FIGURE 141-1. Clinical examination of the scrotum.
Orchiometry
The volume of the testis is determined by comparison with an orchiometer (normal: 15 to 35 mL).59 In the absence of varicoceles, the right and left testes are approximately equal in size. Testicular volume is related to body size and number of sperm per ejaculate. Because seminiferous tubules occupy more than 90% of the volume of the testes, impairment of spermatogenesis is commonly associated with reduced testicular size. Testicular atrophy suggests severe impairment of spermatogenesis.
Testicular Abnormalities
Pain on palpation or excessive tenderness suggests inflammation. Loss of normal testicular sensation may occur with chronic inflammations, neuropathy, or neoplasia. Reduced consistency or softness of the testes is a feature of reduced spermatogenesis. Abnormalities of shape and hard lumps suggest tumors or scars.
Epididymal Abnormalities
Palpable abnormalities include congenital absence of the vas or other failures of development, enlargements of the heads or nodules in the tails of the epididymides with obstruction, spermatoceles, and other cysts and tumors. In men with very small testes (<5 mL), small epididymides suggest severe androgen deficiency, and normal-size epididymides suggest postpubertal testicular atrophy or a severe seminiferous epithelial disorder, such as Klinefelter syndrome.
Vasal Abnormalities
Abnormalities of the vas include absence, nodules and gaps with vasectomy, and thickening or beading of the vas with severe postinflammatory scarring, as from tuberculosis.
Miscellaneous Abnormalities
Incidental scrotal findings include scars from surgery, scrotal dermatitis, and pubic fat pads around the genitals in extreme obesity. Inguinal hernias and lipomas and encysted hydroceles of the cord are palpated above and behind the epididymis. Cysts (“hydatids”) of the appendix testis or epididymis are typically anterior to the head of the epididymis. Spermatoceles and cysts of the paradidymis are in the head or body of the epididymis. Retroversion of the testes is common where the vas and epididymis are anterior rather than posterior to the testes. Hydroceles of mild degree are common. A tense hydrocele may hide a testicular tumor. Unilateral absence of the vas may be associated with ipsilateral agenesis of the kidney and ureter on the same side. Many of these anomalies have little relationship with infertility.
Checking for Varicocele
With the man standing up, the scrotum can be inspected for swelling of the pampiniform plexus and a cough or Valsalva impulse seen or palpated by holding the spermatic cords between the thumb and index finger of each hand and elevating the testes toward the external inguinal ring (see Fig. 141-1). This maneuver reduces the risk of confusing contractions of the cremaster muscles with venous impulses. Varicocele size is graded: cough impulse without palpable enlargement of the spermatic cord (grade 1), palpable enlargement (grade 2), and visible enlargement (grade 3). Although predominantly a left-sided condition, varicoceles may occasionally be on the right side.
The accuracy and reproducibility of clinical examination, even for structures as accessible as those in the scrotum, may not be high. Varicoceles may vary in size from day to day. Even absence of the vasa may be overlooked. With practice, orchiometry can be repeated to within one orchiometer size.
Semen Analysis and Other Investigations
Investigations are outlined in Table 141-3.
SEMEN ANALYSIS
The most important laboratory investigation in male infertility is semen analysis. The variables assessed and the methods are in the World Health Organization’s laboratory manual.60 A new edition was published in 2010. Automation of semen analysis is in progress and should be used in most specialized laboratories soon.10
It is crucial that the laboratory is experienced in the performance of semen analyses and participates in quality-assurance activities.61 There should be a room nearby for the collection of semen. Semen may be obtained by masturbation or coitus using a special nontoxic condom. Ordinary latex contraceptive condoms are unsatisfactory because the rubber usually immobilizes the sperm.62 If these methods of collection are not possible, postcoital examination of midcycle cervical mucus may give some information about the likelihood of adequate semen quality if many motile sperm are found. In contrast, a negative postcoital test on its own is of little diagnostic value because conception can occur in the same cycle.63
The man should be provided with a wide-mouth, sterile, and nontoxic collection jar and written instructions about collection and delivery to the laboratory. A period of abstinence from ejaculation from 2 to 5 days, delivery of the sample to the laboratory within 1 hour of collection, and avoidance of exposure to lubricants or extremes of temperature are specified.
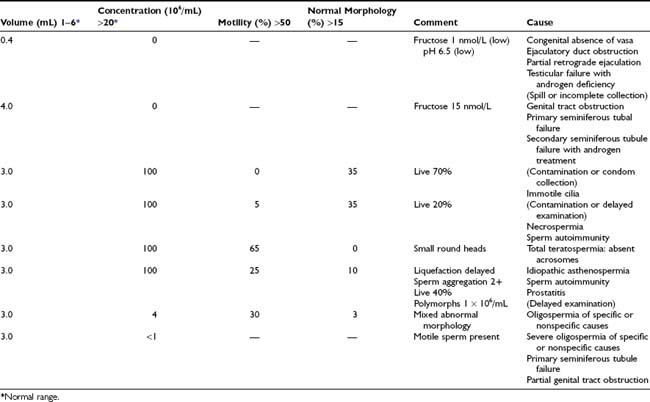
Because of the variability of results, several semen analyses at intervals of 2 or more weeks are necessary in a man with an abnormality in the first test. Even with complete collection of samples, there is variability caused by counting error, other technical errors, and differences in the ejaculate from day to day (Fig. 141-2).60,64 These large variations need to be remembered when interpreting results of semen analysis.
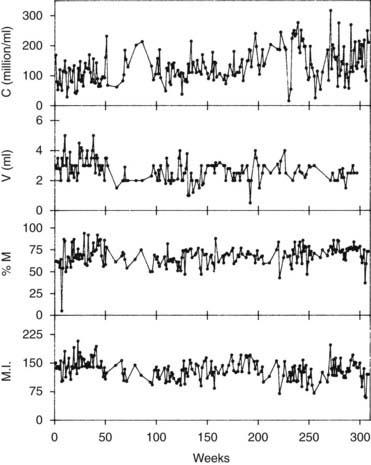
FIGURE 141-2. Variability of semen analysis results in a fertile sperm donor. C, Sperm concentration; M, total motility; M.I., motility index-product of grade and percentage of sperm with progressive motility graded 0 to 3; V, semen volume.
(Data from Mallidis C, Howard EJ, Baker HWG: Variation of semen quality in normal men, Int J Androl 14:99–107, 1991. Used by permission, Blackwell Scientific Publications.)
To check for retrograde ejaculation, urine collected immediately after ejaculation is centrifuged and the pellet examined for sperm.
Assays of semen constituents from the accessory glands and testis are available: zinc and acid phosphatase from the prostate, fructose from the seminal vesicles, neutral α-glucosidase, glycerophosphocholine, and l-carnitine from the epididymis and inhibin B from the Sertoli cells. Prostatic fluid is acid (pH approximately 6.0), but the ejaculate is alkaline because of the admixture with seminal vesicle fluid. Semen biochemistry is of limited usefulness in clinical practice. Some examples are given in Table 141-5.
Immunobead Test
Tests for sperm antibodies should be done routinely on all men being evaluated for infertility, because no semen analysis pattern is characteristic of sperm autoimmunity.35,60 The immunobead test (IBT) with beads binding to more than 50% of motile sperm is regarded as positive, but there is usually more than 70% to 80% immunoglobulin A (IgA) bead binding with clinically significant sperm autoimmunity. Tail tip–only IBT binding is not significant.65 The mixed antiglobulin reaction test is an alternative to the IBT.60 The indirect IBT in which normal donor sperm are exposed to test serum or seminal plasma can be used to test men with too few motile sperm for the direct IBT. An alternative screening method for sperm autoimmunity in men with sperm in the semen would be to perform a sperm–mucus penetration test.60
Sperm–mucus penetration tests can be performed by postcoital examination of sperm in cervical mucus collected at midcycle or after estrogen treatment (ethinyl estradiol, 50 µg twice daily for 4 days) to produce mucus of equivalent quality.60 In vitro capillary mucus penetration (Kremer) tests are particularly important for evaluating the significance of sperm autoantibodies; failure of sperm to penetrate more than 2 cm in 1 hour indicates severe sperm autoimmunity with a poor prognosis and a high likelihood of failed fertilization with standard IVF.35,65
Sperm Function Tests
A number of tests of sperm function are available to examine the human fertilization process (Fig. 141-3). These are only performed in specialist laboratories. If simpler approaches or active preparations of zona pellucida (ZP) or sperm receptor proteins become available, they will be widely used to improve the assessment of human sperm. IVF has permitted many conventional and new tests of sperm function to be examined. Groups of sperm variables that are independently significantly related to the proportion of oocytes that fertilize in vitro can be determined by regression analysis.66 This approach has confirmed the importance of sperm morphology in the ability of sperm to interact with the coverings of the oocyte.
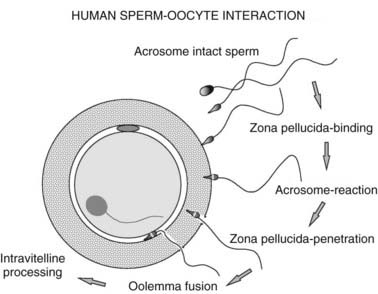
FIGURE 141-3. Stages of human fertilization. Spermatozoa swim through the surrounding medium and cumulus mass (not shown) and bind to the surface of the zona pellucida. The acrosome reaction is stimulated by zona proteins and the acrosome-reacted sperm penetrates the zona, enters the perivitelline space, and binds to the oolemma via the equatorial segment. Oocyte processes surround the sperm head, and it enters the ooplasm and decondenses. Infertility could result from defects of any of these processes. For example, abnormal sperm, particularly with defective head morphology, bind poorly to the zona.
Human Sperm–Zona Pellucida Binding Ratio Test
Because the number of sperm bound to the ZP is strongly related to the fertilization rate, human sperm–ZP interaction tests have been developed using oocytes that failed to fertilize in vitro.66 These oocytes can be used either fresh or after storage in concentrated salt solutions. Because the ZP binding capacity is variable, control (fertile donor) and test sperm are labeled with different fluorochromes (fluorescein and rhodamine). After incubation with equal numbers of control and test sperm, the oocytes are aspirated through a wide bore pipet to dislodge loosely adherent sperm, and the numbers of sperm tightly bound to the ZP are counted with a fluorescence microscope. Results are expressed as a ratio of the number of test and control sperm bound to the ZP of four oocytes. An alternative method is to cut the zonae and expose half to the test and the other half to control sperm (Hemizona assay).67
Human Sperm–Zona Pellucida Penetration Test
It is difficult to determine the number of sperm penetrating the ZP when many sperm are bound to the surface. The sperm bound to the surface of the ZP can be sheared off by repeatedly aspirating the oocyte with a pipet with an internal diameter less than the diameter of the oocyte (120 µm). The sperm penetrating the ZP or perivitelline space can then be counted easily, and the results of this test are the most predictive of fertilization rates with standard IVF.66
Zona Pellucida–Induced Acrosome Reaction Test
Sperm dislodged from the ZP can be stained with a fluorescein-labeled lectin such as Pisum sativum agglutinin or an antibody specific for the acrosomal contents to determine the proportion that are acrosome reacted. This test is useful for diagnosis of disordered ZP-induced acrosome reaction.66
Human Sperm–Oolemma Binding Ratio Test
Sperm-oolemma binding has been studied in a similar way to the sperm-ZP binding test, using oocytes that have had the ZP removed.66
Interpretation of Semen Analysis Results
Table 141-5 shows various patterns of abnormality of semen quality and their common causes. It is always important to consider whether the result is spurious. Repeated tests are necessary to establish an average and to determine the variability within an individual man (see Fig. 141-2).
Variations in Semen Volume and Appearance
Low semen volume suggests incomplete collection, short duration of abstinence from ejaculation before the test, absence or obstruction of the seminal vesicles, or androgen deficiency. High semen volume (>8 mL) may be seen in association with oligospermia but is of little practical significance. Hemospermia is usually the result of minor bleeding from the urethra, but serious conditions, such as genital tract tumors, must be excluded. Discoloration of the semen may indicate inflammation of accessory sex organs. The semen may be yellow with jaundice or Salazopyrin (sulfasalazine) administration. Defects of liquefaction and viscosity are relatively common and presumably result from malfunction of the accessory sex organs. Although these may cause problems with semen analysis and preparation of sperm for assisted reproductive technology (ART), they are probably of little relevance to fertility. Sperm agglutination is common with sperm autoimmunity but can also occur for other reasons.
Azoospermia
The total absence of sperm from the semen needs to be confirmed in repeated tests with vigorous centrifugation of the semen and careful examination of the pellet.60 Rarely, an illness or difficulty with collection will cause transient azoospermia; however, this can also occur for unexplained reasons. With severe spermatogenic disorders and some obstructions, sperm may be present in the semen intermittently. If any live sperm can be found, these can be cryopreserved for intracytoplasmic sperm injection (ICSI).
Oligospermia
Sperm concentrations of less than 20 million/mL are classified as oligospermic.60 This figure probably derives mainly from the work of MacLeod and Gold, who found that only 5% of fertile men had sperm concentrations less than 20 million/mL.68 Recent studies of fertile men generally support 20 million/mL as a clinically useful figure, although the new edition of the WHO semen analysis manual will suggest lowering the cutoff to 14 million/mL and also lower reference values for sperm motility and normal morphology.9,69–71 There is a correlation between sperm concentration and other aspects of semen quality. Both motility and morphology are usually poor with oligospermia.
Asthenospermia
Asthenospermia is defined as less than 50% sperm motility or less than 25% with rapid progressive motility.60 Spurious asthenospermia caused by exposure of sperm to rubber (particularly condoms), spermicides, extremes of temperature, or long delays between collection and examination should be excluded. Low sperm motility is a frequent accompaniment of oligospermia and is often also associated with a mixed picture of morphologic defects suggesting defective spermiogenesis.
Specific ultrastructural defects of the sperm can be evaluated by electron microscopy when there is zero sperm motility or extreme asthenospermia (less than 5% motile sperm).43,72 Absent dynein arms, other axonemal defects, mitochondrial abnormalities, disorganized fibrous sheath or outer dense fibers, or normal ultrastructure may be found. Standard semen analyses usually show normal sperm concentrations and morphology, but there may be tail abnormalities: short, straight, or thick tails or midpiece defects. Viability tests help to distinguish this group of patients from those with necrospermia.73 Patients with structural defects in the sperm may be able to be treated by ICSI. Asthenospermia may also be associated with sperm autoimmunity. The causes of other motility defects of moderate degree are unidentified.
Necrospermia
It is important to distinguish necrospermia from other types of severe asthenospermia, because some patients produce pregnancies despite low sperm motility.33,73–75 Necrospermia is characterized by usually less than 20% to 30% total motility, less than 5% progressive motility, and a viability test less than 30% to 40%, indicating a high proportion of dead sperm. Other causes of severe asthenospermia such as sperm autoimmunity and collection problems must be excluded. Necrospermia may fluctuate in severity, particularly with changes in coital frequency.73,75 Characteristic of necrospermia is an improvement of sperm motility with increased frequency of ejaculation. The condition may be caused by defective storage of sperm in the tails of the epididymides or stasis in the genital tract, and it also occurs with chronic spinal cord injury and with adult polycystic kidney disease associated with cysts in the region of the ejaculatory ducts.33,74 There are ultrastructural features of degeneration in the ejaculated sperm but normal structure of late spermatids in testicular biopsies.73,74 Treatment with antibiotics may have a beneficial effect, but this is not proved. The couple should have intercourse once or twice every day for 3 to 4 days up to the time of ovulation.
Teratospermia
Teratospermia is a reduced percentage of sperm with normal morphology assessed by light microscopy.60 It is important to distinguish mixed abnormalities of sperm morphology from those in which all or the majority of sperm show a single uniform defect, such as spherical heads with absence of the acrosomes (globospermia) and pinhead sperm. Pinhead sperm result when the centrioles from which the sperm tails develop are not correctly aligned opposite the developing acrosome. On spermiation, the sperm heads are disconnected from the tails and absorbed during epididymal transit so that there are only sperm tails in the ejaculate, the cytoplasmic droplet on the midpiece giving the pinhead appearance.76 Both these conditions cause sterility but are extremely rare.
In general, human spermatozoa are very variable in appearance and the microscopic assessment of sperm morphology is highly subjective and difficult to standardize between laboratories. Only a small proportion (<25%) of the motile sperm from fertile men are capable of binding the ZP in vitro, and this zona binding capacity is closely related to the morphology of the sperm head.77 The morphometric characteristics of the sperm that bind to the ZP may be useful as a standard for sperm morphology.10,78 Various histologic assessments of morphology have been used. The simplest is to record as normal only those sperm that have no shape defects in head, midpiece, or tail regions.60 In the strict morphology approach, although size measurements are set, the sperm are assessed by eye and those marginally abnormal are assigned abnormal. Automated methods involving image analysis by computer have been developed that could overcome the between-laboratory variability and greatly improve the predictive value of semen analysis for natural conception.10,78
Before the introduction of ICSI, the percentage of sperm with normal morphology assessed by strict criteria after washing the sperm and adjusting the concentration to 80 million/mL provided one of the most useful predictors of fertilization rates with standard IVF.60 There was a progressive reduction in oocytes fertilized from 60% to 20% as abnormal morphology increased from less than 70% to more than 95%.79 Patients with high proportions of sperm with abnormal morphology are now treated by ICSI because of the risk of failure of fertilization with standard IVF. ICSI results are independent of sperm morphology.
HORMONE ASSESSMENT
While it is not necessary to perform hormone measurements routinely in all infertile men, follicle-stimulating hormone (FSH), LH, and testosterone may be valuable in men with sperm counts of less than 10 million/mL. Normal FSH levels in patients with azoospermia, normal testicular volume, and normal virilization may help distinguish genital tract obstruction from a spermatogenic disorder. However, some men with primary seminiferous tubule failure have normal FSH levels, and a normal FSH is found in about 50% with germ cell arrest at the primary spermatocyte stage. Rarely, high FSH levels are seen with normal spermatogenesis.80 To distinguish primary from secondary hypogonadism, measurement of FSH, LH, and testosterone is useful in men with reduced testicular volume and signs of androgen deficiency. Inhibin B measurement may provide additional information about the state of spermatogenesis.81
Prolactin should be measured in men with galactorrhea or androgen deficiency and loss of libido.82 Other hormone investigations are occasionally required, such as thyroid function tests with hyperprolactinemia, 17-hydroxyprogesterone measurements with congenital adrenal hyperplasia, estradiol with liver disease or tumors, hCG with tumors and estrogen excess, and pituitary function tests for panhypopituitarism.36
CHROMOSOME AND GENETIC STUDIES
At present, it is reasonable to screen all infertile men with otherwise unexplained primary spermatogenic defects with average sperm concentrations less than 10 million/mL by karyotype and Yq microdeletion testing. All patients should be counseled about the possibility of transmitting known and unknown genetic defects.
Karyotypes are performed in men with clinical evidence of primary testicular failure and small testes to confirm a clinical diagnosis of Klinefelter syndrome. Usually the karyotype is 47,XXY, but there may be higher numbers of X chromosomes or a sex-reversal 46,XX karyotype.83–85 Although most men with Klinefelter syndrome produce no sperm in the semen, some are oligospermic and very rarely fertile.83 Also, sperm for ICSI may be obtained by testicular biopsy in 50% of patients.84,85 Defective spermatogenesis may occur with 47,XYY, but the clinical picture is much less uniform than it is for Klinefelter syndrome. The extra sex chromosome is deleted early in gametogenesis because the sperm, embryos, and children generally have normal karyotypes. However, an increased rate of sex chromosomal and autosomal aneuploidy has been noted in studies of sperm from XXY and XYY men.85,86 Some Y-chromosome abnormalities, such as small Y chromosomes and an isochromosome of two short arms, are associated with severe spermatogenic damage. Microdeletions in the long arm of the Y chromosome have been found in 3% to 15% of men with severe primary spermatogenic disorders.11,12,20,29,30 Sons of men with these microdeletions have the same microdeletions.87 There is an increased frequency of autosomal abnormalities with defective spermatogenesis, particularly balanced autosomal translocations (reciprocal and Robertsonian), which may be transmitted in unbalanced form to offspring.88
Cystic fibrosis gene studies are important for evaluation of patients with congenital absence of the vas, as well as their partners.89 If the woman has a cystic fibrosis gene mutation, preimplantation genetic diagnosis of their embryos can be offered. Androgen-receptor defects have also been found in some men with unexplained primary spermatogenic disorders. Mutations in the gene impairing androgen-receptor activity produce androgen insensitivity, which has a variable phenotypic expression from testicular feminization to otherwise normal males with gynecomastia or hypospermatogenesis and oligospermia.26 Increases in the number of CAG repeats in exon 1 over approximately 40 are associated with Kennedy disease (progressive spinobulbar atrophy), and men with this condition may be infertile.
Other specific genetic tests and family studies may be indicated on clinical grounds (see Table 141-4).
TESTICULAR BIOPSY
Testicular biopsies are necessary to assess spermatogenesis in men with presumed genital-tract obstruction. A significant proportion of men with azoospermia, normal testicular size, and normal FSH are found to have severe spermatogenic disorders.7 Some severe spermatogenic defects may be incomplete, and because ICSI can be performed if sperm can be obtained from the testes, diagnostic testicular biopsies should be offered to men with severe primary spermatogenic tubule disorders with persistent azoospermia. If any elongated spermatids can be found, it should be possible to perform ICSI. However, if no elongated spermatids are seen in the diagnostic biopsies, it still may be possible to find spermatids by more extensive sampling of testicular tissue with open biopsies (see later).
It is most important that tissue for histology is removed from the testes with minimal damage and placed in a suitable fixative, such as Bouin’s or Steive’s solution. Standard formalin fixatives destroy the cytoarchitecture.
Testis biopsies may be performed under local or general anesthesia. Needle biopsy many obtain only isolated cells, but these may be sufficient for diagnosis based on cytology or for flow cytometry. The technique shown in Fig. 141-4 usually provides sufficient material for a histologic diagnosis of the state of the seminiferous epithelium, despite some deformation artifacts.90 It is also useful for obtaining testicular sperm for ICSI.91 Complications include failure to obtain tissue, particularly with fibrosed or small (<5 mL) testes, minor bleeding in the skin and testis, and rarely hematoma or reactions to the local anesthetic.
FIGURE 141-4. Fine-needle tissue aspiration biopsy of the testis. A, Local anesthetic is injected around the vas to block testicular sensation. B, A 21-gauge butterfly needle is inserted into the testis. An assistant applies suction to the needle tubing via a syringe, and the operator makes thrusting movements of the needle into the substance of the testis. The appearance of some tissue fluid in the tubing (1 to 2 mm) usually indicates adequate tissue has entered the needle. While maintaining the suction, the needle is removed carefully, and any seminiferous tubules protruding from the needle are grasped with fine forceps to avoid their falling back into the puncture hole. C, Seminiferous tubule sections sucked into the needle are expelled into culture medium. Portions can be sent for histology or used for extraction of sperm for intracytoplasmic sperm injection (ICSI) by stripping the seminiferous tissue out of the connective tissue membrane of the seminiferous tubule.
For clinical purposes, testicular histology is classified as follows: normal or hypospermatogenesis (all the cellular elements of spermatogenesis are present but in reduced numbers), germ cell arrest (the initial cellular elements of spermatogenesis are present but at a certain stage, the process stops, most often at the primary spermatocytes), Sertoli-cell-only syndrome or germ cell aplasia (the tubules contain Sertoli cells but no germ cells), hyalinization (the cellular elements have disappeared, leaving only thickened seminiferous tubule walls as in Klinefelter syndrome), and immature testis (no gonadotropin stimulation, prepubertal appearance).92 Examples are shown in Fig. 141-5. Other classifications such as partial or incomplete maturation arrest and partial germ cell aplasia cause confusion in the literature and should not be used.93
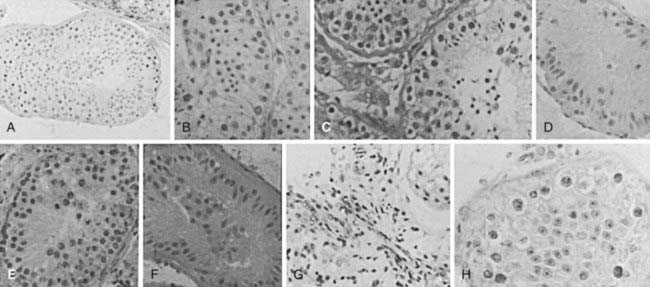
FIGURE 141-5. Testicular histology from fine-needle aspiration samples. A, Normal (×200); B, mild hypospermatogenesis (×500); C, moderate hypospermatogenesis; D, severe hypospermatogenesis; E, germ cell arrest at the primary spermatocyte stage; F, Sertoli-cell-only syndrome; G, Leydig-cell tumor in an undescended testis with hyalinized Sertoli-cell-only tubules; H, carcinoma in situ, only transformed spermatogonia and Sertoli cells present.
OTHER INVESTIGATIONS
Ultrasonography is useful to check for tumors in the testes, particularly when the testes are difficult to palpate because of a tense hydrocele.94 It can also be used to measure testicular size and confirm the presence and nature of cysts or other abnormalities in the scrotum. Some argue scrotal ultrasound should be performed routinely in infertile men to exclude impalpable malignant tumors in the testes.95 Doppler blood-flow assessment is valuable in assessing a painful swollen testis for torsion or inflammation and for evaluating varicoceles. Other tests of a varicocele, including thermography, technetium scans, and venography may be performed but, as pointed out later, the value of treating varicoceles to improve fertility is uncertain. Rectal ultrasound may demonstrate cysts in the prostate, enlarged seminal vesicles, or dilated ejaculatory ducts associated with distal genital tract obstructions.96 Clinical suspicion of the presence of a pituitary tumor should be followed up by appropriate radiology. Abdominal imaging is necessary to check the position of impalpable testes.
Management of Specific Conditions
This section addresses the management of sperm autoimmunity, male genital tract obstructions, coital disorders, genital tract inflammation, and varicocele. Treatment of gonadotropin deficiency and androgen replacement therapy are covered in Chapters 122 and 139.
SPERM AUTOIMMUNITY
Sperm autoimmunity is present in 6% of infertile men.35 They have sperm coated with antibodies to the extent that sperm function is impaired, particularly sperm–mucus penetration and sperm–zona pellucida binding, resulting in severe infertility. Natural pregnancy rates without treatment are very low, and fertilization rates with standard IVF are low or zero. Other men have positive IBT, sometimes with tail tip–only binding and normal or only marginally impaired mucus penetration.65 These low-level sperm autoantibodies are probably irrelevant to the infertility, and other causes of the couple’s infertility should be sought. Sperm autoimmunity can be treated by glucocorticoids in immunosuppressive doses. Antibody levels fall, and semen quality improves in about 50% of patients; about 25% produce natural conceptions during a 4- to 6-month course of treatment.35 There are significant risks of severe side effects, particularly aseptic necrosis of bone. The superior results of ICSI make this treatment obsolete and only useful in exceptional circumstances.
GENITAL TRACT OBSTRUCTION
Clinical Characteristics
Most men with genital tract obstruction have azoospermia, normal testicular size, normal virilization, and normal serum FSH levels. However, some have combined obstruction and spermatogenic disorders or partial obstructions and severe oligospermia. There may be a history of an event that caused the obstruction, such as epididymitis with gonorrhea or associated respiratory disease. Because a few men with normal spermatogenesis have elevated FSH levels and some spermatogenesis may occur in association with a severe spermatogenic disorder, all patients should be offered further investigation. The presence of sperm antibodies in blood serum by indirect IBT indicates sperm are being formed but is an adverse prognostic factor for successful surgery.
With bilateral congenital absence of the vasa or ejaculatory duct obstruction, semen volume, pH, and fructose levels are low. The semen also does not have its characteristic smell and does not form a gel after ejaculation because it contains only prostatic and urethral fluid. Rectal ultrasound shows absent or atrophic seminal vesicles with bilateral congenital absence of the vasa; but with ejaculatory duct obstruction, the seminal vesicles and ejaculatory ducts are dilated, and the cause of the obstruction may be obvious, such as a cyst of the prostatic utricle.96 Testicular biopsy is usually normal, but there may be some reduction in spermatogenesis, either as a coincidence or as a result of the obstruction, particularly after vasectomy.97
Pathophysiology
Degeneration of the Wolffian duct structures occurs with cystic fibrosis gene mutations but can be of variable extent. Although most often only the heads of the epididymides are palpable, some men with bilateral congenital absence of the vasa have parts or all of the epididymides and scrotal vasa present, with absent or atrophic pelvic vasa and seminal vesicles. Young’s syndrome, which is now rare, is not related to cystic fibrosis gene mutations. The pathology shows inspissated material in the head of the epididymis, and there are lipid inclusions in the epithelial cells.41,42 Since some men with this syndrome have fathered children, the blockage may develop in adulthood.
Postinflammatory obstructions after gonorrhea typically occur in the tail of the epididymis, whereas nonspecific bacterial inflammation produces more widespread destruction, and tuberculosis usually causes multiple obstructions in the epididymides and vasa or destruction of the prostate and seminal vesicles. Back-pressure blowout obstructions in the epididymis are frequent after vasectomy. Iatrogenic causes of genital tract obstruction include inadvertent epididymectomy during testicular biopsy, vasal damage during hernia repair or pelvic or lower abdominal surgery such as renal transplantation, and ejaculatory duct obstruction from prostatectomy or complicated bladder catheterization.
Differential Diagnosis
Men with persistent azoospermia, normal testicular size, normal virilization, and normal FSH levels can be assumed to have obstruction until proved otherwise. As many as a third of men with this clinical picture are found to have a serious spermatogenic disorder on testicular biopsy, despite the normal serum FSH level.7 There are rare instances of normal men who show azoospermia on single occasions or over a short period.6,13 This “spurious azoospermia” must be excluded before surgery is contemplated. Once diagnosis of obstruction is confirmed, it is necessary to determine the feasibility of surgery. Intratesticular and caput-epididymal obstructions have a poor prognosis, but cauda-epididymal and vasal obstructions can often be treated successfully with surgery.98 Distal obstructions are important to diagnose because they may be reversed at transurethral endoscopy.96 It is important to test for sperm in urine after ejaculation in patients with possible ejaculatory duct obstruction, because partial retrograde ejaculation can produce the same the semen characteristics as ejaculatory duct obstruction (see Table 141-5). Also, large cysts causing ejaculatory duct obstruction can affect the bladder neck and cause retrograde ejaculation.
Treatment
General Management
Female partners of men with bilateral congenital absence of the vasa should be screened for cystic fibrosis gene mutations and the couple counseled accordingly. Preimplantation or prenatal genetic diagnosis may be performed if mutations are found in both partners. The woman should be investigated in detail to ensure her potential fertility before surgery is contemplated in the man. The prognosis of the procedure and the availability of other forms of treatment, including donor insemination, should be discussed with the couple. Sperm retrieval for ICSI, either from the testis or other parts of the genital tract, is an alternative to surgery.91 ICSI is also used when reconstructive surgery is not possible, the female partner has an infertility problem, or the couple cannot wait 6 to 12 months to have a reasonable attempt at conceiving naturally after surgery. For ICSI, sperm may be obtained by testicular biopsy or percutaneous sperm aspiration from the epididymis under local anesthesia. If a spermatocele is present, usable sperm may be obtained by direct puncture through the scrotal skin. It may be possible to combine vasoepididymostomy with sperm aspiration for cryopreservation or ICSI.
Epididymal and Vasal Surgery
Treatment of male genital tract obstructions is best undertaken by specialist microsurgeons.98 The testis is exposed and the most proximal (to the testis) level of obstruction determined. The patency of the vas is determined by syringing with saline or by vasography. The vas or epididymal tubule is opened proximal to the obstruction, and if possible, the presence of motile sperm is demonstrated by microscopy. Microsurgical anastomosis between the ends of the vas or between the vas and the epididymal tubule is then undertaken.
Results
Vasovasostomy and vasoepididymostomy for caudal blocks produce relatively good results, with 50% to 80% of patients having sperm present in the semen. However, less than half of these produce a pregnancy within the first year.98 There may be continuing obstruction, sperm autoimmunity, or coexisting spermatogenic disorders. The results of vasoepididymostomy for proximal blocks are poor. Although sperm may appear in the semen, pregnancies are extremely uncommon after vasoepididymostomy for caput epididymal blocks. In contrast, the results of ICSI with testicular or epididymal sperm, fresh or after cryopreservation, are similar to those obtained with sperm from semen.91
COITAL DISORDERS
Male coital disorders affecting fertility include erectile dysfunction (impotence), failure of ejaculation, and retrograde ejaculation. Many men have problems with sexual performance after first learning about their infertility, but this usually ameliorates with time. Infrequent and poorly timed intercourse may result from incorrect advice, low libido, or the psychological reaction to infertility.6
Impotence
Impotence may be associated with low libido from androgen deficiency with primary or secondary hypogonadism. Impotence related to vascular or neurologic abnormalities (diabetic autonomic neuropathy or pelvic nerve damage) is uncommon in men presenting with infertility.7 Selective impotence at the time of ovulation may indicate psychological problems and ambivalence about having children.
Failure of Ejaculation
Failure of ejaculation is usual with chronic spinal cord injury and may also be caused by antihypertensive and psychotropic drugs but otherwise is an infrequent cause of infertility in most societies.74,99 Healthy men who cannot ejaculate with intercourse may be able to produce semen by masturbation, with a vibrator, or other stimulation.
Retrograde Ejaculation
Retrograde ejaculation occurs when the bladder neck fails to contract at the time of ejaculation so that all or most of the semen passes into the bladder. Usually, there is an obvious cause: prostatic surgery, diabetic neuropathy, pelvic nerve damage, or spinal cord injury. Retrograde ejaculation is diagnosed by the finding of sperm in urine passed after ejaculation.
Differential Diagnosis
Recognition of a coital disorder is crucial, so all infertile patients must discuss their sexual history in detail. Once recognized, the contribution of organic and psychological factors needs to be evaluated.
General Treatment
An optimistic prognosis can be given provided that live sperm can be obtained. The couple is advised about the various techniques that might be used for collecting the sperm for artificial insemination or other ART. The woman’s potential fertility must be evaluated.
Specific Treatment
A drug that may be contributing to the sexual disorder (e.g., antihypertensive, tranquilizer) should be stopped temporarily or permanently.36 Impotence may respond to sex behavior therapy, administration of type 5 phosphodiesterase inhibitors, intrapenile injections of vasodilators, and physical approaches with pumps and rubber occlusion devices to initiate and maintain erections or penile implants; but these are seldom needed in men with infertility. Some men with failure of ejaculation or retrograde ejaculation may be able to ejaculate during intercourse with a full bladder or after the administration of type 5 phosphodiesterase inhibitors, imipramine, or cholinergic antihistamines such as brompheniramine or ephedrine.99 Others require more powerful stimulation with vibrators or electroejaculation.74 If these are unsuccessful, sperm may be collected by needle biopsy of the testis.100
Use of Collected Semen
If semen can be obtained by masturbation or by wearing nontoxic condoms to collect nocturnal emissions, the couple can be taught to inseminate samples at home. The timing of ovulation can be determined by calendar and either symptoms of ovulation or luteinizing hormone surge detected with a urinary luteinizing hormone dipstick kit. Cryopreservation of samples for AIH or ICSI may also be possible.
Assisted Ejaculation
Ejaculation may be stimulated by applying a vibrator to the underside of the penis near the frenulum of the glans. Vibrators with a 2-mm pitch and frequency of 60 Hz or more are most effective. Men with complete spinal cord injuries below T10 are unlikely to respond and will require electroejaculation. Modern electroejaculation equipment is safe. The probe includes a thermal sensor, and proctoscopy is performed before and after the procedure to ensure there are no burns or other damage to the rectum. A balloon catheter in the bladder is used to prevent retrograde ejaculation.74
Semen obtained by assisted ejaculation from able-bodied men or in the acute stages of spinal cord injuries is often normal.74 In contrast, with chronic spinal cord injury, there is frequently low volume, high sperm concentration, and poor motility.74 As with necrospermia, repeated ejaculation over several days can improve sperm motility. If the semen quality is too poor for AIH or the risks associated with electroejaculation are considered unacceptable, aspiration of sperm from the testis and ICSI produces good results. Assisted ejaculation may cause autonomic hyperreflexia with chronic spinal cord injuries above T6.74 The resulting uncontrolled hypertension may cause cerebral hemorrhage. Careful monitoring of blood pressure and prophylactic nifedipine treatment usually prevents serious problems. Men without complete sensory deprivation require general anesthesia for electroejaculation.
Retrieval of Sperm with Retrograde Ejaculation
Motile sperm may be obtained from the urine after retrograde ejaculation.101 Urinary pH is adjusted to above 7 and osmolality to between 200 and 400 mOsm/kg by administration of 80 g of sodium bicarbonate and 2.0 to 2.5 L of water daily for 3 days before the expected time of ovulation. On the day of ovulation, the man ejaculates and passes urine. Sperm are recovered from the urine by centrifugation, washed, and resuspended in an IVF culture medium. The final pellet is resuspended in approximately 0.5 mL of culture medium for insemination. It is also possible to cryopreserve the sample obtained. If this method fails, electroejaculation and catheterization of the bladder could be considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree