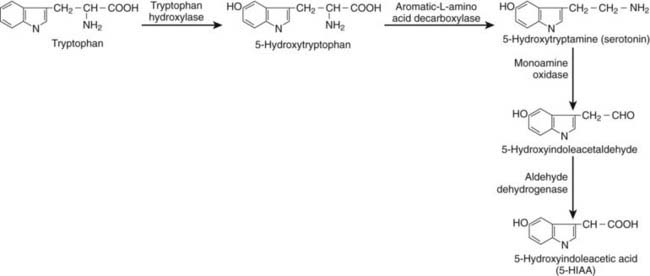
FIGURE 153-1. Schematic illustration of tryptophan metabolism in a neuroendocrine cell (enterochromaffin [EC]-cell).
CLASSIFICATION
Carcinoid tumors have been traditionally classified according to their embryonic origin into foregut, midgut, or hindgut carcinoids.23 This classification from 1963 by Williams and Sandler has been debated, and a new classification system has emerged. In 2000, the World Health Organization (WHO) revised the histopathologic classification system of gastroenteropancreatic (GEP) NE tumors. It was decided that the almost 100-year-old concept of carcinoid is no longer adequate to cover the entire structural and biological spectra of the disseminated NE system neoplasms. Instead, the general terms neuroendocrine tumor and neuroendocrine carcinoma were introduced. Based on a combination of the classic growth and microscopic structural criteria and the value of immunohistochemistry using the proliferation index (PI) Ki67 or the conventional mitotic index, benign NE tumors are distinguished from those with uncertain malignant potential and from NE neoplasms displaying low-grade and high-grade malignancy (i.e., highly and poorly differentiated NE carcinomas, respectively).23
For the GEP NE neoplasms, the current classification is:
As a rule, an NE tumor belonging to group 1 shows all the classic structural features of a benign neoplasm. It is small (<2 cm), well delineated, mostly restricted to the mucosa and the submucosa, displays no angioinvasion, and is composed of highly differentiated NE cells with a PI less than 2%. Typical examples are a classic carcinoid of the tip of the appendix or an incidentally detected trabecular carcinoid of the lower rectum. Some ileal “classic” carcinoids also belong to this group of neoplasms.
The neoplastic lesions of group 2 are usually larger than 2 cm, display widely invasive growth, often have angioinvasion, but are still composed of highly differentiated NE cells with a PI slightly greater than 2%. Typical examples are an ileal classic carcinoid, when discovered by means of clinical symptoms, and some so-called foregut carcinoids (lung carcinoids). The NE tumors of group 3 represent a neoplasm with all the characteristic features of a highly malignant carcinoma, being large with extensive angioinvasion (and metastases) and composed of neoplastic NE cells that display severe atypia and a PI high above 15%. Typical examples are the small cell (“oat cell”) carcinomas of the bronchi.
Recently a tumor-node-metastasis (TNM) staging of gastrointestinal neuroendocrine tumors, including a proposal for a grading system, has been established by the European Neuroendocrine Tumor Society (ENETS) (Table 153-1). A grade I tumor shows PI less than 2%, a grade II tumor, PI 2% to 20%, and a grade III, PI above 20%.24
Table 153-1. TNM Classification for Endocrine Tumors of Lower Jejunum and Ileum (European Neuroendocrine Tumor Society)
| T—Primary Tumor | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | In situ tumor/dysplasia (<0.5 mm) |
| T1 | Tumor invades lamina propria or submucosa and <1 cm |
| T2 | Tumor invades muscularis propria or subserosa or >1 cm |
| T3 | Tumor penetrates serosa |
| T4 | Tumor invades adjacent structures |
| For any T, add (m) for multiple tumors. | |
| N—Regional Lymph Nodes | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| M—Distant Metastasis | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastases |
| M1 | Distant metastasis |
Histologic classification is based largely on characteristic cytochemical staining. Carcinoid tumors are routinely stained with antibodies to CgA, synaptophysin, and neuron-specific enolase (NSE; NE markers). The NE tumors belonging to the midgut region, so-called classic carcinoids, are also stained for serotonin. Besides staining with the specific NE markers described earlier, current histopathologic analysis should include staining for the proliferation marker Ki67 and, if possible, also for the expression of somatostatin receptors, which are particularly important for decisions about future treatment. Carcinoid tumors exhibit substantial differences in terms of genotype and phenotype. NE tumors of the foregut area, mainly pulmonary, frequently show losses of chromosome 11q, which represent a characteristic alteration of these tumors.25 Both typical and atypical carcinoids of the lungs show loss of heterozygosity at 11q13, a region that harbors the MEN1 gene. Atypical carcinoids also show loss of heterozygosity at 3p14-p21.3. Tumor biology parameters such as CD44, nm-23, and Ki67 may provide valuable prognostic information and help to identify the patients at risk of disease-related death.26 Recent studies show that carcinoid tumors of the lung and GI tract may develop via different molecular pathways. Inactivation of several different tumor-suppressor genes on chromosome 18 may be important for the biological behavior of the GI tumors.27 Analysis of rectal carcinoids reveals no abnormalities in proliferation but impaired apoptosis. Transforming growth factor α (TGF-α) and epithelial growth factor receptor (EGFR) autocrine mechanisms have been implicated. Poorly differentiated carcinoid tumors demonstrated frequent loss of heterozygosity for p21, DCC, and APC tumor-suppressor genes.28 Expression of thyroid transcription factor 1 may indicate a bronchial origin of the carcinoid.29 Small intestinal carcinoids overexpress the neoplasia-related genes NAP1L1 (mitotic regulation) MAGE-D2 (adhesion) and MTA1 (estrogen antagonism), genes involved in tumor development and metastasization.30 Familial midgut carcinoids are rare, but bronchial carcinoids may be part of the MEN1 syndrome.31
Clinical Features of Carcinoid Tumors
NE tumors of the foregut region usually have a low serotonin/5-hydroxytryptamine (5-HT) content and often secrete precursors of 5-HT such as 5-hydroxytryptophan (5-HTP) but also histamine and a multitude of polypeptide hormones.32 Such polypeptide hormones include corticotropin-releasing factor (CRF), adrenocorticotropin (ACTH), growth hormone–releasing hormone (GHRH), antidiuretic hormone (ADH), gastrin, somatostatin, glucagon, tachykinins, and CgA. The carcinoid syndrome is sometimes seen in patients with a lung carcinoid with secretion of serotonin, 5-HTP, or histamine.33,34 Midgut carcinoids are responsible for the majority of patients with the classic carcinoid syndrome seen in 30% to 50% of patients.34,35 They contain peptides and amines and secrete serotonin, tachykinins, and CgA. Hindgut NE tumors only rarely have a carcinoid syndrome, but they contain GI hormones such as CgA, pancreatic polypeptide, peptide YY, and somatostatin.
The Carcinoid Syndrome
The classic carcinoid syndrome includes flushing (80%), diarrhea (70%), abdominal pain (40%), valvular heart disease (40% to 45%), telangiectasia (25%), wheezing (15%), and pellagra-like skin lesions (5%).34–36 The carcinoid syndrome, first described in 1954 by Thorson and colleagues,6 has the following features: A malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid insufficiency without septal defects), peripheral vasomotor symptoms, bronchial constriction, and an unusual type of cyanosis. One year later, Dr. William Bean37 gave this colorful description of the carcinoid syndrome:
This witch’s brew of unlikely signs and symptoms, intriguing to the most fastidious connoisseur of clinical esoterica—the skin underwent rapid and extreme changes resembling in clinical miniature the fecal phantasmagoria of the aurora borealis.
Lembeck5 had already in 1953 demonstrated the presence of serotonin in carcinoid tumor, and most of the symptoms of the carcinoid syndrome were ascribed to the release of serotonin. In 1986, the release of tachykinins from carcinoid tumors and the significance of the carcinoid flush were reported.8
FLUSHING
Four different types of flushing have been described in the literature. The first type (Fig. 153-2) is the diffuse, erythematous flush, usually affecting the face, neck, and upper chest (i.e., normal flushing area). This flush is commonly of short duration, lasting from 1 to 5 minutes, and is related to early stages of malignant midgut carcinoids. The second type (Fig. 153-3) is violaceous flush, which affects the same areas of the body and has roughly the same time course or sometimes lasts a little longer. These patients also may have facial telangiectasia. This flush is related to the later stages of malignant midgut carcinoids and is normally not felt by the patients because they have become accustomed to the flushing reaction. The third type (Fig. 153-4) is prolonged flushing, lasting for hours up to several days. It sometimes involves the whole body and is associated with profuse lacrimation, swelling of the salivary glands, hypotension, and facial edema. These symptoms are usually associated with malignant bronchial carcinoids. Finally, the fourth type (Fig. 153-5) of flushing reaction is bright red, patchy flushing, which is seen in patients with chronic atrophic gastritis and ECLomas (derived from enterochromaffin-like cells) of the gastric mucosa, with evidence of increased histamine production.33–38

FIGURE 153-3. Patient with midgut carcinoid with chronic flushing (type 2) and characteristic telangiectasia of the nose.
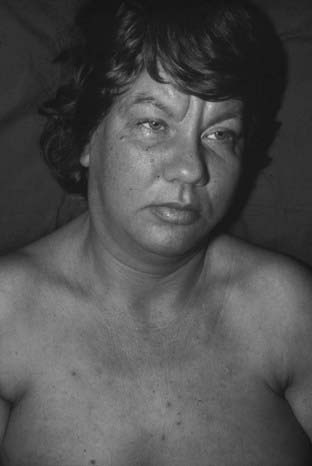
FIGURE 153-4. Patient with a lung carcinoid with production of histamine as well as serotonin. Notice the lacrimation and swollen lips in addition to the flush (type 3).

FIGURE 153-5. Patient with gastric carcinoid (enterochromaffin-like [ECL]-oma) with histamine production and typical bright-red flushing (type 4).
The facial flushing associated with carcinoid tumors should be distinguished from other causes of flushes (Tables 153-2 to 153-5). The carcinoid flush is provoked by spicy food, alcohol, and physical and psychological stress and is often worse in the morning. Patients with idiopathic flushes usually have a long history of flushing, starting rather early in life and sometimes with a family history without occurrence of a tumor. Menopausal flushes usually involve the whole body and might be related to release of calcitonin gene–related peptide (CGRP), with transient vasodilation, a so-called dry flush. Another type of menopausal symptom is the wet flush, which includes epinephrine-induced sweating.39,40 Proposed mediators of flushing in the menopause are CGRP, histamine, prostaglandins, serotonin, lysyl-bradykinin, and substance P. Estrogen is known to have an impact on the production and release of different signaling substances such as noradrenaline and β-endorphin. Low estrogen levels cause lower β-endorphin activity, which in turn enhances the release of gonadotropin-releasing hormone (GnRH), which gives rise to high luteinizing hormone (LH) levels.41 Postmenopausal women in whom a true carcinoid syndrome is developing can tell the difference between the two types of flushes. Sometimes patients with medullary thyroid carcinoma have brief flushes provoked by alcohol. In patients with watery diarrhea, hypokalemia, achlorhydria syndrome (WDHA; vasoactive intestinal peptide [VIP]omas), a purple-red constant flushing of the whole body may develop. This flushing reaction is related to the vasodilator effects of VIP. Flushes seen in mastocytosis are related to release of histamine from mast cell granules. Mastocytosis is a rare disease of mast cell proliferation that occurs both cutaneously and systemically.42,43 In addition to the content of histamine and heparin, the mast cell granules contain arachidonic acid, which can be released and metabolized to prostaglandin D2 (PGD2). Involvement of skin in mastocytosis causes urticaria pigmentosa, which is present in 99% of all mastocytosis (Darier’s sign). The diagnosis is based on urinary histamine metabolite determination, urinary PGD2 metabolites, and a tissue biopsy.44
Table 153-2. Flushing Reaction: Differential Diagnosis
Table 153-3. Drugs Causing Flushes
Table 153-4. Food Causing Flushes
Table 153-5. Neurologic Disorders Causing Flushes
The exact cause of flushing in the carcinoid syndrome is not yet fully elucidated. It was previously thought to be related to excess production of serotonin, but some patients with high levels of serotonin do not have any flushing, and serotonin antagonists such as methysergide, cyproheptadine, and ketanserin have little effect on the flushing.34,35,38,45 In a study from our group in which we measured the release of tachykinins (neuropeptide K and substance P) during flushing provoked by pentagastrin or alcohol, a clear correlation was found between the onset and intensity of the flushing reactions and the release of tachykinins.8 Furthermore, when the release of the tachykinins was blocked by prestimulatory administration of octreotide, very little or no flushing was observed in the same patient.8 Others also reported tachykinins as possible mediators of the carcinoid flush.46,47 Another mediator of the flushing reaction may be bradykinin, which also has been shown to be released during provoked flushing.36,48 Histamine may be a mediator of the flushes seen in both lung carcinoids and gastric carcinoids. Tachykinins, bradykinins, and histamine are known vasodilators, and somatostatin analogs may relieve flushing by reducing circulating levels of these agents.49 An interesting theory by Furschgott and Zawadski50 is that indirect vasodilation is mediated by endothelium-derived relaxing factor or nitric oxide released by 5-HTP during platelet activation and may be a possible cause of flushing.50
DIARRHEA
Diarrhea occurs in 30% to 80% of patients with the carcinoid syndrome. Its pathophysiology is poorly understood but is probably multifactorial. A variety of tumor products, including serotonin, tachykinins, histamine, kallikrein, and prostaglandins can stimulate peristalsis, electromechanical activity, and tone in the intestine. A secretory diarrhea may occur.51,52 Malabsorption may result from intestinal resections, lymphangiectasia secondary to mesenteric fibrosis, bacterial overgrowth secondary to a tumor partially obstructing the small bowel, or rapid intestinal transit. Increased secretion by the small bowel, malabsorption, or accelerated transit may overwhelm the normal storage and absorptive capacity of the proximal colon and result in diarrhea, which may be aggravated if the reabsorptive function of the colon is impaired.53 In patients with increased urinary 5-HIAA and carcinoid syndrome, the transit time in the small bowel and colon is reduced in comparison with that of normal subjects; the volume of the ascending colon is smaller than that in normal subjects, and the postprandial colonic tone is markedly increased. These findings indicate that in patients in whom the carcinoid syndrome is associated with diarrhea, major alterations in gut motor function occur that affect both the small intestine and colon.54 Because many patients with carcinoid tumors undergo wide resection of the small intestine at an earlier stage, they may be affected by the symptoms of short bowel syndrome.
CARCINOID HEART DISEASE
Carcinoid heart disease occurs in 57% to 77% of patients with the carcinoid syndrome but is hemodynamically significant in a much smaller percentage (<10%). The carcinoid heart lesions are characterized by plaquelike fibrous and cardiac thickening that classically involves the right side of the heart (approximately 10% affecting the left side). The finding of new collagen beneath the endothelium of the endocardium is almost pathognomonic for carcinoid heart disease. Echocardiography can demonstrate early lesions in about 70% of patients with carcinoid syndrome, whereas routine clinical examination detects them in only 5% to 10%.55–59 Currently, carcinoid heart disease is a rare event, possibly owing to earlier diagnosis of carcinoid tumors but also because of more effective treatment, such as somatostatin analogs and α interferons, which block the release of substances causing the fibrosis. The most common lesion is tricuspid insufficiency, followed by tricuspid stenosis, pulmonary regurgitation, and pulmonary stenosis. Patients with carcinoid heart disease have significantly higher 5-HIAA levels and tachykinin levels, suggesting that these substances released into the circulation might play a role.55 Substances inducing fibrosis are thought to be released directly into the right side of the heart and then neutralized or degraded through the pulmonary circulation, because rather few patients have similar lesions on the left side. However, patients with lung carcinoid occasionally displayed the same fibrotic changes on the left side. Serotonin has been found to modulate cell proliferation in valvular subendocardial cells, and human heart valves have been shown to express serotonin receptors (-HT1B, 1D, 2A and 2B). Long-term administration of serotonin in rats gives rise to cardiac fibrosis.60 The transforming growth factor β (TGF-β) family of growth factors is up-regulated in carcinoid fibrous plaques on the right side of the heart, and these growth factors are known to participate in matrix formation and collagen deposition.61 One substance that might induce TGF-β locally could be serotonin.62 Another possible mediator might be insulin-like growth factor 1 (IGF-1), which is released from carcinoid tumor cells. Treatment with somatostatin analogs down-regulates circulating IGF-1 and may prevent further development of carcinoid heart disease.
BRONCHIAL CONSTRICTION
True asthma is rare in patients with carcinoid syndrome. The causative agent for bronchial constriction is not known, but both tachykinins and bradykinins have been suggested as mediators. These agents can constrict smooth muscle in the respiratory tract and also cause local edema in the airways.63,64
OTHER MANIFESTATIONS OF THE CARCINOID SYNDROME
Fibrotic complications other than heart lesions may be found in patients with carcinoid tumors. These include intraabdominal and retroperitoneal fibrosis, occlusion of the mesenteric arteries and veins, Peyronie’s disease of the penis, and carcinoid arthropathy. Intraabdominal fibrosis can lead to intestinal adhesions and bowel obstruction and is indeed a more frequent cause of bowel obstruction than is the primary carcinoid tumor. Retroperitoneal fibrosis can result in ureteral obstruction that impairs kidney function and sometimes requires treatment with ureteral stents. Narrowing and occlusion of arteries and veins by fibrosis is potentially life threatening. Ischemic loops of small bowel occasionally have to be removed, which ultimately causes short bowel syndrome. Other rare features of the carcinoid syndrome are pellagra-like skin lesions, with hyperkeratosis and pigmentation, myopathy, and sexual dysfunction.65,66
Diagnosis of Carcinoid Tumors and the Carcinoid Syndrome
BIOCHEMICAL DIAGNOSIS
In all types of carcinoid tumors, high levels of CgA, pancreatic polypeptide (PP), and α and β human chorionic gonadotropin subunits may be found.26,31,67–72 Patients with NE tumors of the midgut carcinoids have increased 5-HT production, and elevated 5-HT levels can be measured directly in the plasma or indirectly via the 5-HT metabolite, U5-HIAA, in the urine (Fig. 153-6).32 Elevated 24-hour urinary 5-HIAA levels have 73% sensitivity and 100% specificity in predicting the presence of a carcinoid tumor of the midgut area. Patients with foregut carcinoids rarely secrete 5-HT but may release ACTH, GHRH, histamine, and high levels of CgA.26 Patients with hindgut carcinoids rarely have elevated levels of tumor markers, even in the presence of metastatic tissues. However, increased levels of PP and CgA are found in a majority of these patients.73 Plasma CgA is increased in more than 80% of patients with NE GI tumors and is the best general tumor marker (Fig. 153-7). It also appears to correlate with tumor load and can therefore be used to predict prognosis, particularly in patients with classic midgut carcinoids.74 Elevation of CgA levels can precede radiographic evidence of occurrence in different types of GI NE tumors. This may prove useful in diagnosis; CgA may be elevated in 93% of patients with metastatic pulmonary carcinoid.26 The sensitivity and specificity are approximately 92% and 96%, respectively, which is better than for neuron-specific enolase (NSE) or α–human chorionic gonadotropin (hCG) testing.75 False-positive tests can occur in patients with reduced kidney function or inflammatory bowel disease. Chronic atrophic gastritis also gives high CgA levels. It must be remembered that treatment with somatostatin analogs can reduce the levels of CgA but does not necessarily reduce the tumor mass.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree