FIGURE 16-1. Demonstration of clonality of pituitary adenomas. Southern blots from extracts of gonadotroph adenomas (c lanes) and peripheral leukocytes (d lanes) of five women who were heterozygous for the HPRT gene. The peripheral leukocytes from all five women expressed both alleles of the HPRT gene, but the adenomas expressed only one allele or the other.
(From Alexander JM, Biller BMK, Bikkal H, Zervas NT, Arnold A, Klibanski A: Clinically nonfunctioning pituitary tumors are monoclonal in origin. J Clin Invest 86:336–340, 1990.)
Specific mutations have been identified in association with hereditary pituitary adenomas in multiple endocrine neoplasia type I (MEN 1), Carney complex, and familial isolated acromegaly associated with mutations of aryl hydrocarbon receptor interacting protein (AIP). In MEN 1,42 a mutation of the MEN1 gene results in decreased expression of the tumor suppressor gene menin and development of adenomas of the pituitary, parathyroids, and pancreas. All pituitary adenoma types can occur in MEN 1, most commonly lactotroph and somatotroph adenomas, and rarely including gonadotroph adenomas37,43 and those identified only as clinically nonfunctioning.44–46 In the Carney complex, about half the patients have germ line inactivating mutations in the regulatory subunit type I of the c-AMP-dependent protein kinase A gene (PRKARIA).47 The resulting phenotype consists of somatotroph adenomas, myxomas of the heart, skin, and breast, spotty skin pigmentation (multiple skin lentigines and blue nevi), schwannomas, ovarian cysts, and adrenal, testicular, and thyroid tumors. Mutations of AIP have been found in familial acromegaly in Finland48 but infrequently in familial acromegaly in other countries.
About 40% of somatotroph adenomas are associated with mutations of the gene encoding the alpha subunit of the G stimulatory protein (Gsα), and as a consequence, constitutively activating adenylyl cyclase and increasing cAMP, which is mitogenic to somatotroph cells, thereby resulting in somatotroph adenomas.49 Mutations that cause other pituitary adenomas, including gonadotroph adenomas, are not known. Investigators have searched for other mutations that might be causally related to development of other pituitary adenomas, but none of these has been clearly associated with the pathogenesis of any pituitary adenoma. Three genes have been identified that might be related to the pathogenesis of pituitary adenomas. One is the pituitary tumor transforming gene (PTTG), which was cloned from GH4 cells, a rat pituitary tumor cell line.50 It is overexpressed in the majority of human pituitary adenomas of all cell types compared with nonadenomatous pituitary tissue.51 Another is a truncated form of the fibroblast growth factor receptor 4, which has been identified in all types of human pituitary adenomas. A third is the MEG3 tumor suppressor gene, expression of which is selectively lost in nonfunctioning adenomas by hypermethylation.52
External hormonal stimulation from the hypothalamus is unlikely to be a primary cause of gonadotroph adenomas but might have a secondary effect on adenoma growth and probably has an effect on adenoma secretion, since administration of the GnRH antagonist Nal-Glu GnRH to patients who have gonadotroph adenomas and supranormal serum FSH concentrations lowers FSH levels to normal.53
Clinical Features
NEUROLOGIC FEATURES
Clinically nonfunctioning sellar masses, by definition, do not cause florid syndromes of hormonal excess and as a result often grow unrecognized until they become so large as to cause neurologic symptoms, including abnormalities of vision and oculomotor function (see Table 16-2).
Visual Abnormalities
A mass within or near the sella that stretches the optic chiasm or nerves sufficiently may result in visual impairment. Masses that arise below the optic chiasm, such as pituitary adenomas, may extend superiorly to elevate and then stretch the chiasm sufficiently to cause visual field abnormalities, initially of the upper, outer quadrants (bilateral superior quadrantopsia) and then of the outer halves (bilateral hemianopsia), before much later affecting central vision and visual acuity. Masses that arise above the chiasm may first cause asymmetric impairment of inferior visual fields. They may be reversible once the masses have been partially or completely excised.
Oculomotor Abnormalities
Masses that arise within the sella and extend into a cavernous sinus may affect one or more of the oculomotor nerves on that side, resulting in diplopia, impaired extraoculomotor movements, and/or ptosis. A dramatic example of this phenomenon is the sudden development of these findings following pituitary apoplexy, the sudden occurrence of bleeding into the pituitary or into a preexisting adenoma.
Other
Other neurologic manifestations of sellar masses are headaches, cerebrospinal fluid leakage and meningitis, and hydrocephalus. Any kind of sellar mass can cause a headache; those that grow more rapidly are more likely to do so. CSF rhinorrhea is uncommon. When it does occur, it is the result of an aggressive pituitary adenoma that extends inferiorly and erodes the cribriform plate, allowing the leakage of CSF, which in turn predisposes to retrograde infection and meningitis. Hydrocephalus is also uncommon. When it occurs it is often the result of a suprasellar lesion that obstructs the fourth ventricle.
ENDOCRINOLOGIC FEATURES
Hormonal Excess—Gonadotroph Adenomas
Serum Concentrations of Gonadotropins and Their Subunits
Although gonadotroph adenomas are typically clinically nonfunctioning, because they secrete inefficiently and because their secretory products—intact gonadotropins and their subunits—usually do not cause a clinical syndrome, they often can be identified by their basal and stimulated secretory products (Table 16-4). Gonadotroph adenomas often basally secrete sufficient intact FSH to result in a supranormal serum FSH concentration. In a series of 38 men who had clinically nonfunctioning pituitary adenomas, 10 had supranormal serum FSH concentrations.54 The degree of FSH elevation may range from minimal to 10 times the upper limit of normal. Intact FSH secreted by gonadotroph adenomas appears to be normal or nearly normal in size,55 charge,56 and biological activity in vitro.57 In contrast, gonadotroph adenomas uncommonly produce supranormal serum concentrations of intact LH, but when they do, the serum testosterone concentration is elevated.58–60 About 15% of men who have gonadotroph adenomas have supranormal basal serum concentrations of gonadotropin subunits α, FSHβ, or LHβ.54 Administration of synthetic TRH to patients who have gonadotroph adenomas often produces an increase in the serum concentrations of intact gonadotropins and their subunits, especially of the LHβ subunit.54,61
Table 16-4. Hormonal Criteria for the Diagnosis of Gonadotroph Adenomas
| Men | Women |
|---|---|
| Supranormal Basal Serum Concentrations of | |
| FSH | FSH but not LH |
| α LHβ, or FSHβ subunits | Any subunit relative to intact FSH and intact LH |
| LH and testosterone | FSH and estradiol |
| Supranormal Response to TRH of | |
| FSH | FSH |
| LH | LH |
| LHβ (most common) | LHβ (most common) |
Clinical Syndromes
Gonadotroph adenomas sometimes result in recognizable clinical syndromes (Table 16-5). One syndrome that is being recognized with increasing frequency is ovarian hyperstimulation when a gonadotroph adenoma secretes intact FSH.62–66 Continuous secretion of FSH by the adenoma, in contrast to cyclical secretion by normal gonadotroph cells, results in very large ovaries, oligomenorrhea, and multiple large cysts and widened endometrial stripe, all detected by pelvic ultrasound (Fig. 16-2). This clinical picture can be mistaken for polycystic ovarian syndrome, but administration of a superactive GnRH analog to a patient with the gonadotroph adenoma results in increased, rather than decreased, FSH secretion and ovarian size and function.67 Typically in these patients, the serum FSH concentration is elevated and the LH concentration is suppressed, and the concentrations of α subunit and estradiol are elevated. The estradiol concentration is often higher than 500 pg/mL and sometimes as high as 2000 pg/mL. Excision of the gonadotroph adenoma can lead to restoration of normal gonadotropin secretion and ovarian function, and pregnancy can occur.67,68
Table 16-5. Clinical Syndromes Associated With Gonadotroph Adenomas
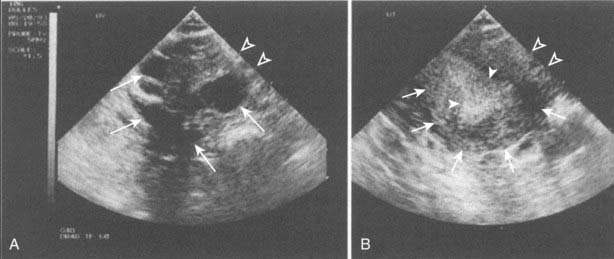
FIGURE 16-2. Ultrasound of ovaries (A) and uterus (B) in a 39-year-old woman who had a gonadotroph adenoma secreting FSH and causing ovarian hyperstimulation. In A, the closed arrows indicate large ovarian cysts. In B, the thin arrows indicate the uterus, and the wide arrows indicate the thickened endometrium. The distance between the open arrows is 1 cm.
(From Djerassi A, Coutifaris C, West VA, Asa SL, Kapoor SC, Pavlou SN, Snyder PJ: Gonadotroph adenoma in a premenopausal woman secreting follicle-stimulating hormone and causing ovarian hyperstimulation. J Clin Endocrinol Metab 80:591–594, 1995.)
Other clinical presentations of gonadotroph adenomas are less common. One is pituitary apoplexy following GnRH or GnRH analog administration to patients with a gonadotroph adenoma. Recent reports describe discovery of previously unrecognized gonadotroph adenomas when GnRH superactive analogs were administered to treat prostate cancer.69–71 Enlargement of a gonadotroph adenoma, without apoplexy, was also reported when a superactive GnRH analog was administered for prostate cancer.72 Another presentation is large testicular size in a hypogonadal man. Yet another presentation is premature puberty in a boy whose gonadotroph adenoma secretes intact LH.73,74
Hormonal Excess—Other Pituitary Adenomas
Although corticotroph, somatotroph, and lactotroph adenomas often secrete efficiently and therefore usually result in classic clinical syndromes, a minority of these adenomas secrete inefficiently and do not cause a classic clinical syndrome and therefore present as a clinically silent sellar mass. In some of these, subtle features of the syndrome can be detected, and in others no clinical features can be detected, but the serum concentration of the pituitary or target gland hormone is elevated. The latter are termed clinically silent adenomas.4,5
Hormonal Deficiencies
The large size of many sellar masses commonly causes hormonal hyposecretion due to compression of the pituitary, stalk, or hypothalamus, but these deficiencies often do not compel the patient to seek medical attention. The deficiency most likely to lead a patient to seek medical attention is that of vasopressin, due to damage to the hypothalamus or infundibulum, resulting in diabetes insipidus. Patients who have deficiencies of anterior pituitary hormones, when questioned, however, often do report symptoms. Even in the absence of symptoms, testing for hypocortisolism, hypothyroidism, and hypogonadism may detect deficiencies.
Diagnosis
Establishing the diagnosis of a clinically nonfunctioning sellar mass usually proceeds from recognizing that a patient’s visual abnormality or other neurologic symptom could be caused by a sellar lesion, to confirming the presence of the lesion by an imaging procedure, to characterizing the lesion by its hormonal features. Alternatively, the sellar mass may have been found incidentally (“incidentaloma”) when an MRI was performed because of symptoms unrelated to the mass, in which case the next step is to attempt to characterize the lesion by its hormonal features.
UTILITY OF DIAGNOSING A CLINICALLY NONFUNCTIONING SELLAR MASS
Identifying the specific type of sellar mass is desirable in distinguishing a pituitary from a nonpituitary lesion and in providing a marker by which to monitor the treatment response. Distinguishing the lesion as of pituitary rather than nonpituitary origin is of value because it influences treatment. If surgery is indicated, for example, a pituitary lesion is almost always approached transsphenoidally, no matter how large, because it is situated below the diaphragm sella, but a meningioma should be approached transcranially if it arises above the diaphragm sella. If the lesion is a somatotroph adenoma, identified by an elevated serum concentration of IGF-1, medical treatment may be an option. Finding a tumor marker such as an elevated IGF-1, characteristic of a somatotroph adenoma, or an elevated serum FSH, characteristic of a gonadotroph adenoma, not only identifies the lesion as of somatotroph or gonadotroph origin but also provides a means by which to follow the response to treatment. For example, when the serum FSH concentration is elevated prior to surgery, the decrease after surgery correlates with reduction in adenoma mass seen by imaging.75
IMAGING OF THE SELLAR REGION
Magnetic resonance imaging is currently the best imaging technique for the sellar region. If MRI shows a lesion that is clearly above the sella and distant from the pituitary, it is safe to say it is not a pituitary lesion, but for a lesion within the sella, with or without extrasellar extension, MRI does not distinguish a pituitary adenoma from other sellar lesions or one kind of pituitary adenoma from another (Fig. 16-3). Some MRI features suggest a greater likelihood of one type of lesion than another, but none is pathognomonic. For example, a sellar mass with a thin rim of tissue that emits a strong signal after gadolinium administration and a core that emits little signal suggests a cystic lesion, such as a Rathke’s cleft cyst, but a largely cystic pituitary adenoma cannot be excluded. Another example is a sellar mass that is somewhat irregular in shape and emits a heterogeneous signal, which is typical of a craniopharyngioma, but a pituitary adenoma can also present similarly. A converse example is an invasive lesion of the clivus, which is typical of a chordoma, but some pituitary adenomas extend primarily into the clivus and therefore can be mistaken for a chordoma. Because of the limitations of imaging in distinguishing types of sellar lesions, endocrinologic testing is invariably necessary.
ENDOCRINOLOGIC TESTS
Sellar mass lesions should be evaluated by measurement of serum concentrations of pituitary hormones and related target gland hormones to determine if the lesion is of pituitary or nonpituitary origin, and if pituitary, the cell of origin. A prolactin concentration above 100 ng/mL, and especially above 200 ng/mL, suggests a lactotroph adenoma76; an elevated IGF-1 concentration suggests a somatotroph adenoma even if the patient does not exhibit features of acromegaly; an elevated 24-hour urine cortisol suggests a corticotroph adenoma even if the patient does not exhibit features of hypercortisolemia; and an elevated serum T4 associated with a TSH value that is not suppressed suggests a thyrotroph adenoma.
Suspicion that the lesion is a gonadotroph adenoma depends on the absence of findings suggestive of another adenoma type, as well as the presence of specific combinations of intact gonadotropins and their subunits (see Table 16-4). The combinations differ somewhat in men and women. In a man who has a pituitary macroadenoma, elevated basal serum concentrations of intact gonadotropins and/or their subunits alone or in combination with responses of any of these to TRH is strong evidence that the adenoma is of gonadotroph origin. An elevated basal FSH concentration is common. In a woman of postmenopausal age, elevated basal serum concentrations of intact FSH or gonadotropin subunits are usually of little diagnostic value, because either the adenoma or the nonadenomatous postmenopausal gonadotroph cells could be the source. However, a gonadotroph adenoma is likely if intact FSH is markedly elevated but LH is not at all elevated, or if one of the gonadotropin subunits is distinctly elevated but intact FSH and LH are not elevated.61 More commonly, however, the diagnosis depends on finding an LHβ subunit response to TRH. In a woman of premenopausal age, ovarian hyperstimulation, including elevated serum estradiol concentration, as discussed earlier, elevated FSH out of proportion to LH levels, or elevated basal α subunit concentration all point to the gonadotroph nature of the sellar mass.
Gonadotroph adenomas can usually be readily distinguished from pituitary enlargement due to gonadotroph hyperplasia that results from longstanding primary hypogonadism. The pituitary enlargement seen with primary hypogonadism is not as prominent as that observed with gonadotroph adenomas at the time of presentation. In primary hypogonadism, LH as well as FSH is elevated, and neither intact gonadotropins nor their subunits respond to TRH.77
HISTOLOGIC EVALUATION
Even when the identity of a sellar mass cannot be determined in vivo, pathologic examination of excised tissue can usually make this identification. Pituitary adenomas have a characteristic appearance that differs from that of the normal pituitary and of other sellar masses. By light microscopy, pituitary adenoma cells do not exhibit the normal pituitary glandular pattern but are arranged in cords or sheets,78,79 sometimes interspersed with varying amounts of fibrous tissue. In an adenoma, cells are usually homogenously similar in size, but cell size varies considerably among adenomas. Immunostaining for pituitary hormones (GH, ACTH, prolactin, FSHβ, LHβ and TSHβ, and α) usually identifies the adenoma type.
APPROACH TO THE PATIENT WITH A SELLAR MASS
The clinical approach to the patient harboring a pituitary mass is compounded by the observation that the incidence of incidental silent pituitary microadenomas discovered at autopsy is between 10% and 20%. Pituitary cysts, hemorrhages, and infarctions are also not uncommonly discovered at autopsy. With the widespread sensitive imaging techniques, asymptomatic pituitary lesions are being identified with increasing frequency.80 Pituitary abnormalities compatible with the diagnosis of pituitary microadenomas are detectable in about 10% of the normal adult population. Considering the differential diagnosis of the intrasellar mass discussed previously, and recognizing that most observed lesions represent pituitary adenomas, several issues should be considered in the management of these masses. Of particular concern is whether the mass is hormonally functional, and whether local mass effects are apparent at the time of diagnosis or develop in the future.80
Evaluation of pituitary mass function is important, as the onset of symptoms and signs related to disordered hormone secretion are often insidious and may remain unnoticed for years. Clinical evaluation for changes compatible with ACTH, GH, or PRL hypersecretion or hyposecretion may reveal long-term serious systematic complications, and each may require distinct therapeutic approaches.81 In the absence of clinical features of a humoral hypersecretory syndrome, recommendations for cost-effective laboratory screening are debatable. The incidence of hormone-secreting tumors in asymptomatic subjects with incidental pituitary masses is low, and low-grade asymptomatic hormone hypersecretion (e.g., for PRL or α subunits) carries questionable long-term risk.
In the absence of evidence for hormone oversecretion, the presence of (or potential for) local compressive effects must be considered. The risk for macroadenoma enlargement towards a compressive macroadenoma is low, so that a decision to operate may be confidently postponed. For hypothalamic or parasellar masses of uncertain origin, a histologic tissue examination may be the only direct approach to yielding an accurate diagnosis. Although distinguishing MRI or CT features may be helpful in the differential diagnosis of the nonpituitary sellar mass, the final diagnosis usually remains elusive until pathologic confirmation is obtained. The benefits of pituitary surgery must also be weighed carefully against the potential side effects,82 although endoscopic approaches may now facilitate safer access to sellar tissue for histologic diagnosis or resection.83 If surgery is not indicated, subsequent imaging studies can determine the lesion’s slow growth rate, if any. In the absence of tumor growth, the interval between scans may be prolonged, and surgery should not be recommended in these asymptomatic cases. When an incidentally asymptomatic macroadenoma is diagnosed, visual field and pituitary function should be comprehensively evaluated. If these are found to be normal, imaging should be repeated. Progressive enlargement or impingement of vital structures will indicate the need for surgical intervention.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree