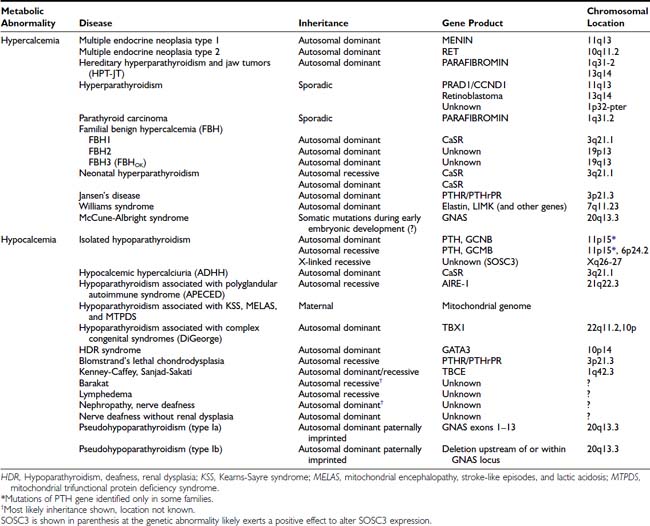
FIGURE 60-1. Regulation of extracellular fluid (ECF) calcium (Ca2+) by parathyroid hormone (PTH) action on kidney, bone, and intestine. A decrease in ECF Ca2+ is sensed by the calcium-sensing receptor, leading to an increase in PTH secretion, which predominantly acts directly on kidney and bone that possess the PTH/PTHrP receptor (PTHR1; see Fig. 60-3). The skeletal effects of PTH are to increase (+) osteoclastic bone reabsorption, but because osteoclasts do not express the PTHR1, this action is mediated via the osteoblasts, which do have this receptor and in response release cytokines and factors that activate osteoclasts. In the kidney, PTH stimulates (+) the 1α-hydroxylase (1α) to increase the conversion of 25-hydroxy vitamin D [25(OH)D] to the active metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D]. In addition, PTH increases (+) reabsorption of Ca2+ from the renal distal tubule and inhibits reabsorption of phosphate from the proximal tubule, leading to hypercalcemia and hypophosphatemia. PTH also inhibits Na+/H+ antiporter activity and bicarbonate reabsorption, causing a mild hyperchloremic acidosis. Elevated 1,25(OH)2D acts on the intestine to increase (+) absorption of dietary calcium and phosphate; it is important to note that PTH does not appear to have a direct action on the gut. In response to hypocalcemia and the increase in PTH secretion, all of these direct and indirect actions of PTH on the kidney, bone, and intestine will help to increase ECF Ca2+, which in turn will act via the calcium-sensing receptor to decrease PTH secretion.
Regulation of Calcium Homeostasis
DISTRIBUTION AND METABOLIC ACTIONS OF CALCIUM
The total body content of calcium in a normal adult is approximately 1000 g, of which nearly all exists within the crystal structure of bone mineral; less than 1% is soluble in extracellular and intracellular fluid.1,2 On average, bone mineral closely approximates the composition of hydroxyapatite [Ca10(PO4)6(OH)2], which means that 6 mmol of phosphate are released with every 10 mmol of calcium mobilized during bone resorption (or about 1 : 2 on a mg/mg basis).1,3
In blood, calcium is partly bound to proteins. Albumin accounts for about 70% of the protein-bound fraction.4 Another portion (about 6%) is associated with diffusible ion complexes.1 Thus normally, only half of total plasma calcium is freely ionized, but it is this fraction that is most important physiologically and subject to stringent endocrine regulation. The normal ranges of total and ionized serum calcium in the adult are 8.5 to 10.5 mg/dL and 1.17 to 1.33 mmol, respectively.5 The usual 2 : 1 ratio of total to ionized calcium may be disturbed by disorders such as metabolic acidosis (calcium binding to proteins is reduced at acid pH) or by changes in serum protein concentrations, as in starvation, cirrhosis, dehydration, or multiple myeloma. When precise knowledge of the ionized calcium concentration is clinically important, direct measurement with calcium-selective electrodes should be performed.
Serum calcium is higher during infancy/childhood and adolescence than in the adult6 but does not change at puberty or, in women, during the menstrual cycle. During pregnancy, total serum calcium and albumin decline progressively, but ionized calcium is minimally affected.7,8 Fetal calcium content rises dramatically during the third trimester (to about 30 g at term),9 and fetal serum total and ionized calcium concentrations both are higher than maternal levels, consistent with active placental transport of calcium.10 Despite daily losses of 200 to 300 mg/d of calcium in breast milk, lactating women maintain normal levels of ionized calcium in blood by increasing intestinal calcium absorption in response to augmented production of 1,25(OH)2D.11,12
Calcium entry into cells is strongly favored by a steep electrochemical gradient. Thus, the cell interior is electronegative, and cytosolic free calcium is in the range of 10−7 M, which is 10,000-fold lower than extracellular calcium concentrations. Calcium traverses the plasma membrane through various channels, including voltage-, receptor- and store-operated forms, the regulation of which is complex and tissue specific.13,14 Intracellularly, nearly all (99%) calcium is sequestered in pools within mitochondria, endoplasmic reticula, or sarcoplasmic reticula or is tightly bound to the inner surface of the plasma membrane.15 Sequestered calcium, especially that within the endoplasmic reticulum, may be released rapidly into the cytosol following activation of cell-surface receptors. In this way, it plays a critical role in signal transduction and in controlling calcium entry via store-operated channels. The extremely low cytosolic free calcium concentration is maintained by active calcium transport into intracellular pools or by extrusion out of the cell via high-affinity, low-capacity Ca2+/H+ adenosine triphosphatases (ATPases) and low-affinity, high-capacity Na+/Ca2+ exchangers driven by the transmembrane sodium gradient.16
With the discovery of a G protein–coupled calcium-sensing receptor (CaSR) expressed in parathyroid, renal epithelial, and other cells, it has become clear that calcium can act as an extracellular ligand to directly control cellular function.17 The principal actions of the CaSR with respect to calcium homeostasis include suppression of PTH secretion and, in the thick ascending loop of the renal tubule, inhibition of calcium, magnesium, and NaCl reabsorption. Extracellular calcium also is utilized directly for normal matrix mineralization in bone and cartilage and is required for activation of important circulating or extracellular enzymes and proteases. Intracellular calcium exerts a broad range of effects via interaction with key enzymes and effector molecules, including kinases, phosphatases, calmodulins, transcription factors, ion channels (including calcium channels), and troponins and other proteins involved in contraction, microtubule and microfilament assembly, and motility. The steep gradients between intracellular calcium and both extracellular and intracellularly sequestered calcium are crucial for normal neuromuscular activity and provide the potential required for rapid transients and waves of cytosolic free calcium that serve key second-messenger functions in both excitable and nonexcitable cells.14,18
CALCIUM ABSORPTION
Intestinal calcium absorptive efficiency in humans ranges broadly between 20% and 70%, declines steadily with age, and is strongly influenced by previous calcium intake, the presence of other nutrients, pregnancy, lactation, overall calcium balance, and the availability of vitamin D.19–23 Fecal calcium includes the residual fraction of dietary calcium that is not absorbed, as well as a contribution from secreted calcium present in bile and other digestive juices (“endogenous fecal calcium”). Endogenous fecal calcium in humans normally amounts to 100 to 200 mg/d and is relatively unaffected by changes in dietary or serum calcium.24,25
Intestinal calcium absorption is adjusted physiologically in response to variations in calcium intake, as shown by radiocalcium kinetic and balance studies in normal subjects.26,27 Obligate renal and intestinal excretion of calcium is such that calcium balance cannot be maintained if dietary calcium consistently falls below 200 to 400 mg/d, even though the percentage of calcium absorbed may be very high (i.e., 70%). As calcium intake increases, overall calcium absorption (in mg/d) rises, but the fractional absorption of ingested calcium declines progressively such that within the physiologic range of calcium intake, total net calcium absorption tends to plateau at approximately 400 mg/d. Consequently, urinary calcium excretion tends also to plateau at higher intakes. Additional buffering of changes in dietary calcium results from control of renal tubular calcium reabsorption and skeletal calcium release, but regulation of intestinal absorptive efficiency is critical for calcium balance.26 The mechanisms of this inverse regulation of intestinal absorptive efficiency by changes in calcium availability are considered in the following discussion.
MECHANISMS AND SITES OF CALCIUM ABSORPTION
Calcium is absorbed throughout the intestine. In terms of rates of transport per unit length of mucosa, absorption is most efficient in the duodenum and proximal jejunum, which exhibit the highest levels of vitamin D–dependent calcium binding proteins and in which lower luminal pH (5 to 6) promotes dissociation of calcium from complexes with food constituents and other ions.28,29 On the other hand, longer residence times in the more distal small bowel segments may allow absorption of a larger proportion of total calcium intake in the distal jejunum and ileum.30,31 The ileum, for example, may become an important site of net calcium absorption during dietary restriction of calcium or when the residence time of luminal contents in more proximal bowel segments is reduced.30,32
Transcellular calcium absorption necessarily involves three steps: entry into the cell, diffusion across the cell, and extrusion from the cell (see Chapter 58). The first step, entry through the apical brush border, occurs via a member of the vanilloid (TRPV) superfamily of channels, namely TRPV6 (previously referred to as CaT1 or ECaC2).33,34 Another member of this family, TRPV5, appears to be dominantly involved in calcium reabsorption in the kidney (see later). The TRPV6 and TRPV5 genes are located on chromosome 7q33-35. Their structure includes six transmembrane regions, a short hydrophobic region between segments 5 and 6 that likely forms the calcium pore, and large intracellular domains at the N- and C-termini.33 The pore region is highly selective for calcium,35,36 and mutations in this region have been shown to abolish calcium permeability.37 Posttranslational modification and glycosylation of TRPV6 occurs, but the functional effects of these modifications are not well defined. Similarly, the mechanisms regulating the activity of these molecules are not well understood. TRPV6 and TRPV5 expression varies significantly between species and in humans, and both genes are expressed in a variety of tissues.38 Specifically, TRPV5 seems to be the major isoform expressed in the kidney, whereas TRPV6 is most highly expressed in the small intestine (restricted to the apical surface of epithelial cells).39 Given the important role of 1,25(OH)2D3 and other hormonal regulators in calcium absorption, the effects of these hormones on TRPV6 expression has recently been investigated. 1,25(OH)D3 clearly increases TRPV6 expression in vitro (see Chapter 58).40 Furthermore, mice with targeted inactivation of the vitamin D receptor demonstrate a 90% reduction in TRPV6 expression and a threefold reduction in intestinal calcium absorption.41 Additionally, calcium exposure appears to independently affect TRPV6 expression insofar as high calcium intake decreases TRPV6 expression, and low intake increases it.41,42 Finally, TRPV6 expression is decreased in mice with targeted inactivation of their estrogen receptor-α gene and is increased in mice receiving exogenous estradiol administration.43
After calcium has entered the cell, it must be transported through the cytoplasm and extruded from the cell against a steep gradient. The mechanism by which calcium moves through the cell involves the small cytosolic protein calbindin D9k. Calcium entering the cell via the apical TRPV6 channel becomes tightly associated with the calbindin, which buffers the relatively large mass of entering calcium and minimizes its impact upon cytosolic free-calcium concentrations. The calbindin/calcium complex then diffuses across the cytosol to the basolateral membrane. Free calcium then dissociates into the low-cytosolic calcium environment maintained immediately subjacent to the basolateral membrane by high-affinity membrane Ca2+-ATPases located there. Finally, these calcium ATPases actively extrude calcium out of the cell.29 The known cellular concentrations and kinetic properties of the calbindin D9k molecule would support observed rates of duodenal calcium transport at submicromolar concentrations of cytosolic free calcium.44 Indeed, the importance of this buffered diffusional process predicts that enterocyte calbindin D9k content is likely to be a major determinant, along with TRPV6 activity, of the overall rate of enterocyte calcium transport.
RENAL CALCIUM EXCRETION
Regulation of renal calcium excretion is an important mechanism for homeostatic control of blood ionized calcium in the face of fluctuations in filtered load, as derived from intestinal calcium absorption and net bone resorption. When urinary calcium is viewed as a function of the amount of calcium actually absorbed by the gut (i.e., after regulation of intestinal absorptive efficiency has been factored out), the precision with which the kidney adjusts tubular calcium reabsorption to residual changes in filtered load becomes obvious. Ordinarily, the daily load of calcium filtered at the glomerulus (the product of the glomerular filtration rate45 and ultrafilterable calcium46) is approximately 10,000 mg/d in adult humans, which means that the extracellular calcium pool is completely filtered several times a day. Since urinary calcium excretion (and net intestinal calcium absorption) is approximately 200 mg/d, only 2% of filtered calcium is excreted normally. This high ratio of filtered to excreted calcium affords ample opportunity for finely tuned hormonal control of calcium excretion, even though it may be difficult to measure the small changes involved.
SITES AND MECHANISMS OF RENAL CALCIUM REABSORPTION
Calcium is reabsorbed at multiple sites and by different mechanisms along the nephron. As in the intestine, the challenge of transporting calcium across the renal epithelium requires that adequate rates of transport be achieved without substantially increasing the concentration of calcium within the cytosol of the epithelial cell. This is accomplished mainly by use of paracellular diffusional mechanisms that are supplemented in some nephron segments by active transcellular transport, especially in response to hormonal stimulation.
Approximately 60% to 70% of tubular calcium reabsorption, like that of sodium, occurs in the proximal tubule.46 Proximal tubular calcium reabsorption occurs mainly via passive diffusion along paracellular pathways, down the ambient (lumen-positive) electrochemical gradient.47 Another 20% to 25% of the filtered calcium load is reabsorbed in Henle’s loop.46 This occurs mainly by paracellular diffusion in the cortical thick ascending limb (cTAL),48–50 although active transcellular transport may play a minor role as well.51,52 Calcium (and magnesium) reabsorption in this segment is severely impaired in patients with homozygous inactivating mutations in claudin 16 (CLDN16) (previously referred to as paracellin-1) or claudin 19 (CLDN19) protein, strongly expressed in tight junctions of the cTAL and presumably required for normal divalent cation conductance.53–56 Hypercalcemia suppresses renal tubular calcium reabsorption even in the absence of PTH,57 an effect likely due to direct activation of basolateral CaSRs in the cTAL (and possibly other nephron segments).58 In the cTAL, activated CaSRs lower apical K+ exit by inhibiting renal outer medullary K+ channels (ROMKs), thereby blunting Na-K-2Cl cotransporter (NKCC2) activity and reducing the transepithelial voltage gradient that drives paracellular cation movement.17,59–61 The critical importance of normal rates of Na-K-2Cl reabsorption for adequate calcium transport in the cTAL is highlighted clinically by the occurrence of dramatic hypercalciuria in patients with Bartter’s syndromes, which involve defects in NKCC2, ROMK, or other transporters or channels required for effective Na-K-2Cl reabsorption.62 An analogous Bartter’s-like phenotype may occur in some patients with activating CaSR mutations.63 CaSR activation in the cTAL also antagonizes PTH-stimulated increases in calcium reabsorption, possibly by impairing cyclic adenosine monophosphate (cAMP) generation61 (see later).
Only about 8% to 10% of filtered calcium is reabsorbed in more distal segments of the tubule, but calcium transport in the distal nephron is a key control point for hormonal regulation and involves predominantly transcellular reabsorption.64–66 Cells in the late distal tubule exhibit striking co-localization of several unique proteins that are critical for effective transcellular calcium reabsorption. These include an apical epithelial calcium channel, TRPV5 (formerly ECaC1 or CaT2 and highly homologous to TRPV6, discussed earlier), the cytosolic calcium-binding protein, calbindin D28k, basolateral plasma membrane calcium ATPase(s) (PMCAs), and basolateral NA+/Ca2+ exchanger 1 (NCX1).67–72 As in the intestinal epithelium, these cells admit calcium across their apical membranes via opening of TRPV5. Expression of this channel is up-regulated by PTH, thus enhancing calcium reabsorption (see later). In contrast, high intracellular calcium concentrations and increased calbindin D28k levels reduce calcium reabsorption, possibly via enhanced buffering of subapical Ca2+ ions. Calcium bound to calbindin D28k is ferried across the cell to the basolateral membrane, where it ultimately is extruded against a steep electrochemical gradient, predominantly via NCX1 (70% of Ca2+ flux),73,74 but also by PMCA. As expected, mice lacking TRPV575 or calbindin D28k76 were found to manifest severe hypercalciuria.
Additional calcium absorption may occur in more distal nephron segments, such as cortical and medullary collecting ducts.47 Proteins other than those described above may be involved in renal calcium reabsorption as well, including voltage-operated calcium channels47,77 and Cl− channels, as evidenced by the hypercalciuria seen in Dent’s disease, an X-linked disorder due to inactivating mutations in the Cl− channel ClC-5 that may be involved in endocytic vesicle function and protein trafficking.78,79
REGULATION OF RENAL CALCIUM REABSORPTION
Calcium excretion is affected by a variety of hormones, ions, nutrients, and drugs.80,81 Among these, PTH is the principal physiologic regulator of renal tubular calcium transport, as appreciated clinically by the markedly abnormal overall relation of serum to urinary calcium in hyper- and hypoparathyroidism.80,82,83 PTH acts to enhance tubular calcium reabsorption in the cTAL, distal convoluted tubules (DCT), and connecting tubules (CNT).50,65,84,85 It augments DCT calbindin D28k expression86 and basolateral N+/Ca2+ exchange,87–91 increases the affinity for calcium of the basolateral Ca2+-ATPase,92 and hyperpolarizes the DCT cell,93 which then activates the TRPV5 channel.94 In immortalized murine distal tubular cells, PTH causes insertion into the apical membrane of new dihydropyridine-sensitive membrane calcium channels,77 and it stimulates stretch-activated nonselective cation channels in rabbit CNT.95 These are distinct from TRPV5 channels and may represent other mechanisms of apical calcium entry. PTH increases the surface expression or activity of TRPV5 epithelial calcium channels. In the cTAL, PTH also may increase some form of active transcellular transport,51,52 and it may stimulate paracellular diffusional calcium transport by augmenting the transepithelial voltage gradient, at least in some segments and species.96,97
Studies in patients with inactivating vitamin D receptor mutations have demonstrated that 1,25(OH)2D3 is required for the calcium-reabsorptive response to PTH.98 It furthermore accelerates the increase in DCT cell calcium entry initiated by PTH,99 and it directly increases DCT calcium reabsorption, apparently by driving higher expression of TRPV5, calbindin D28k, and PMCA (see Chapter 58).74,100–103 Another hormone affecting calcium reabsorption is calcitonin. When given in large doses, calcitonin acutely reduces proximal tubular calcium reabsorption by a mechanism independent of PTH;104 however, an important role for calcitonin in the physiologic regulation of calcium reabsorption is thought to be unlikely. Hypercalciuria observed in states of excess growth hormone or cortisol seems likely to be secondary to an increased filtered load of calcium rather than to direct tubular actions of these hormones.105–110 Estrogen treatment of normal postmenopausal women lowers urinary calcium excretion by increasing tubular calcium reabsorption.111,112 In DCT of ovariectomized rats, 17β-estradiol acutely increases mRNA expression of all major components of the calcium transport pathway, including TRPV5, calbindin D28k, NCX1, and PMCA1b.113 Reported effects on tubular calcium reabsorption of insulin,114 glucagon,96 antidiuretic hormone,96 and angiotensin II115 are of uncertain physiologic or clinical significance.
Parathyroid Hormone Gene Structure and Function
The PTH gene is located on chromosome 11p15 and consists of 3 exons which are separated by 2 introns.116 Exon 1 of the PTH gene is 85 bp in length and is untranslated (Fig. 60-2), whereas exons 2 and 3 encode the 115-amino-acid pre-proPTH peptide (see Chapter 56 for detailed discussion). Exon 2 is 90 bp in length and encodes the initiation (ATG) codon, the prehormone sequence, and part of the prohormone sequence. Exon 3 is 612 bp in size and encodes the remainder of the prohormone sequence, the 84 amino acids comprising the mature PTH peptide and the 3′ untranslated region.117 The 5′ regulatory sequence of the human PTH gene contains a vitamin D response element 125 bp upstream of the transcription start site, which down-regulates PTH mRNA transcription in response to vitamin D receptor binding.118,119 PTH gene transcription (as well as PTH peptide secretion) is also dependent upon the extracellular calcium and phosphate concentration,120,121 although the presence of specific upstream “calcium or phosphate response element(s)” has not yet been demonstrated.122,123 The secretion of mature PTH from the parathyroid chief cell is regulated through a G protein–coupled calcium-sensing receptor, which is also expressed in renal tubules and several other tissues, albeit at lower abundance. PTH mRNA is first translated into a pre-proPTH peptide. The “pre” sequence consists of a 25-amino-acid signal peptide (leader sequence) which is responsible for directing the nascent peptide into the endoplasmic reticulum to be packaged for secretion from the cell.124 The “pro” sequence is 6 amino acids in length, and although its function is less well defined than that of the “pre” sequence, it is also essential for correct PTH processing and secretion.124 After the 84 amino acid–containing mature PTH peptide is secreted from the parathyroid cell, it is cleared from the circulation (with a short half-life of about 2 minutes) via nonsaturable hepatic and renal uptake (see Chapter 56).
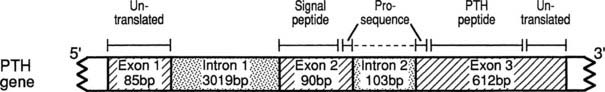
FIGURE 60-2. Schematic representation of the PTH gene, PTH mRNA, and PTH peptide. The PTH gene consists of 3 exons and 2 introns; the peptide is encoded by exons 2 and 3. The PTH peptide is synthesized as a precursor, which contains a presequence and a prosequence. The mature PTH peptide, which contains 84 amino acids, and larger carboxyl-terminal PTH fragments are secreted from the parathyroid cell.
(From Parkinson D, Thakker R: A donor splice site mutation in the parathyroid hormone gene is associated with autosomal-recessive hypoparathyroidism, Nat Genet 1:149–153, 1992.)
PTH mediates its actions through a receptor it shares with PTH-related peptide (PTHrP also known as PTHrH, PTH-related hormone).125,126 This PTH/PTHrP receptor (Fig. 60-3) is a member of a subgroup of G protein–coupled receptors, and its gene is located on chromosome 3p21.3.127,128 The PTH/PTHrP receptor is highly expressed in kidney and bone, where it mediates the endocrine actions of PTH. However, during embryonic and postnatal development, the PTH/PTHrP receptor is most abundantly expressed in chondrocytes of the metaphyseal growth plate, where it mediates predominantly the autocrine/paracrine actions of PTHrP.129,130 Mutations involving the genes that encode PTH, the calcium-sensing receptor, the PTH/PTHrP receptor, and Gsα all affect the regulation of calcium homeostasis and can thus be associated with genetic disorders characterized by hypercalcemia or hypocalcemia (Table 60-1).
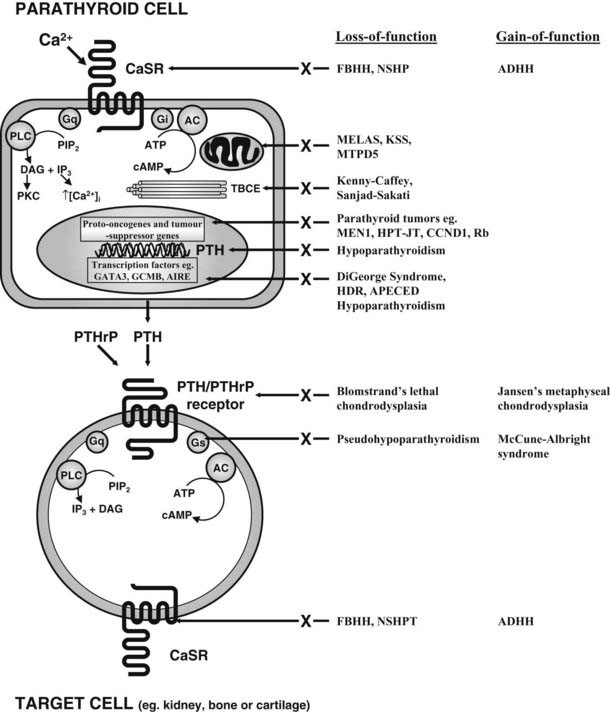
FIGURE 60-3. Schematic representation of some of the components involved in calcium homeostasis. Alterations in extracellular calcium are detected by the calcium-sensing receptor (CaSR), a 1078-amino-acid G protein–coupled receptor. The PTH/PTHrP receptor, which mediates the actions of PTH and PTHrP, is also a G protein–coupled receptor. Thus, Ca2+ and PTH and PTHrP involve G protein–coupled signaling pathways, and interaction with their specific receptors can lead to activation of Gs, Gi and Gq, respectively. Gs stimulates adenylcyclase (AC), which catalyses the formation of cAMP from ATP. Gi inhibits AC activity, and cAMP stimulates PKA, which phosphorylates cell-specific substrates. Activation of Gq stimulates PLC, which catalyses the hydrolysis of the phosphoinositide (PIP2) to inositol triphosphate (IP3), which increases intracellular calcium, and diacylglycerol (DAG), which activates PKC. These proximal signals modulate downstream pathways, resulting in specific physiologic effects. Abnormalities in several genes, which lead to mutations in proteins in these pathways, have been identified in specific disorders of calcium homeostasis (see Table 60-1).
Hypercalcemic Diseases
Similar to the findings in other tumor syndromes, the abnormal expression of an oncogene or the loss of a tumor-suppressor gene can result in abnormal proliferative activity of parathyroid cells. The molecular exploration of these genes has provided important novel insights into the pathogenesis of different forms of hyperparathyroidism. Oncogenes are genes whose abnormal expression may transform a normal cell into a tumor cell. The normal form of the gene is referred to as a proto-oncogene, and a single mutant allele may affect the phenotype of the cell; these genes may also be referred to as dominant oncogenes (Fig. 60-4A). The mutant versions (i.e., the oncogene), which are usually excessively or inappropriately active, may arise because of point mutations, gene amplifications, or chromosomal translocations. Tumor-suppressor genes, also referred to as recessive oncogenes or anti-oncogenes, normally inhibit cell proliferation, whereas their mutant versions in cancer cells have lost their normal function. In order to transform a normal cell into a tumor cell, both alleles of the tumor-suppressor gene must be inactivated. Inactivation arises by point mutations or, alternatively, by small or larger intragenic deletions that can involve substantial genomic portions or a whole chromosome. Larger deletions may be detected by cytogenetic methods, by Southern blot analysis, or by PCR-based analysis of polymorphic markers. Compared to genomic DNA from other cells (e.g., leukocytes), genomic DNA from the patient’s tumor cells typically lack certain chromosomal regions, and this finding is consequently referred to as loss of heterozygosity (LOH) (see Fig. 60-4B). Finding LOH, therefore, suggests an inactivating mutation or deletion in the other allele.
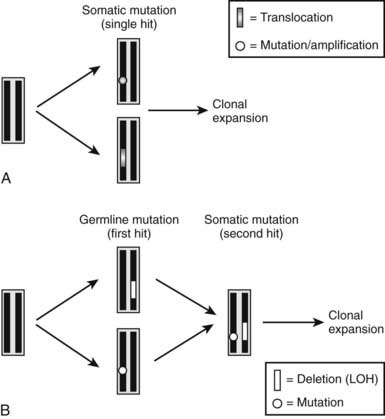
FIGURE 60-4. Schematic illustration of molecular defects that can lead to development of parathyroid tumors. A, Somatic mutation (point mutation or translocation) affecting a proto-oncogene (e.g., PRAD1 or RET) results in a growth advantage of single parathyroid cell and thus its clonal expansion. B, Inherited single point mutation or deletion affecting a tumor-suppressor gene (first hit) makes the parathyroid cell susceptible to a second, somatic “hit” (point mutation or deletion, i.e., LOH), which then leads to clonal expansion of a single cell.
PARATHYROID TUMORS
Parathyroid tumors may occur as an isolated and sporadic endocrinopathy or as part of inherited tumor syndromes,131 such as the multiple endocrine neoplasias (MEN) or hereditary hyperparathyroidism with jaw tumors (HPT-JT),132 or in response to chronic overstimulation, as in uremic hyperparathyroidism.133 Genetic analyses of kindreds with MEN1 and MEN2A and of tumor tissue from patients with single parathyroid adenomas have shown that some of the molecular mechanisms known to be involved in tumor genesis can also be responsible for the development of hyperparathyroidism.
Our current understanding indicates that sporadic parathyroid tumors are caused by single somatic mutations that lead to the activation or overexpression of proto-oncogenes such as parathyroid adenoma 1 (PRAD1) or RET (see Fig. 60-4A). Furthermore, different tumor-suppressor genes affecting the parathyroid glands are predicted to be located on several different chromosomes, and in a significant number of patients, LOH has been documented for one of these loci. For all these somatic mutations, a single point mutation or a deletion provides a growth advantage to a single parathyroid cell and its progeny, leading to their clonal expansion.
In hereditary forms of the disease, two distinct, sequentially occurring molecular defects are observed. The first “hit” (point mutation or deletion) is an inherited genetic defect, which affects only one allele that comprises a gene encoding an anti-oncogene (see Fig. 60-4B). Subsequently, a somatic mutation or deletion affecting the second allele occurs in a single parathyroid cell, and because of the resulting growth advantage, this mutation leads to its monoclonal expansion and thus the development of parathyroid tumors. Examples of this latter molecular mechanism in the development of hyperparathyroidism are the inactivation of tumor-suppressor genes such as the multiple endocrine neoplasia type 1 (MEN1) gene, the hyperparathyroidism–jaw tumor (HPT–JT) gene, and the retinoblastoma (Rb) gene (see Table 60-1).
PARATHYROID ADENOMA 1 AND PTH GENES
Investigations of the PTH gene in sporadic parathyroid adenomas detected abnormally sized restriction fragment length polymorphisms (RFLPs) with a DNA probe for the 5′ part of the PTH gene in some adenomas,134 indicating disruption of the gene. Further studies of the tumor DNA demonstrated that the first exon of the PTH gene (see Fig. 60-2) was separated from the fragments containing the second and third exons, and that a rearrangement had occurred, juxtaposing the 5′ PTH regulatory elements with “new” non-PTH DNA.135 This rearrangement was not found in the DNA from the peripheral leukocytes of the patients, thereby indicating that it represented a somatic event and not an inherited germline mutation. Investigation of this rearranged DNA sequence localized it to chromosome 11q13, and detailed analysis revealed that it was highly conserved in different species and expressed in normal parathyroids and in parathyroid adenomas. The protein expressed as a result of this rearrangement, which was designated PRAD1, was demonstrated to encode a 295-amino-acid member of the cyclin-D family of cell-cycle regulatory proteins. Cyclins were initially characterized in the dividing cells of budding yeast, where they controlled the G1 to S transition of the cell cycle, and in marine mollusks, where they regulated the mitotic phase (M-phase) of the cell cycle.136 Cyclins have also been identified in man and have an important role in regulating many stages of cell-cycle progression. Thus PRAD1, which encoded a novel cyclin referred to as cyclin D1 (CCND1), is an important cell-cycle regulator, and overexpression of PRAD1 may be an important event in the development of at least 15% of sporadic parathyroid adenomas.137,138
Interestingly, more than 66% of the transgenic mice overexpressing PRAD1 under the control of a mammary tissue-specific promoter were found to develop breast carcinoma in adult life,139 and expression of this proto-oncogene under the control of the 5′ regulatory region of the PTH gene resulted in mild to moderate chronic hyperparathyroidism.137,138 Taken together, these findings in transgenic animals provide further evidence for the conclusion that PRAD1 can be involved in the development of a significant number of parathyroid adenomas.
In addition to the rearrangement of the PTH gene in some parathyroid adenomas, a nonsense mutation of the PTH gene, Arg83Stop, that occurred in association with LOH of the PTH locus has been reported in a parathyroid adenoma.140 The patient, who had presented with hypercalcemia and an undetectable serum PTH concentration, showed heterozygosity in the peripheral-blood leukocytes with wild-type and mutant alleles, but the parathyroid adenoma had a loss of the wild-type allele and retention of the mutant (Arg83Stop) allele, which predicts that the tumor secretes only a PTH peptide that is truncated after the 52nd amino acid. Following removal of the parathyroid adenoma, normocalcemia was restored.140 These findings demonstrate that PTH nonsense mutations, which result in truncated forms of PTH not recognizable by standard hormone assays, may be associated with parathyroid adenoma, and that endogenously produced N-terminal PTH fragments can be biologically active.140
MULTIPLE ENDOCRINE NEOPLASIA 1 GENE
MEN1 is characterized by the combined occurrence of tumors of the parathyroids, pancreatic islet cells, and anterior pituitary (Table 60-2).141,142 Parathyroid tumors occur in 95% of MEN1 patients, and the resulting hypercalcemia is the first manifestation of MEN1 in about 90% of patients. Pancreatic islet cell tumors occur in 40% of MEN1 patients; gastrinomas, leading to the Zollinger-Ellison syndrome, are the most common type and also the important cause of morbidity and mortality in MEN1 patients. Anterior pituitary tumors occur in 30% of MEN1 patients, with prolactinomas representing the most common type. Associated tumors, which may also occur in MEN1, include adrenal cortical tumors, carcinoid tumors, lipomas, and cutaneous angiofibromas and collagenomas.142,143 The gene causing MEN1 was localized to a less than 300-kb region on chromosome 11q13 by genetic mapping studies that investigated MEN1-associated tumors for LOH and by segregation studies in MEN1 families.144 The results of these studies, which were consistent with Knudson’s model for tumor development, indicated that the MEN1 gene represented a putative tumor-suppressor gene (see Fig. 60-4B). Characterization of genes from this region led to the identification of the MEN1 gene, which consists of 10 exons that encode a novel 610-amino-acid protein referred to as menin.145,146 Over 1100 germline MEN1 mutations have been identified, and the majority (>80%) are inactivating and are consistent with its role as a tumor-suppressor gene.147,148 These mutations are diverse in their types, and in approximate percentages, 25% are nonsense, 45% are frameshift deletions or insertions, 9% are splice-site mutations, 20% are missense mutations, and 1% are whole or partial gene deletions.144,147–149 In addition, the MEN1 mutations are scattered throughout the 1830-bp coding region of the gene with no evidence for clustering. Correlations between the MEN1 germline mutations and the clinical manifestations of the disorder appear to be absent.147–150 Tumors from MEN1 patients and non-MEN1 patients have been observed to harbor the germline mutation together with a somatic LOH involving chromosome 11q13, as expected from Knudson’s model and the proposed role of the MEN1 gene as a tumor suppressor.148,151–161 The role of the MEN1 gene in the etiology of familial isolated hyperparathyroidism (FIHP) has also been investigated, and germline MEN1 mutations have been reported in 29 families with FIHP.148,151,162–164 The sole occurrence of parathyroid tumors in these families is remarkable, and the mechanisms that determine the altered phenotypic expressions of these mutations remain to be elucidated.
Table 60-2. Multiple Endocrine Neoplasia Syndromes, Characteristic Tumors, and Associated Genetic Abnormalities*
| Type (Chromosomal Location) | Tumors |
|---|---|
| MEN1 (11q13) | |
* Autosomal-dominant inheritance of the multiple endocrine neoplasia (MEN) syndromes has been established.
The function of menin has been investigated by identifying its interactions with other proteins, and by under- or overexpression in in vitro studies (see Chapter 151). Menin has no homology to any known proteins or sequence motifs other than three nuclear localization signals (NLSs) in its C-terminal segment. Subcellular localization studies have shown that menin is predominantly a nuclear protein in nondividing cells, but in dividing cells, it is found in the cytoplasm. Menin has been shown to interact with a number of proteins that are involved in transcriptional regulation, genome stability, cell division, and proliferation (see Chapter 151).148
The functional role of menin as a tumor suppressor also has been investigated, and studies in human fibroblasts have revealed that menin acts as a repressor of telomerase activity via hTERT (a protein component of telomerase).165 Furthermore, overexpression of menin in the human endocrine pancreatic tumor cell line (BON1) resulted in an inhibition of cell growth166 that was accompanied by up-regulation of JunD expression but down-regulation of delta-like protein 1/preadipocyte factor-1, proliferating cell nuclear antigen, and QM/Jif-1, which is a negative regulator of c-Jun.166 These findings of growth suppression by menin were observed in other cell types. Thus, expression of menin in the RAS-transformed NIH3T3 cells partially suppressed the RAS-mediated tumor phenotype in vitro and in vivo.167 Overexpression of menin in CHO-IR cells also suppressed insulin-induced AP-1 transactivation, and this was accompanied by an inhibition of c-Fos induction at the transcriptional level.168 Furthermore, menin reexpression in Men1-deficient mouse Leydig tumor cell lines induced cell cycle arrest and apoptosis.169 In contrast, depletion of menin in human fibroblasts resulted in their immortalization.165 Thus, menin appears to have a large number of functions through interactions with proteins, and these mediate alterations in cell proliferation.
MULTIPLE ENDOCRINE NEOPLASIA 2 GENE (c-ret)
MEN2 describes the association (see Table 60-2) of medullary thyroid carcinoma (MTC), pheochromocytomas, and parathyroid tumors.141,144 Three clinical variants of MEN2 are recognized: MEN2a, MEN2b, and MTC-only (see Table 60-2). MEN2a is the most common variant, and the development of MTC is associated with pheochromocytomas (50% of patients), which may be bilateral, and parathyroid tumors (20% of patients). MEN2b, which represents 5% of all MEN2 cases, is characterized by the occurrence of MTC and pheochromocytoma in association with a Marfanoid habitus, mucosal neuromas, medullated corneal fibers, and intestinal autonomic ganglion dysfunction leading to multiple diverticula and megacolon. Parathyroid tumors do not usually occur in MEN2b. MTC-only is a variant in which medullary thyroid carcinoma is the sole manifestation of the syndrome. The gene causing all three MEN2 variants was mapped to chromosome 10cen-10q11.2, a region containing the c-ret proto-oncogene which encodes a tyrosine kinase receptor with cadherin-like and cysteine-rich extracellular domains, and a tyrosine kinase intracellular domain.170,171 Specific mutations of c-ret have been identified for each of the three MEN2 variants. Thus in 95% of patients, MEN2a is associated with mutations of the cysteine-rich extracellular domain, and mutations in codon 634 (Cys→Arg) account for 85% of MEN2A mutations. However, a search for c-ret mutations in sporadic non-MEN2a parathyroid adenomas revealed no codon 634 mutations.172,173 MTC-only is also associated with missense mutations in the cysteine-rich extracellular domain, and most mutations are in codon 618. However, MEN2b is associated with mutations in codon 918 (Met→Thr) of the intracellular tyrosine kinase domain in 95% of patients. Interestingly, the c-ret proto-oncogene is also involved in the etiology of papillary thyroid carcinomas and in Hirschsprung’s disease. Mutational analysis of c-ret to detect mutations in codons 609, 611, 618, 634, 768, and 804 in MEN2a and MTC-only, and codon 918 in MEN2b, has been used in the diagnosis and management of patients and families with these disorders.171,174
HYPERPARATHYROIDISM–JAW TUMOR SYNDROME GENE
The HPT–JT syndrome is an autosomal-dominant disorder characterized by the development of parathyroid adenomas and carcinomas, and fibro-osseous jaw tumors.175,176 In addition, some patients may also develop uterine tumors and renal abnormalities, which include Wilms’ tumors, renal cysts, renal hamartomas, renal cortical adenomas, and papillary renal cell carcinomas.177 Other tumors, including pancreatic adenocarcinomas, testicular mixed germ cell tumors with a major seminoma component, and Hurthle cell thyroid adenomas have also been reported in some patients.132,177 It is important to note that the parathyroid tumors may occur in isolation and without any evidence of jaw tumors, and this may cause confusion with other hereditary hypercalcemic disorders such as MEN1, familial benign hypercalcemia (FBH) (which is also referred to as familial hypocalciuric hypercalcemia [FHH]), and FIHP.178 HPT–JT can be distinguished from FBH, because in FBH, serum calcium levels are elevated during the early neonatal or infantile period, whereas in HPT–JT, such elevations are uncommon in the first decade. In addition, HPT–JT patients, unlike FBH patients, will have associated hypercalciuria. The distinction between HPT–JT patients and MEN1 patients, who have only developed the usual first manifestation of hypercalcemia (>90% of patients), is more difficult and is likely to be influenced by operative and histologic findings and by the occurrence of other characteristic lesions in each disorder. It is important to note that HPT–JT patients will usually have single adenomas or a carcinoma, but MEN1 patients will often have multiglandular parathyroid disease. The distinction between FIHP and HPT–JT in the absence of jaw tumors is difficult but important; HPT–JT patients may be at a higher risk of developing parathyroid carcinomas.179–181 These distinctions may be helped by the identification of additional features, and a search for jaw tumors, renal, pancreatic, thyroid, and testicular abnormalities may help to identify HPT–JT patients. The jaw tumors in HPT–JT are different from the brown tumors observed in some patients with primary hyperparathyroidism and do not resolve after parathyroidectomy.178 Indeed, ossifying fibromas of the jaw are an important distinguishing feature of HPT–JT from FIHP, and the occurrence of these may occasionally precede the development of hypercalcemia in HPT–JT patients by several decades. The gene causing HPT–JT is located on chromosome 1q31.2 and consists of 17 exons that encode a ubiquitously expressed and evolutionarily conserved 531-amino-acid protein, designated parafibromin.132,182 This gene, CDC73, is also referred to as HRPT2 (i.e., hyperparathyroidism type 2). HRPT2 mutations associated with HPT–JT were found to be scattered throughout the 1593-bp coding region, with the majority (>80%) predicting a functional loss through premature truncation. A genotype-phenotype correlation was not apparent from these analyses.177,182,184 The observation of LOH involving the chromosome lq31.2 region in HPT–JT-associated tumors indicated that parafibromin may be acting as a tumor suppressor, consistent with Knudson’s two-hit hypothesis.177,178,182 This was supported by the observations of germline and somatic HRPT2 mutations in HPT–JT-associated tumors.177,182–185 Similar germline and somatic HRPT2 mutations have also been found in sporadic parathyroid carcinomas, and the frequency of such mutations is high, ranging from 67% to 100%183,186; however, the frequency of HRPT2 mutations in sporadic parathyroid adenomas is low at 0% to 4%, indicating that HRPT2 mutations likely confer an aggressive growth potential to the parathyroid cells.182–184,186,187 HRPT2 mutations and allelic imbalances have also been identified in sporadic renal tumors,188 and a loss or down-regulation of HRPT2 expression has been reported in both breast and gastric cancers.189,190 These studies indicate that HRPT2 and its encoded protein, parafibromin, play a critical role in inherited and sporadic parathyroid cancers as well as other nonhereditary solid tumors. The role of parafibromin, which is predominantly a nuclear protein with a monopartite NLS,191 was not readily apparent, inasmuch as it has no homologies to known proteins. However, the approximately 200 amino acids of the C-terminal domain shared over 25% sequence identity with the yeast Cdc73 protein which is a component of the yeast polymerase-associated factor 1 (PAF1) complex, a key transcriptional regulatory complex that interacts directly with RNA polymerase II.182,192,193 Studies of the PAF1 complex in yeast and Drosophila, as well as in mammalian cells, have revealed that parafibromin, as part of the PAF1 complex, is a mediator of the key transcriptional events of histone modification, chromatin remodeling, initiation and elongation, and the wnt/β-catenin signaling pathway.192–194 Studies of a mouse deleted for HRPTt2 have revealed that HRPT2 expression and the PARAFIBROMIN/PAF complex directly regulate genes (e.g., H19, IgF1, Igf3, Igfbp4, Hmga1, Hmga2, and Hmga3) that are involved in cell growth and apoptosis.195
LRP5 AND THE WNT/β-CATENIN PATHWAY
Aberrant Wnt/β-catenin signaling with an accumulation of β-catenin in the cytoplasm and nucleus is associated with several types of tumor development (e.g., adenomatous polyposis coli and colorectal cancer). Investigations of this pathway in parathyroid tumors have revealed that β-catenin accumulation occurs in parathyroid adenomas and in parathyroid tumors associated with chronic renal failure.196 In addition, a protein-stabilizing mutation, Ser37Ala, in exon 3 of β-catenin was detected in over 7% of parathyroid adenomas but not parathyroid tumors of chronic renal failure, from Swedish patients197,198 but not North American199 or Japanese200 patients. The Ser37Ala β-catenin mutations were homozygous in the parathyroid adenomas, which had a higher expression of β-catenin and the nonphosphorylated active form of β-catenin.198 In addition, MYC, which is a direct target of the Wnt/β-catenin signaling pathway in colorectal cancer cells and a critical mediator of the early stages of intestinal neoplasia, was also overexpressed, and the stable activity of endogenous β–catenin was found to be necessary for MYC and cyclin D1 expression.196 Stability of β-catenin is regulated by Wnt ligands, which bind to the cell-surface frizzled receptors and LRP5 and LRP6 co-receptors that alter phosphorylation of several intracellular second messengers and consequently accumulation of nonphosphorylated β-catenin. Investigation of the Wnt signaling pathway in parathyroid tumors revealed that over 85% of adenomas and 100% of tumors from chronic renal failure patients have a shorter LRP5 transcript, which contained an in-frame deletion of 142 amino acids (residues 666 to 809) that encompassed the third YWTD beta propeller domain between the second and third epidermal growth factor repeats.197 This internally truncated LRP5 receptor activated β-catenin signaling in parathyroid tumors by a mechanism that may involve an impaired inhibitory action of the WNT antagonist, DKK1.197 The parathyroid tumors expressing the internally truncated LRP5 receptor did not harbor the β-catenin stabilizing mutation, Ser37Ala, and those that had the stabilizing β-catenin mutation did not express the truncated LRP5 receptor.197 Thus, it seems that the presence of the stabilizing β-catenin mutation and the expression of the truncated LRP5 receptor are mutually exclusive. However, these studies demonstrate an important role for the WNT β-catenin signaling pathway in parathyroid tumorigenesis.
Rb GENE
The Rb gene, which is a tumor-suppressor gene201 located on chromosome 13q14, is involved in the pathogenesis of retinoblastomas and a variety of common sporadic human malignancies including ductal breast, small cell lung carcinoma, and bladder carcinomas. Allelic deletion of the Rb gene has been demonstrated in all parathyroid carcinomas and in 10% of parathyroid adenomas202,203 and was accompanied by abnormal staining patterns for the Rb protein in 50% of the parathyroid carcinomas but in none of the parathyroid adenomas.202 These results demonstrate an important role for the Rb gene in the development of parathyroid carcinomas and may be of help in the histologic distinction of parathyroid adenoma from carcinoma.202 The Rb protein may also be secondarily involved in parathyroid tumorigenesis by its interaction with the retinoblastoma-interacting zinc finger protein 1, RIZ1.204 The findings of extensive deletions of the long arm of chromosome 13 (including the Rb locus) in some parathyroid adenomas and carcinomas,203 and similar findings in pituitary carcinomas,205 suggest that other tumor-suppressor genes on chromosome 13q may also have a role in the development of such tumors.
RETINOBLASTOMA-INTERACTING ZINC FINGER PROTEIN 1 GENE ON CHROMOSOME 1P
Loss of heterozygosity studies have revealed allelic loss of chromosome 1p32-pter in 40% of sporadic parathyroid adenomas.206 This region is estimated to be about 110 cM, equivalent to about 110 million base pairs (Mbp) of DNA, but additional studies narrowed the interval containing this putative tumor-suppressor gene(s) to an approximately 4-cM (i.e., about 4-Mbp) region.207 Investigations of one candidate gene, retinoblastoma-interacting zinc finger protein 1 (RIZ1), have revealed that over 25% of parathyroid tumors had LOH of the RIZ1 locus, and that over 35% of parathyroid tumors had hypermethylation of the RIZ1 promoter region.204 Moreover, the RIZ1 promoter hypermethylation was related to LOH in these tumors, indicating that these two events may represent the “two hits” (see Fig. 60-4B) required for tumor development in Knudson’s hypothesis for tumorigenesis.204
NONSYNDROMIC FAMILIAL ISOLATED HYPERPARATHYROIDISM
FIHP may represent an incomplete manifestation of a syndromic form such as MEN1, FHH, or HPT-JT.163,164,182,208 Twenty-nine MEN1, nine HRPT2 and five CaSR germline mutations have been reported to date in FIHP kindreds.164 However, it is important to note that the genetic etiology of nonsyndromic FIHP in the majority of families remains to be elucidated.209,210 Thus, studies of 32 kindreds with nonsyndromic FIHP for mutations of the MEN1, CaSR, and HRPT2 genes have revealed that only one family harbored a germline mutation, and this involved the HRPT2 gene that encodes parafibromin.209,210 However, studies of 10 other FIHP kindreds have indicated that another locus, referred to as HRPT3, on chromosome 2p13.3-p14, is likely to be involved in the etiology of nonsyndromic FIHP.211 Thus, the genes and their underlying abnormalities that lead to nonsyndromic FIHP remain to be identified.
HYPERPARATHYROIDISM IN CHRONIC RENAL FAILURE
Chronic renal failure is often associated with a form of secondary hyperparathyroidism that may subsequently result in the hypercalcemic state of “tertiary” hyperparathyroidism. The parathyroid proliferative response in this condition led to the proposal that the autonomous parathyroid tissue might have undergone hyperplastic change and therefore be polyclonal in origin. However, studies of X-chromosome inactivation in parathyroids from patients on hemodialysis with refractory hyperparathyroidism have revealed at least one monoclonal parathyroid tumor in over 60% of patients.133 In addition, LOH involving several loci on chromosome Xp11 was detected in one of these parathyroid tumors, thereby suggesting the involvement of a tumor-suppressor gene from this region in the pathogenesis of such tumors.133 Interestingly, none of the parathyroid tumors from these patients with chronic renal failure had LOH involving loci from chromosome 11q13. This unexpected finding of monoclonal parathyroid tumors in the majority of patients with “tertiary” hyperparathyroidism suggests that an increased turnover of parathyroid cells in secondary hyperparathyroidism may possibly render the parathyroid glands more susceptible to mitotic nondisjunction or other mechanisms of somatic deletions, which may involve loci other than those—MEN1 and PRAD1—located on chromosome 11q13. In addition, as noted above, parathyroid tumors from patients with chronic renal failure have been shown to accumulate β-catenin and to have a truncated form of the LRP5 receptor, which lacks 142 amino acids.196,197
DISORDERS OF THE CALCIUM-SENSING RECEPTOR
Three hypercalcemic disorders due to mutations and/or reduced activity of CaSR have been reported,208,212–217 which are FBH, also referred to as FHH, neonatal severe hyperparathyroidism (NSHPT), and autoimmune hypocalciuric hypercalcemia (AHH) (Table 60-3).
Table 60-3. Diseases Associated With Abnormalities of the Extracellular Calcium-Sensing Receptor (CaSR)
| CaSR Abnormality and Disease | CaSR Genotype |
|---|---|
| Loss-of-function CaSR mutation | |
| Familial benign hypercalcemia | Heterozygous |
| Neonatal severe primary hyperparathyroidism | Heterozygous or homozygous (mutant) |
| Gain-of-function CaSR mutation | |
| Autosomal-dominant hypocalcemic hypercalciuria | Heterozygous |
| Bartter syndrome type V | Heterozygous |
| CaSR autoantibodies | |
| Autoimmune hypocalciuric hypercalcemia | Homozygous (normal) |
| Acquired hypoparathyroidism | Homozygous (normal) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree