primary diagnosis as appropriate [e.g., “diabetes mellitus type 2 uncomplicated”; “diabetes mellitus type 1; BDR, NEPH”]): BDR, Background diabetic retinopathy; CAD, coronary artery disease diagnosed by history, electrocardiogram (ECG), or stress ECG; HTN, hypertension, defined as blood pressure ≥ 140/90 mm Hg consistently; NEPH, diabetic nephropathy, defined as ≥ 0.5 g protein in 24-hr urine collection and/or serum creatinine consistently ≥ 1.2 mg/dL (≥106 mol/L); NEUR, neuropathy, defined as known gastroparesis when not pregnant, orthostatic hypotension, or sensory abnormalities in lower extremities detected at bedside examination; PDR, proliferative diabetic retinopathy.
Gestational Diabetes Mellitus
GDM is subclassified to distinguish between those with FPG values within the normal range for pregnancy (i.e., < 95 mg/dL [5.3 mmol/L]) and those with values exceeding the limits of normal (i.e., ≥ 95 mg/dL [5.3 mmol/L]). Those with higher FPG are at greater risk for progress to a diagnosis of diabetes outside of pregnancy,76 and some have arbitrarily initiated pharmacologic therapy in those with elevated FPG in the diagnostic OGTT.77 Patients are often seen who had GDM in a previous pregnancy but had no postpartum evaluation of glucose metabolism. Others may have had impaired fasting glucose or IGT postpartum, but not DM. From the perspective of proper classification and epidemiology, the diagnosis of GDM should not be assigned to such patients in a subsequent pregnancy. However, for purposes of clinical management, it is appropriate to stratify them on the basis of FPG (see earlier) and to designate them as GDM class A1, previous GDM, or as GDM class A2, previous GDM.
Preexisting Diabetes
We subdivide patients with preexisting DM into those with presumed type 1 or type 2 DM. Historically, the White classification78 was devised to predict pregnancy risk in type 1 DM based on age at onset and duration of diabetes, in combination with microvascular or macrovascular complications. In the present era, fetal loss is uncommon, and the degree of metabolic control throughout pregnancy and the presence or absence of vascular complications, independent of maternal age or duration of DM, are more specific predictors of maternal or fetal morbidity. Therefore, we currently use the White classification only for descriptive purposes. To assist in the estimation of maternal and fetal risks, we designate pregnancies in women with DM as uncomplicated (no known vascular or neuropathic complications) or complicated (one or more complications). Abbreviations for the specific complication(s) are added as a postscript. Hare79 proposed a similar although not identical classification.
Physicians would like to provide pregnant women who have complications of diabetes prospective answers to two questions: Will pregnancy accelerate or worsen preexisting complications? Does the diabetic complication per se contribute to the risk of adverse pregnancy outcome? In many cases, evidence is not sufficient to offer specific advice. These complicated issues have been reviewed in detail in a recent technical report from the American Diabetes Association.43 In the next several paragraphs, we comment on those situations for which the greatest amount of specific information is available.
Retinopathy
Diabetic retinopathy may worsen during gestation. The risk is present primarily in women with active proliferative changes or severe preproliferative retinopathy. Patients with mild background retinopathy or inactive laser-treated proliferative disease rarely experience progression of consequence. An association has been found between worsening retinopathy during pregnancy and the severity of hyperglycemia at enrollment80,81 and the magnitude of improved glycemic control achieved in the first half of gestation.80 This worsening during pregnancy may be analogous to the transient deterioration observed in nonpregnant subjects after the initiation of “tight” control of diabetes.82 Data from the Diabetes Control and Complications Trial82 indicate that pregnancy per se adds independently to the risk of transient progression of retinopathy, and the increased risk of progression may continue during the first postpartum year. Hypertension in pregnancy also is associated with progression of diabetic retinopathy.83 Regardless of the mechanisms involved, women with preexisting retinopathy should be advised of the potential for deterioration and the need for close ophthalmologic follow-up before conception, during pregnancy, and in the postpartum period. Although photocoagulation therapy can be used effectively during gestation, those with active proliferative disease should be advised to postpone pregnancy until photocoagulation treatment has stabilized the retinal condition.
Nephropathy
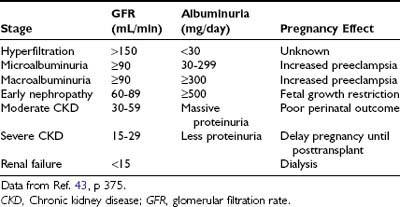
Diabetic nephropathy (24-hour urine protein ≥ 0.5 g or reduced creatinine clearance) increases risks for both the mother and offspring.43,84 Worsening proteinuria (twofold to threefold increase), hypertension, premature labor, and a need for early induction are common outcomes. The risks of these complications increase with stage of nephropathy (Table 146-3). Most women experience little permanent effect on renal function, despite transient but substantial increases in proteinuria.84,85 Occasionally, patients experience deterioration in renal function that continues in the postpartum period.43 Whether this decline is related to pregnancy or reflects the natural progression of renal impairment is uncertain. The number of subjects with severe diabetic nephropathy is too small to gain definitive information at any single center.
Neuropathy
Diabetic neuropathy is commonly found in patients with longstanding diabetes. Little is known about the effect of pregnancy on progression of diabetic neuropathy. However, autonomic neuropathy may contribute to maternal morbidity and adverse pregnancy outcome.43,86,87 Gastroparesis may result in marked glucose lability, inadequate nutrition, and maternal pulmonary aspiration. Bladder dysfunction may increase risk for urinary tract infection and worsening renal function.
Cardiovascular Disease
Both systolic and diastolic blood pressure may increase in pregnancy in type 1 diabetic women.88 In dated studies, myocardial infarction was associated with 50% mortality.89,90 An increased risk for myocardial infarction and congestive heart failure is also found in the postpartum period. The number of subjects with either longstanding type 1 or type 2 DM who experience coronary artery disease during pregnancy is small. At this time, an efficient, cost-effective strategy for detection and treatment of cardiovascular disease before and during pregnancy is not available.43
DIAGNOSIS OF GESTATIONAL DIABETES MELLITUS
Detection
The optimal cost-effective strategy for the detection and diagnosis of GDM has been the subject of much controversy for decades. In the United States and a number of other countries, the standard procedure is to do a screening blood glucose (50-gm glucose challenge test [GCT]) followed by a 3-hour OGTT in those with a positive GCT. In some other countries, an OGTT is performed as the only blood glucose test in women with a history of GDM risk factors. It is anticipated that translation of the results of the HAPO Study5 and the increasing prevalence of GDM9,10 (see Epidemiology section) will lead to a less complicated screening and diagnostic algorithm for all pregnant women that will be widely if not globally adopted. Previously diagnosed and undiagnosed type 2 DM have also increased in prevalence8 and are generally asymptomatic. Testing blood glucose concentrations in all women at the first obstetric appointment and serially throughout pregnancy is not cost effective in most populations. However, those with undiagnosed DM or IGT before conception have hyperglycemia in the first trimester and may benefit from early diagnosis and initiation of therapy. Therefore, it is important to have a comprehensive strategy for GDM diagnosis that is tailored to the population served. Participants in the Fourth and Fifth International Workshop Conferences on Gestational Diabetes Mellitus recommended that an assessment of risk for GDM as outlined in Table 146-4 be performed during the first prenatal visit.11,75
Table 146-4. Screening Strategy for Detecting Gestational Diabetes Mellitus11,75
We anticipate that strategies for detection and diagnosis of GDM will change when the HAPO Study results are applied to clinical practice, but in the authors’ opinion, universal blood glucose testing should continue to be performed in the interim unless the patient population includes a sizable proportion of women who qualify as low risk. Two points from Table 146-4 deserve emphasis. First, it is critical that women be defined as “low risk” only if they have all of the low-risk characteristics. Second, those who are identified as “high risk” should have their initial blood glucose testing at the first prenatal visit. If normal, they are to be retested at 24 to 28 weeks’ gestation. Women at “average risk” should receive a glucose challenge test (GCT) for the first time at 24 to 28 weeks’ gestation. Those with values of 140 mg/dL (7.8 mmol/L) or more go on to a diagnostic oral glucose tolerance test (OGTT).
It is important that glucose measurements on serum or plasma be made with certified laboratory techniques. Although measurement of capillary blood glucose with portable meters and reagent strips is convenient and rapid, a within-test variability of 10% to 15% markedly reduces both the sensitivity and specificity of this approach.11,75 Measurements of random blood glucose,91 hemoglobin A1c,92 or fructosamine93 also are not sufficiently sensitive for screening purposes.11 Although more palatable, screening with alternative agents such as jelly beans may be less sensitive in detecting GDM.94 Second-trimester amniocentesis for amniotic fluid insulin demonstrates reasonable sensitivity for early diagnosis of GDM95,96 but is invasive and potentially has adverse consequences.
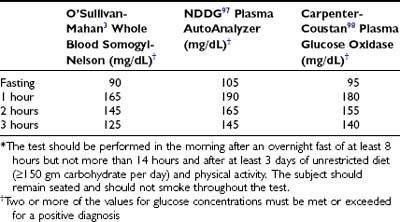
Diagnosis
Patients with an abnormal GCT receive a 3-hour 100-g OGTT, interpreted according to the criteria of O’Sullivan and Mahan3 (extrapolated to venous plasma from whole blood). These criteria for the diagnosis of GDM, initially established over 40 years ago, with minor modifications remain in widespread use today. These criteria were chosen to identify women at high risk for development of diabetes following pregnancy, not to identify pregnancies at increased risk for adverse perinatal outcomes. Others use criteria for GDM that are the same as those used to classify glucose tolerance in nonpregnant persons.4 When the National Diabetes Data Group (NDDG) developed the classification and diagnosis of DM in 1979,97 the AutoAnalyzer colorimetric (ferricyanide-based) analytic method for glucose was the “gold standard.” Currently, glucose assays are primarily enzymatic (glucose oxidase or hexokinase). Carpenter and Coustan98 derived values for interpretation of a 100-g OGTT that more accurately extrapolates the O’Sullivan results to glucose oxidase–based methods. This results in lower plasma glucose values for the diagnosis of GDM than those recommended by the NDDG and about a 50% increase in the number of women with a diagnosis of GDM.9 Several studies found that pregnancy-associated complications occurred in equal frequency in the additional pregnancies defined as GDM with the plasma glucose cut-off values recommended by Carpenter and Coustan and in those cases that also met the higher NDDG criteria.99–101 The Fourth International Workshop Conference on GDM12 and the American Diabetes Association12 endorsed the Carpenter-Coustan translation of the O’Sullivan-Mahan criteria for interpretation of the 100-g OGTT (Table 146-5). The American Diabetes Association also approved criteria for interpretation of a 2-hour 75-g OGTT that are identical to the Carpenter and Coustan levels used in the 100-g OGTT.98 These criteria are loosely based on studies of populations in the United States, Europe, Brazil, and Australia,11,102,103 but they lack validation from perinatal outcomes in a large number of pregnancies.
A number of investigators have also provided evidence that adverse pregnancy outcomes are common in women with plasma glucose levels during the OGTT that are lower than those currently used in the United States for the diagnosis of GDM. As indicated previously, the HAPO Study results showed continuous associations between perinatal outcome and maternal glycemia across the full subdiabetic range.5 In our opinion, caregivers should continue to apply the diagnostic criteria they presently use until results from the HAPO Study and others lead to new clinical guidelines.
HETEROGENEITY OF GESTATIONAL DIABETES MELLITUS
All forms of diabetes first recognized in pregnancy are defined as GDM. However, patients with GDM exhibit substantial phenotypic and genotypic heterogeneity. Based on clinical features and follow-up studies, GDM is commonly regarded as a precursor of type 2 DM.
Phenotypic Heterogeneity
Substantial heterogeneity has been observed among women with GDM in age, weight, severity of carbohydrate intolerance, and insulin secretion.76 Those who have GDM are older and heavier than their “normal” counterparts yet span the age and weight spectrum of the obstetric population. FPG levels tend to increase in GDM in parallel with the degree of obesity, but lean women with elevated FPG levels are frequently encountered. Fasting plasma immunoreactive insulin (IRI) is greater in obese subjects of both control and GDM groups (except in GDM subjects with elevated FPG).76 Controlling for the effects of age and weight indicates that the short-term (15-minute) and long-term (3-hour integrated) IRI response is attenuated in both obese and lean women with GDM, and the insulinopenia is most pronounced when the fasting plasma glucose level is elevated.76 A small number of gravida with GDM display well-preserved IRI responses to oral glucose.76 Similar heterogeneity exists with respect to the first and second phases of IRI secretion in response to intravenous glucose in GDM.17 Insulin response to mixed meals also is heterogeneous in markedly obese patients with GDM. Severe insulin resistance is characteristic of late normal pregnancy, and several reports indicated that on average, subjects with GDM are even more resistant.17,18,104 In some studies, the issue is confounded by differences in age and weight and potentially by the effects that even mild fasting hyperglycemia may exert on insulin sensitivity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree