FIGURE 51-1. Association between mean glucose level as measured by level of glycated hemoglobin (HbA1c) and presence of retinopathy in older patients (55 to 75 years) with type 2 diabetes (n = 185).
(From Nathan DM, Singer DE, Godine JE, et al: Retinopathy in older Type II diabetics: association with glucose control. Diabetes 35:797–801, 1986.)
Nephropathy
The natural history of diabetic nephropathy, although duration dependent, extends over many more years than retinopathy before clinical expression becomes evident.43,44 A minimum of 12 years, and more often 15 to 18 years, of type 1 diabetes is required before the development of clinical-grade (dipstick positive, i.e., ≥500 mg/24 hours) proteinuria, the first incontrovertible sign of end-stage renal disease. After the development of clinical-grade proteinuria, creatinine clearance declines over 5 to 10 years, terminating in end-stage renal disease.45
The reluctance to perform kidney biopsies early in the course of diabetes for documentation of microscopic changes in the glomerulus and the less than perfect correlation between microscopic changes and clinical course have led to reliance on surrogate markers of evolving nephropathy. “Incipient” nephropathy, as demonstrated by microalbuminuria (generally >20 mg to 30 mg, and <300 mg, of urinary albumin per 24 hours, or >30 µg per mg creatinine in a spot urine test), has been identified as a predictor or marker for the development of end-stage renal disease in retrospective studies of type 146–49 and type 2 diabetes.50 Unfortunately, microalbuminuria can vary considerably in individuals over time, with levels fluctuating from abnormal to normal values. Therefore, abnormal urinary albumin excretion, as defined above, in at least two of three urine collections within a 6-month period has been suggested as a definition of “persistent” microalbuminuria.51 Although the presence of or changes in microalbuminuria have been used as renal end points in many observational studies and clinical trials, a recent study has demonstrated that microalbuminuria may revert to normoalbuminuria more commonly than was previously appreciated, even in the absence of specific interventions.52
For several reasons, investigators have suspected that the association between glucose control and nephropathy may be more complex than that observed with retinopathy. The occurrence of nephropathy in no more than 40% of patients with type 1 diabetes and 25% of patients with type 2 diabetes suggests that variables other than glycemia are operant. Hypertension and family history of hypertension are mediators of nephropathy.53,54 In addition, candidate and genome-wide approaches have identified genetic risk factors for diabetic nephropathy.55,56
The association between levels of glycemia and nephropathy has been more difficult to establish than for retinopathy. Potential reasons for the difficulty in establishing a relationship between glycemia and nephropathy include the following: (1) the development of renal failure may influence glycemic control (e.g., alterations in sensitivity to insulin with development of hypertension and effects of antihypertensive medications on glycemia); (2) uremia, anemia, and transfusions may interfere with, or influence, the accuracy of measurements of glycated hemoglobin; and (3) given the long duration of diabetes before the development of renal failure, infrequent measurements of glycated hemoglobin, representing a relatively brief period of exposure, may not be predictive of the development of nephropathy. Even with these potential problems, studies have demonstrated an association between the derived glycemic index and an increase in creatinine level over time,26 or between mean levels of glycated hemoglobin, measured over 7 years, and risk of microalbuminuria in type 1 diabetes.57
Neuropathy
Quantitative electrophysiologic measures of nerve conduction have been available for more than 40 years and should have contributed to the examination of the association between glycemia and neuropathy. Unfortunately, the complex relationship between neurophysiologic studies and symptomatic clinical diabetic neuropathy has complicated the study of glucose control and neuropathy. For example, the early observation that insulin treatment of new-onset type 1 diabetes reversed slowed motor nerve conduction within 6 weeks in asymptomatic patients supported an acute effect of hyperglycemia on nerve conduction and cast doubt on the role of electrophysiologic testing.58 The absence of histologic data from peripheral nerve biopsies has been a major impediment to our understanding of diabetic neuropathy. A modest association between glycemia and motor and sensory nerve conduction has been documented in type 159 and type 260 diabetes.
Cardiovascular Disease
Cardiovascular disease (CVD), including atherosclerosis-based coronary artery, cerebrovascular, and peripheral artery disease resulting in myocardial infarctions, stroke, and foot ulcers that may require amputations, is a less specific consequence of diabetes than are the microvascular and neuropathic complications already discussed. Nevertheless, the risk for CVD is increased by twofold to fivefold in diabetic versus nondiabetic men, and by even more in diabetic versus nondiabetic women.61,62 The relatively greater impact of diabetes on CVD in women than in men may be due to the relatively greater impact of diabetes on CVD risk factors in women than in men.63,64 Both type 1 and type 2 diabetes are associated with increased risk for CVD, with the former imposing a greater relative risk than the latter. The absolute risk for CVD is far greater in persons with type 2 than type 1 diabetes, owing to their greater age and higher coincidence of other CVD risk factors, including hypertension, dyslipidemia (typically a high level of triglycerides and low high-density lipoprotein [HDL]-cholesterol level), and obesity. Autonomic neuropathy and especially diabetic nephropathy increase the risk for CVD in all persons with diabetes. 65
INTERVENTIONAL-CLINICAL TRIALS
Type 1 Diabetes
At best, observational studies can only indicate associations between glycemic control (and other confounders) and complications. The implementation of randomized, controlled clinical trials has facilitated our understanding of cause and effect in the pathogenesis of diabetic complications. Treatments designed to achieve near-normal glucose control (“intensive therapy”) were compared with conventional therapies, and their differential effects on the development and progression of complications were studied. In type 1 diabetes, intensive treatment regimens took advantage of the introduction and refinement of methods for self-monitoring of blood glucose levels and of improved methods of physiologic replacement of insulin, such as continuous subcutaneous insulin infusion (CSII) with pumps and multiple daily injection (MDI) regimens.17 Four well-designed randomized studies,21,66–68 set the stage for the larger and more comprehensive DCCT.19,69 All of these preliminary trials were secondary intervention studies that included only subjects with retinal lesions at baseline and a relatively long mean duration of diabetes. The duration of these trials ranged from 8 to 60 months, and included 30 to 100 subjects. (By contrast, the DCCT studied 1441 subjects with a mean follow-up of 6.5 years.) The total number of patient-years of study was less than 800 in the four previous secondary intervention trials combined. (The total number of patient-years for the secondary intervention component of the DCCT was almost 5000 at study end in 1993.) Except for the Oslo study,68 which included two intensive treatment groups, the studies compared type 1 diabetic patients randomly assigned to conventional treatment versus patients randomly assigned to CSII66,67 or MDI.21 The results of the Kroc,66 Steno,67,70 and Oslo,68,71 studies were similar with regard to retinopathy. In the first 6 to 12 months, a transient worsening of retinopathy occurred among patients receiving intensive treatment. Of the early trials, only the Stockholm Diabetes Study demonstrated a beneficial effect of intensive therapy over time.21
Diabetes Control and Complications Trial
In 1993, the DCCT ended the 60-year debate regarding the relationship between metabolic control and long-term complications. DCCT investigators reported consistent, unequivocal salutary effects of intensive diabetes management on the development and progression of the microvascular and neurologic complications of type 1 diabetes mellitus.19
Design
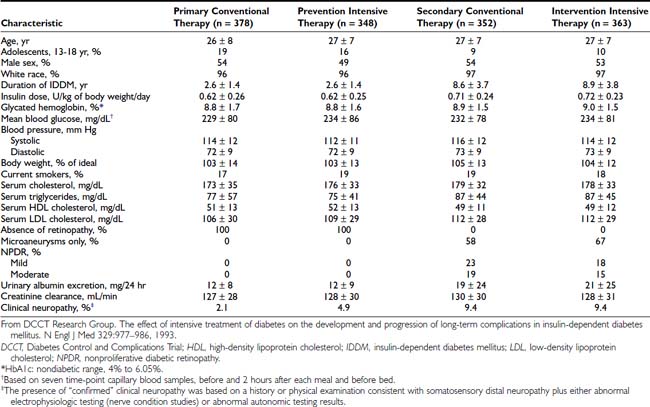
The DCCT, initiated in 1983, was designed to answer definitively whether intensive diabetes management would affect the development and/or progression of long-term complications in type 1 diabetes, and at what cost.69 The DCCT addressed primary prevention and secondary intervention of chronic complications by including two parallel studies. The primary prevention study determined whether intensive therapy aimed at achieving glycemic levels as close to the nondiabetic range as possible would prevent the development or slow the progression of complications in type 1 diabetic patients aged 13 to 39 with 1 to 5 years of diabetes duration and no evidence of retinopathy or nephropathy. The secondary intervention study determined whether intensive therapy would prevent the progression of complications in type 1 diabetic patients with 1 to 15 years of diabetes duration and at least one microaneurysm but no more than moderate nonproliferative retinopathy. They could have as much as 200 mg of albumin excretion per 24 hours (although only a small fraction had this level of albuminuria at baseline). The baseline characteristics of the two study cohorts are shown in Table 51-1. Study patients also were selected based on an assessment that they would accept random assignment of therapy, and that they were likely to continue to participate in a long-term study. On average, these patients were probably more motivated than the usual patient with type 1 diabetes. The DCCT also examined the costs, both financial and adverse events, associated with intensive compared with conventional therapy.
Intensive Treatment and Metabolic Goals
Primary Prevention and Secondary Intervention cohorts were randomly assigned to conventional therapy (designed to mimic the usual diabetes therapy at that time with one or two daily injections of insulin and daily glucose monitoring) or to intensive therapy (designed to normalize blood glucose control). Conventional therapy had the clinical goals of avoiding any symptoms of hyperglycemia or hypoglycemia, but no specific numeric blood glucose targets. Intensive therapy had the goal of achieving blood glucose control as close to the nondiabetic range as possible, including pre-meal blood glucose levels between 70 and 120 mg/dL (3.9 to 6.7 mMol/L), peak postprandial levels less than 180 mg/dL (10 mMol/L), and hemoglobin HbA1c levels in the nondiabetic range (<6.05%). In order to reach these goals, patients assigned to intensive therapy used three or more insulin injections per day or insulin pump therapy, guided by frequent self-monitoring of blood glucose levels and adjusted based on meal size, composition, and exercise. (See Table 51-2 for a description of the intensive regimen.)
Table 51-2. Diabetes Control and Complications Trial Intensive Therapy
| Self-Monitoring of Blood Glucose |
| Glycated Hemoglobin |
| HbA1c every 3 months measured centrally with HPLC assay (nondiabetic range, 4% to 6.05%) |
| Insulin |
| Supervision |
CSII, Continuous subcutaneous insulin infusion; HPLC, high-performance liquid chromatography; SMBG, self-monitoring of blood glucose.
Results
Detailed results of intensive compared with conventional therapy in the DCCT have been reported extensively.19,72–90 The initial report19 summarized the major results, whereas subsequent reports presented expanded analyses of the effects of intensive therapy on long-term complications, including retinopathy,72–74 nephropathy,75 neuropathy,76–78 and macrovascular disease and its risk factors79; the effects of intensive therapy on quality of life,80 neurobehavioral outcome,81 and residual insulin secretion82; the implementation83 and adverse effects of intensive therapy84,85; the cost-benefit analysis of intensive therapy compared with conventional therapy86; the results of intensive therapy on pregnancy87; and the association among glycemia, long-term complications, and other risk factors.88–90 A long-term follow-up study of the DCCT cohort, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, is in its 15th year, as of 2008, and is providing further insight into the long-term consequences of intensive therapy.91–98
Adherence and Metabolic Results
Over the 6.5-year mean follow-up time of the DCCT (range, 3 to 9 years), compliance was excellent, with more than 99% of the cohort completing the trial.19 In addition, virtually no crossover between assigned treatments was noted. Subjects adhered to their assigned treatment for more than 97% of study time. Intensive therapy decreased HbA1c to a nadir of approximately 6.9% by 6 months and maintained mean HbA1c levels during the remainder of the trial that were approximately 2% lower than with conventional treatment (7% vs. 9%) (Fig. 51-2). Of note, intensive therapy did not consistently normalize HbA1c, achieving levels that were, on average, four standard deviations above the nondiabetic mean. Lower glycemia achieved with intensive therapy was accompanied by a threefold increase in hypoglycemia.85
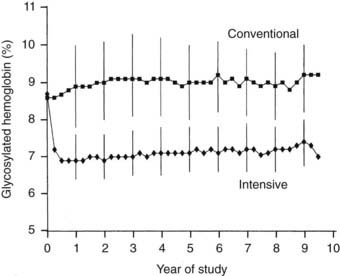
FIGURE 51-2. Hemoglobin A1c results during the Diabetes Control and Complications Trial (DCCT). Medians of all quarterly measurements with the 25th and 75th percentiles of the yearly values (vertical lines) are shown. The differences between treatment groups became significant by 3 months and remained significantly different over the course of the study (P < .001).
(From DCCT Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993.)
Retinopathy
Retinopathy was evaluated every 6 months by seven-field stereoscopic fundus photography, which was graded in a central reading unit. The principal outcome in the primary prevention study was the development of a sustained (seen on two consecutive exams) three-step or greater progression on a retinopathy severity scale adopted from the Early Treatment of Diabetic Retinopathy Study (ETDRS).99 Similarly, the principal outcome in the secondary intervention study was a sustained progression of three or more steps from the baseline level. Intensive therapy reduced the development of these end points by 76% in the Primary Prevention study and by 54% in the Secondary Intervention study compared with conventional therapy (Fig. 51-3). Other retinopathy outcomes and the effects of intensive therapy are shown in Table 51-3.
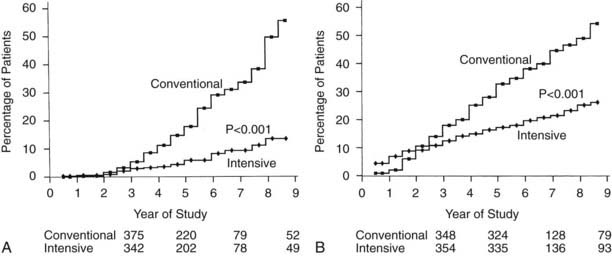
FIGURE 51-3. Cumulative incidence of Diabetes Control and Complications Trial primary retinopathy outcomes (three-step change in retinopathy from baseline value, sustained for at least 6 months) measured by fundus photography, comparing intensive and conventional therapy groups. A, Primary prevention cohort: intensive therapy reduced the cumulative incidence by 76% (P < .001). B, Secondary intervention cohort: intensive therapy reduced the cumulative incidence by 54% (P < .001).
(From DCCT Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993.)
Table 51-3. Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) Results
| Complication | During DCCT (6.5-year follow-up) | During EDIC (4.5-year follow-up after end of DCCT*) |
|---|---|---|
| Risk Reduction with Intensive Compared With Conventional Therapy, % | ||
| Retinopathy | ||
| Three-step progression | 76 | 77 |
| Proliferative | 64 | 76 |
| Macular edema | 46 | 72 |
| Laser therapy | 56 | 71 |
| Nephropathy | ||
| Microalbuminuria | 35 | 53 |
| Albuminuria | 56 | 87 |
| ≥300 mg/24 hours | ||
| Neuropathy | 60 | |
* Adjusted for presence of complication at end of DCCT.
The overall effect of intensive therapy was to decrease all stages of retinopathy included in the DCCT. The benefits were similar in almost all subgroups of patients defined by age, gender, and other baseline characteristics. However, intensive therapy was relatively more effective when initiated early in the course of diabetes (shorter vs. longer duration) and when retinopathy was less severe at baseline.19,72,73 Although intensive therapy reduced the risk somewhat less for more advanced stages of retinopathy than for earlier stages, patients with more advanced retinopathy still benefited from intensive therapy. The beneficial effects of intensive therapy were not seen for the first 3 years of therapy, presumably because of the natural “momentum” of diabetic complications. In addition, intensive therapy was associated with a transient worsening of retinopathy during the first 1 to 2 years of therapy.74 Both of these factors delayed the beneficial effects of intensive therapy in the Secondary Intervention cohort.
The EDIC follow-up has shown further improvement in retinal status in the previous intensive treatment group compared with the previous conventional treatment group (see Table 51-3). The differences in retinal outcomes between the two treatment groups persisted, and even expanded, 4.5 years after DCCT end, even though the vast majority of the previous conventional treatment cohort had changed to intensive therapy and mean HbA1c levels had drifted closer between the two treatment groups.92 The persistent benefit of 6.5 years of DCCT intensive therapy compared with conventional therapy for as long as 10 years after the end of the DCCT,92–94 during which glycemia had become similar between the original treatment groups, led to the concept of “metabolic memory.” Metabolic memory refers to the durable, imprinting effect of previous glycemic control on diabetic complications. It has been demonstrated for retinopathy, nephropathy, and neuropathy.92–94,97,98 Although the mechanism of this phenomenon remains unknown, long-lived glycated proteins may explain how previous glycemia can continue to have effects on microvascular complications.100
The study of families of DCCT volunteers that included more than one person with diabetes has revealed clustering of retinopathy within families.101 The tendency for some families with diabetes to develop retinopathy, while other families do not, most likely is mediated by genetic factors, although some as yet to be identified shared environmental factor could theoretically also play a role. Intensive therapy decreased the development and progression of retinopathy in DCCT volunteers who were members of “high-risk” families, as well as in DCCT volunteers in “low-risk” families.
Nephropathy
Nephropathy was routinely assessed by standardized measurements of albumin excretion and creatinine clearance, based on an annual 4-hour collection. The primary analytic end points for nephropathy are shown in Table 51-3 and Fig. 51-4. As with retinopathy, the risk for progression of nephropathy was reduced by intensive therapy. This included reduction in the development of microalbuminuria (>40 mg/24 hours) and clinical grade albuminuria (>300 mg/24 hours). The small number of patients who developed clinical nephropathy, defined as a creatinine clearance <70 mL/min/1.732 with albumin excretion >300 mg/24 hours, precluded a statistically valid analysis of any difference between treatment groups. However, the number of conventional treatment patients who developed this level of renal dysfunction (n = 5) was more than twice the number of intensive treatment patients (n = 2). The relatively small number of secondary intervention patients who had microalbuminuria at baseline (n = 70) made it difficult to demonstrate a benefit of intensive therapy with regard to slowing progression to clinical grade albuminuria once microalbuminuria had occurred.75 The long-term EDIC follow-up of the DCCT cohort has reinforced the role of intensive therapy in delaying and perhaps preventing diabetic nephropathy83 (see Table 51-3). “Metabolic memory” applies to nephropathy as it does to retinopathy. The widening difference in renal outcomes between the original intensive and conventional treatment groups, as long as 8 years after the end of the DCCT, has further established the benefits of early intervention in preventing nephropathy.92,93
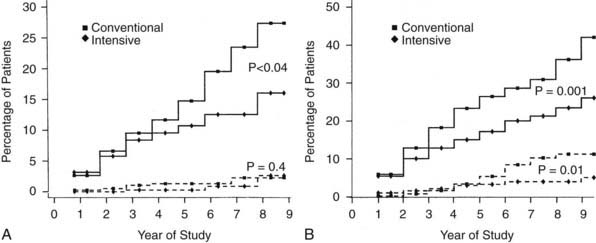
FIGURE 51-4. Cumulative incidence of renal end points in Diabetes Control and Complications Trial, comparing intensive and conventionally treated groups. Albumin excretion rate >40 mg/24 hours is shown with the solid line, and >300 mg/24 hours with the dashed line. A, Primary prevention cohort: intensive therapy reduced the mean risk for developing microalbuminuria (>40 mg/24 hours) by 34% (P < .04). B, Secondary intervention cohort: intensive therapy reduced the mean risk for developing microalbuminuria by 43% (P = .001) and the risk for clinical albuminuria (>300 mg/24 hours) by 56% (P = .01).
(From DCCT Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree