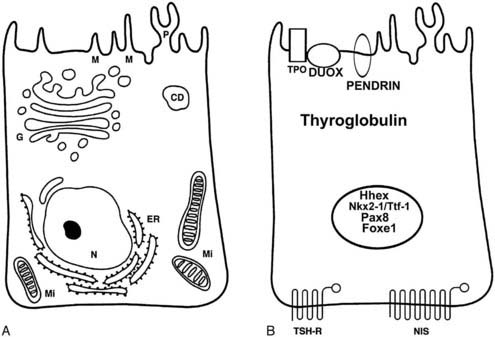
FIGURE 72-1. Gross anatomy of the thyroid. A, Anterior view. B, Posterior view.
In a normal adult, the entire gland is approximately 6 to 7 cm wide, 3 to 4 cm long, and its weight ranges between 15 and 25 g. The thyroid is generally asymmetrical, the right lobe being even twice as large as the left and extending higher and lower in the neck than the left lobe. Interestingly, in patients with dextrocardia, the size of the lobes is reversed, suggesting that the asymmetry of the thyroid lobes could be connected to the position of the heart.2 A thin connective capsule encloses the thyroid. Fibrous septa are occasionally detached from this capsule and penetrate into the parenchyma to produce an incomplete lobulation. This inner capsule is connected to an outer capsule (also called the false capsule of the thyroid) that is continuous with the pretracheal fascia. Blood vessels, the parathyroids, and the recurrent laryngeal nerves are located in the space between the two capsules and are in close contact with the thyroid—the parathyroids on the posterior surface of the gland and the recurrent laryngeal nerves just medial to the lateral lobes.
BLOOD SUPPLY
The thyroid is a highly vascularized organ with four arteries providing the gland with an abundant blood supply. Frequent anastomoses among these blood vessels and an arteriolar network are present on the surface of the gland; from this network, small arteries branch out and enter deeply into the tissue. The capillaries are localized in the interfollicular connective tissue, forming a basketlike network that surrounds each follicle. The capillary endothelial cells are fenestrated, like those of other endocrine glands. Each fenestration is about 50 nm in diameter. Stimulation with thyroid-stimulating hormone (TSH) increases the number and density of fenestrations.3 The veins emerge from the thyroid parenchyma and form a plexus of three groups of veins: the superior, middle, and inferior thyroid veins.
LYMPHATICS
A rich plexus of lymphatic capillaries surrounds the thyroid follicles and communicates with small lymphatic vessels found in the interlobular connective tissue. These deep blood vessels give rise to a surface network of lymphatics that drain into several groups of nodes. The uppermost group of nodes is situated just above the thyroid isthmus and is a systematic group consisting of one to five nodes called the Delphian nodes.2 These are readily felt when involved in cancer or Hashimoto’s thyroiditis. Other more variable groups of nodes in the thyroid area include the pretracheal nodes below the isthmus, those on the thyroid surface, and a last group found along the lateral vein, the recurrent laryngeal nerve, or along the carotid sheath.
INNERVATION
Thyroid innervation is very poor compared to that of other endocrine glands.3 The innervation of the thyroid is provided by sympathetic, parasympathetic, and peptidergic fibers, although few fibers enter the gland.4,5 Both sympathetic and parasympathetic fibers extend throughout the tissue among the follicles in close relation to the follicular cells or around blood vessels.
The regulator peptides detected in the thyroid are produced by both neural crest–derived parafollicular cells and intrathyroid peptidergic nerve fibers.6,7 Some neuropeptides, such as the vasoactive intestinal peptide (VIP), neuropeptide Y,8 substance P, or galanin are exclusively produced in the nerve fibers distributed throughout the thyroid.9 Presumably, these neuropeptides regulate follicular cell functions via a paracrine pathway.
ANATOMIC VARIANTS
The anatomic variants most frequently described in healthy individuals are caused by defects in the regression of the thyroglossal duct. This group of anomalies is generally characterized by the presence of an accessory lobe (pyramidal lobe) attached to the upper part of the isthmus of the thyroid (15% of the population).2 The pyramidal lobe is a rostral-directed stalk that results from the retention and growth of the caudal end of the thyroglossal duct. Other anomalies are associated with a defective atrophy of the duct. In some instances, the entire thyroglossal duct persists as an epithelial cord connecting the foramen cecum of the tongue to the larynx; in other cases, remainders of the duct form isolated or multiple cysts along the line of descent of the duct. Persistent portions of the thyroglossal duct may differentiate into thyroid tissue and form structures called accessory thyroids. The presence of accessory thyroids in addition to a normally located gland is characteristic of this anomaly.
A different developmental defect results in the formation of an ectopic thyroid gland (see “Ectopic Thyroid” later in this chapter). The other frequent anatomic variant (5% of the population) is a thyroid gland that has not developed as a unique mass2 but has the posterior part split into two distinct globes of thyroid tissue. Other anatomic anomalies are rare and found in less than 1% of healthy individuals. These variants include the absence of the isthmus (the thyroid consists of two independent lateral lobes, a physiologic condition in nonmammalian vertebrates10) or the absence of a significant portion of a lateral lobe, frequently the lower half of the left lobe.
The Thyroid Follicle
The thyroid gland displays a peculiar, highly-organized architecture characterized by the presence of spheroidal structures known as follicles. The follicles are supported by an interfollicular extracellular matrix and a capillary network. Thyroid follicles are composed of a single layer of epithelial cells (thyroid follicular cells) that surround a closed cavity (follicular lumen) filled with colloid, a concentrated solution of thyroglobulin.11 The follicle has been defined as the “morphofunctional unit” of the thyroid.12 As described later, thyroid follicular cells express a specific set of genes whose protein products perform functions which are essential for thyroid hormone biosynthesis. However, it is the follicular organization that, together with the polarity of the follicular cells, allows the biochemical steps required for thyroid hormone biosynthesis to occur as a functional chain of events (secretion of proteins in the follicular lumen as exocrine cells do; reabsorption and hydrolysis of proteins; release of hormones into blood by endocrine secretion13). The follicular architecture is absolutely required for thyroid function. In both mice14 and humans,15 T4 is detected only after the differentiation of the follicles.
The follicular cell separates the follicular lumen (where hormone synthesis begins and pre-formed hormone is stored) from the bloodstream, from where iodide has to be uploaded and where hormones will be released at the end of the process. Follicular cells have a specific structural polarity established by molecular mechanisms that create specialized regions in the plasma membrane and cytoplasm.16,17 The surface of the cells is divided into two functionally distinct but physically contiguous regions: an apical and a basolateral domain. The apical domain faces the follicle lumen and displays a differentiated tissue-specific organization that is characterized by the presence of apical microvilli, pseudopods, and by the localization of thyroperoxidase (TPO),18 Na+ or Cl− channels,19,20 pendrin,21 and dual oxidase (Duox).22 The basal domain faces the extracellular matrix and is characterized by the expression of sodium/iodide symporter (NIS),23 Na+/K+ adenosine triphosphatase (ATPase),24 epidermal growth factor (EGF),25 and TSH receptors (TSHR).26 Junctional complexes between cells separate the apical and basal domains to prevent the mixing of the proteins that are sorted asymmetrically. Thyroid hormone synthesis requires basal to apical transport of iodide and thyroglobulin.27 Conversely, hormone secretion is based on apical to basal transport of thyroglobulin. In addition, follicular size is controlled by a bi-directional ion transport system. The basal cell plasma membrane is structurally and functionally connected by integrins to the basal lamina surrounding the follicle.28,29 The basal lamina consists of laminin, type IV collagen, and fibronectin30,31; a very thin connective space—less than 2 µm wide—separates the basal lamina from the endothelial capillary cells.
In the follicles, thyroid cells form a barrier between the extrafollicular space and the lumen. A tight barrier is critical because it promotes cell polarity, guarantees efficient transport, and prevents passive back-diffusion.32 The strong intercellular adhesion is mediated by a junctional complex that consists of tight junctions (zonula occludens), adherens junctions, and desmosomes. The three different types of cell junctions present an apical-to-basal distribution. In fact, the tight junctions are located close to the apical border of the cells, followed by the adherens junctions (located immediately below the tight junctions), and finally by the desmosomal junctions, which are positioned below the adherens junctions and occur on the plasma membrane. The anchoring junctions connect the cytoskeleton of a cell to the cytoskeleton of its neighbor or to the extracellular matrix. All these adhesion structures present the same overall organization: adhesive transmembrane proteins linked to the cytoskeleton by cytosolic adapter proteins.
Among the different types of cell junctions, the tight junctions are the structures that seal cells together. Tight junctions control the permeability of the paracellular space and define the boundary between the apical and basolateral domain of the follicular plasma membrane. They appear as a complex network of anastomosing fibrils consisting of junctional proteins responsible for cell-cell contact. This macromolecular complex interacts with different cellular structures. The major transmembrane proteins in a tight junction are occludin and claudins. The cytoplasmic portion of these proteins interacts with intracellular peripheral membrane proteins, such as ZO-1 and ZO-2,33 which link the junction to the microfilaments. The microtubules can also be functionally linked to the tight junctions via cytoskeletal-associated proteins34 such as cingulin, 7H6 antigen, and actin.
The epithelial adherens junctions, which as already said are located below the tight junctions, are formed by transmembrane adhesion proteins that belong to the cadherins family (cadherins and transmembrane Ca++-dependent adhesion molecules). In the thyroid tissue, as well as in other epithelial tissues,35 E-cadherin accumulates in adherens junctions and plays a crucial role in the induction of these stable adhesions, forming homophilic contacts between neighboring cells. E-cadherin is linked to an intracellular anchor protein, catenin, which in turns binds to the actin cytoskeleton. The catenins participate in intracellular signal transduction pathways,36 creating a functional bridge that couples physical adhesion to intracellular signaling events.
Desmosomal junctions, another type of anchoring junction, are the third type of junction present in epithelia. The transmembrane proteins found in desmosomal junctions are the desmogleins and desmocollins, members of the cadherins superfamily.37 The cytoplasmic tails of these adhesion proteins bind intracellular proteins (plakoglobin, desmosplakin), which in turn bind to the intermediate cytoskeleton filaments. These protein-protein interactions lead to the formation of a network through the tissue that connects different adjacent cells.38
Follicular cells in the epithelia communicate via gap junctions.39 Gap junctions are formed by channel-forming proteins (connexins). These channels allow inorganic ions and other small water-soluble molecules to pass directly from the cytoplasm of one cell to another, coupling the cells both electrically and metabolically.
Primary cultures of porcine thyroid follicular cells provided a useful in vitro model to understand the mechanisms leading to folliculogenesis. Follicular cells freshly isolated from pig thyroid glands and cultured in the absence of TSH aggregate only transiently.40 In the presence of TSH, these cells assemble epithelial junctions, polarize, and organize themselves into follicle-like structures in which apical poles of cells delineate a lumen cavity. The first and essential step for in vitro folliculogenesis is cell aggregation mediated by E-cadherin. During the first few hours in culture, E-cadherin expression increases in the lateral cell surface and accumulates in the subapical regions, where the adherens junctions will be assembled.41 ZO-1 and Na+/K+ ATPase are expressed. The earliest stage of cell-surface differentiation is marked by the redistribution of these two proteins: ZO-1 is recruited around the future pole of the cell, and ATPase is confined to the basal-lateral cell surface. At this stage, apical domain–associated proteins are detected in intracellular vacuoles that later fuse with the cell surface at the nascent apical pole. The follicular lumen is generated in two different steps, both of which are consequences of the polarized phenotype of thyroid cells. The first step is triggered by the lack of adhesive properties of the apical cell surface. The second step, which is required for the control of follicular size, is driven by a bi-directional ion transport system that secrets Cl− in a basal-to-apical direction and, conversely, absorbs Na+ in an apical-basal direction.12
The formation and maintenance of follicle-like structures in cultured porcine thyroid cells is dependent on TSH. In the absence of TSH or cyclic adenosine monophosphate (cAMP) stimulators, follicles undergo a morphologic conversion: thyroid cells spread and form epithelial monolayers.42 The activation of the ERK kinase signaling pathway is involved in this conversion. TSH, acting through its second messenger cAMP, inhibits ERK activation and represses the epithelial conversion.43 In addition, TSH modulates other steps required to maintain follicle organization.44 Stimulation of cAMP/PKA stabilizes E-cadherin-dependent cell-cell adhesions45 and inhibits the production of thrombospondin 1, a matricellular protein which acts as a negative modulator of cell-cell adhesion, thereby inhibiting the dissociation of tight and adherens junctions.46 Furthermore, TSH down-regulates the expression of TGF-β1,47 which induces the loss of epithelial polarization.48 TSH might also control follicular lumen generation because chloride channels, located at the apical pole, are regulated by cAMP.49
One limitation of the data on follicle formation from in vitro studies is that it cannot be directly extrapolated to comprehend tissue follicles in vivo. Follicles in the thyroid gland are surrounded by a basal lamina, which is absent in cultured follicles.41 Furthermore, cultured follicles invert their polarity and manifest a dramatic change in functional properties unless they are cultured with a gel consisting of extracellular matrix proteins.50 In addition, TSH is not required for the formation of follicles in vivo, since folliculogenesis is not affected in mutant mice with impaired TSH/TSHR signaling (see heading “TSHR”).
The Thyroid Cells
In addition to the stromal component, the thyroid gland is composed of three epithelial cell populations (the thyroid parenchyma) of different embryologic origin: (1) the follicular cells, the largest population, which surround the follicular lumen and are responsible for thyroid hormone synthesis; (2) the parafollicular C cells, devoted to calcitonin production; and (3) the epithelial cells, vestiges of the ultimobranchial body.
THYROID FOLLICULAR CELLS
When observed under the light microscope, thyroid follicular cells (TFCs) show a neutrophilic cytoplasm, a basal nucleus, and para-aminosalicylic acid (PAS)-positive vacuoles (phagosomes).51 Follicular cells appear as cuboidal epithelial cells whose height is approximately 15 µm. Cells become flatter (squamous) or higher (columnar) depending on whether or not they undergo TSH stimulation.
Electron microscopy (Fig. 72-2) reveals the characteristic features of cells actively engaged in protein synthesis.52 The rough endoplasmic reticulum and the Golgi apparatus are the dominant organelles in the cell. The apical surface of the cell is covered with thin microvilli or pseudopods that protrude into the follicular lumen. A distinctive feature of follicular cells engaged in protein synthesis is the presence of several vesicles localized in the apical or subapical cytoplasm. The smaller (150 to 200 nm) vesicles are exocytotic vesicles containing newly synthesized thyroglobulin. The fusion of these vesicles with the apical plasma membrane leads to the delivery of thyroglobulin into the follicle lumen. TPO and hydrogen peroxide–producing enzymes are localized to the luminal side of the follicular cell membrane,18 thus allowing the iodination process to occur. The larger vesicles (500 to 4000 nm) are filled with dense material called colloid droplets that are the result of the uptake of the iodinated thyroglobulin stored in the follicular lumen. TSH induces the uptake of thyroglobulin from the follicle lumen, increasing the number of colloid droplets.53 The reabsorption of the colloid involves a macropinocytosis mechanism whose first step is the formation of pseudopods at the apical pole. The pseudopods close, and a portion of the colloid is internalized into the cell.54
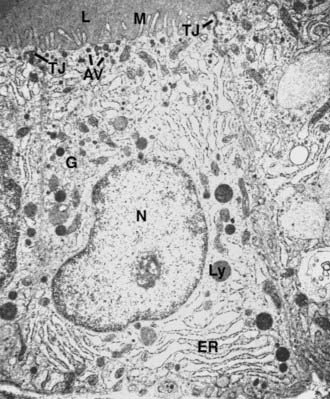
FIGURE 72-2. Electron micrograph of a rat follicular cell. AV, Apical vesicles; ER, endoplasmic reticulum; G, Golgi apparatus; L, lumen; Ly, lysosome; M, microvilli; N, nucleus; Tg, thyroglobulin; TJ, tight junction.
(Courtesy Professor L. Nitsch.)
At the molecular level (Fig. 72-3), a follicular cell can be identified by the presence of a specific set of proteins—and corresponding mRNAs—indispensable for its specialized functions.55 Among such proteins, thyroglobulin (Tg) and TPO are remarkably specific, being detectable exclusively in thyroid follicular cells. Other proteins, such as TSHR, NIS, pendrin, and Duox, though expressed in thyroid follicular cells, are also present in other tissues. The exclusive or prevalent expression of genes necessary for thyroid hormone biosynthesis in thyroid follicular cells appears to be due to a combination of transcription factors unique to this cell type.55 These transcription factors (Nkx2-1/Ttf-1, Pax8, Foxe1) have subsequently been found to be important in controlling not only the differentiation but also the morphogenesis of the gland. The molecular features and the functions of these factors will be described further on (see “Molecular Genetics of Thyroid Gland Development”).
C CELLS
C cells represent the other endocrine cellular type present in the thyroid gland of mammals. They synthesize and secrete calcitonin,56 a polypeptide hormone involved in calcium metabolism. In lower vertebrates, C cells form an organ called the ultimobranchial gland that is separate from the thyroid gland. Quail chick chimera experiments have demonstrated that C cells of avian species derive from neural crest cells,57 which during embryonic development colonize the ultimobranchial body, a transient organ in mammals, to finally disperse into the thyroid gland.58
C cells are known as parafollicular cells because of their distribution among follicular cells. However, in spite of their name, not all C cells are located between follicular cells and the basement membrane (a real parafollicular position); they are also found among the follicles (interfollicular) or in an intrafollicular position. In fact, C cells are found dispersed as individual cells in small groups closely associated to follicular cells, and in more complex structures consisting of both follicular and C cells. The number of parafollicular cells in the thyroid differs among species.59 In humans, the number of C cells decreases with age: the neonatal thyroid has 10 times more C cells than the adult thyroid, where they are fewer than 1% of the follicular cells. These cells are usually distributed in the upper two thirds of the lateral lobe in intrafollicular and parafollicular positions.60
C cells are characterized by clear cytoplasm and small, compact nuclei. Electron microscopy reveals that these cells contain cytoplasmic secretory granules 100 to 200 nm in diameter.61 At the molecular level, C cells are identified by the presence of calcitonin. The calcitonin/calcitonin gene-related peptide (CGRP) gene encodes four proteins: calcitonin, CGRP, katacalcin I and katacalcin II. The splicing of the first three exons to the fourth gives rise to an mRNA that encodes for a protein precursor which is subsequently processed to give calcitonin and a peptide, katacalcin I. CGRP and katacalcin II are the products of alternative splicing and are by far less abundant than calcitonin in C cells. CGRP is a 37-amino-acid vasoactive peptide with unknown effect on calcium metabolism.62 Interestingly, C cells, although different from follicular cells in function and embryologic derivation, express Nkx2-1/Ttf-1,63 which is a distinctive marker of thyroxine-producing cells. Nkx2-1/Ttf-1 is also present in immature C cells, in the migrating ultimobranchial body, and in the cells of the fourth pharyngeal pouch.14,64,65
Parafollicular cells share several biological features with other neuroendocrine cells that originate from the neural crest. Indeed, parafollicular cells express neuroendocrine markers such as neurospecific enolase and chromogranin A and a large number of regulatory peptides and their receptors,66 including somatostatin, serotonin, cholecystokinin2-receptor (CCK2R), gastrin releasing peptide, thyrotropin-releasing hormone (TRH), and helodermin. Whether different subpopulations of parafollicular cells synthesize different sets of regulatory factors has not yet been demonstrated. However, there is some evidence of functional heterogeneity within mammalian parafollicular cells.7 In neonatal rats, 90% of calcitonin-producing cells coexpress somatostatin, while in adults this factor is detected in only 1% of parafollicular cells.66 Also, in humans calcitonin and somatostatin are co-localized in few parafollicular cells.67 However, somatostatin is detected in almost all C-cell carcinomas in rats.66 Similarly, in many human medullary thyroid carcinomas, C cells can express somatostatin.68 The functional relevance of the production of these regulatory peptides by C cells is still unclear. These biologically active peptides might regulate thyroid function in a paracrine pathway, because parafollicular cells are distributed among follicular cells and often tightly adhered to them. Somatostatin, calcitonin, CGRP, and katacalcin inhibit thyroid hormone secretion, while gastrin-releasing peptide and helodermin stimulate this process.7 The expression of CCK2R, which binds cholecystokinin and gastrin,69 and the observation that gastrin induces calcitonin secretion suggest an interrelationship between calcium homeostasis and gastrointestinal hormones. In addition, the presence of CCK2 in thyroid tissues allows one to hypothesize that CCK2 and its receptor are both involved in an autocrine loop required for C-cell function.69
ULTIMOBRANCHIAL BODY–DERIVED EPITHELIAL CELLS
In addition to the common thyroid follicles, other epithelial structures are evident in the mammalian thyroid. These structures, described as a “second kind of thyroid follicles,”70 rarely display a clear follicular organization. The finding that the second kind of thyroid follicles are absent in the avian thyroid (where ultimobranchial bodies never merge with the thyroid) has suggested that these structures represent remnants of the ultimobranchial body of endodermal origin.71
In humans, ultimobranchial-body remnants known as solid cell nests (SCN)72 are frequently present in the thyroid gland and are preferentially located in the middle and upper third of the lobes.72 SCN appear as para- or intrafollicular clusters and cords of epithelial cells clearly separated from the follicles by a basal lamina.
SNC are composed of two cell types: C cells and “main” cells, the most important cell population of these structures. The presence of C cells is consistent with the common ultimobranchial derivation of both SNC and C cells. In most cases, the SNC are found mixed with another structure known as a mixed follicle, in which follicular cells and main cells underline a lumen filled with colloid-like material.72,73 Main cells, polygonal in shape, show squamoid features, with oval nuclei and eosinophilic cytoplasm lacking intercellular bridges. The molecular phenotype of main cells is peculiar. These cells express p63,73 Bcl2 and telomerase.74 They do not express markers of differentiation such as Nkx2-1/Ttf-1, Tg, calcitonin, and CGRP. It is worth noting that recent studies in mouse embryos have revealed that Nkx2-1/Ttf-1–negative, p63-positive cells are present both in the epithelium of the fourth pharyngeal pouch and in the ultimobranchial body, confirming the SNC are ultimobranchial-body remnants.64 The presence in the main cells of both telomerase and p63, a transcription factor present in basal/stem cells of several multilayered epithelia and absent in differentiated cells, has suggested the hypothesis that these cells could be a source of multipotent cells able to differentiate towards either Tg- or calcitonin-producing cells. In addition, it has been suggested that main cells could be the cells of origin of a subset of papillary thyroid carcinoma.75
Development of the Thyroid Gland
The adult thyroid gland in mammals is assembled from two different embryologic structures. This composite origin reflects the dual endocrine function of the gland. The thyroglobulin-producing follicular cells derive from a small group of endodermal cells of the primitive pharynx (the thyroid anlage). The calcitonin-producing parafollicular cells are neural crest–derived cells from the ultimobranchial bodies. The ultimobranchial bodies are transient embryonic structures originated from the fourth pharyngeal pouch. The thyroid anlage and the ultimobranchial bodies migrate from their original sites, reach their final position anterior to the trachea, and fuse to form the definitive thyroid gland. The thyroid follicles derive from the thyroid anlage cells, while the C cells are scattered within the interfollicular space. After this early ontogenetic phase, the thyroid begins to function at a basal level; subsequent differentiation of the hypothalamic nuclei and the organization of the pituitary-portal vascular system guarantee the maturation of the thyroid-system function.76
Data on thyroid organogenesis in humans are scarce and in many cases based on out-of-date reports. In contrast, the morphogenesis and differentiation of the thyroid have been extensively studied in animal models, mostly in the mouse and rat. Studies on patients affected by congenital hypothyroidism with thyroid dysgenesis have confirmed that identical genetic mechanisms are involved in thyroid organogenesis, both in humans and in mice. Furthermore, the recent introduction of zebrafish as a model for the analysis of thyroid development confirmed that the molecular pathways involved in the formation of the thyroid and in the differentiation of follicular cells are essentially conserved among all vertebrates.
Here we will describe in detail the morphologic and molecular aspects of thyroid development in mice (summarized in Fig. 72-4). These data can reasonably be extended to humans; the known relevant differences will be highlighted.
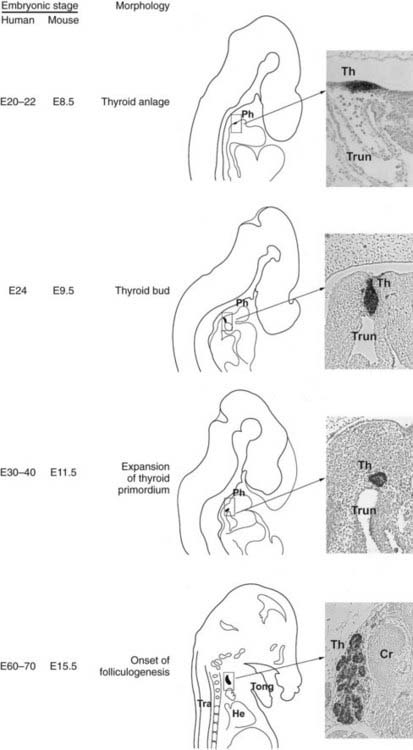
FIGURE 72-4. Thyroid development. In the middle is a schematic representation of the different steps of thyroid organogenesis in mouse embryos; on the left, the correspondent embryonic stages (E, embryonic day) in mice and humans are indicated; on the right relevant sagittal sections of mouse embryos stained with an anti-Nkx2-1/Ttf-1 are shown. Cr, Cricoid cartilage; He, heart; Ph, pharynx; Th, thyroid; Tong, tongue; Tra, trachea; Trun, truncus arteriosus.
SPECIFICATION OF THE THYROID FOLLICULAR CELLS: THE THYROID ANLAGE
Gastrulation involves dramatic changes in the overall structure of the embryo, converting it into a complex three-dimensional structure. The anterior and posterior endoderm invaginates, forming two pockets which extend and fuse, generating a primitive gut tube that runs along the anterior-posterior axis of the embryo. The foregut is the most anterior (cranial) region of this tube. The endoderm of the foregut gives rise to a number of cell lineages which eventually form the epithelial components of thyroid, thymus, lungs, stomach, liver, and pancreas. All organs derived from the gut tube undergo a rather similar process. First, a restricted group of cells differentiate from their neighbors, as can be visualized both by the appearance of specific molecular markers (usually transcription factors) and by the almost simultaneous appearance of a multilayered structure. Subsequently, all organs undergo a morphogenetic process that entails, depending on the organ, proliferation, branching morphogenesis, and/or migration or a combination of these processes. Finally, each organ forms a specific cellular organization that is directly correlated to the organ function (i.e., follicular organization in the case of the thyroid). The first differentiation step in this process will be called throughout this chapter specification, even though the term usually refers to a reversible process; in the case of all organs derived from the gut, it is at present unknown whether the first differentiation event is either reversible or irreversible.
While the inductive mechanisms by which endodermal cells produce liver, pancreas, and lung cell lineage begin to be detailed, the events that commit a group of multipotent endodermal cells to a thyroid fate are almost unknown. The first morphologic consequence of thyroid specification is the appearance of the thyroid anlage (also called thyroid placode). In the mouse (19.5-day-long gestation) it is visible at embryonic day (E)8 to 8.5 and in humans at E22.77 In both the species, the thyroid anlage appears as a midline endodermal thickening in the ventral wall of the primitive pharynx caudal to the region of the first branchial arch that forms the tuberculum impar78 (see Fig. 72-4). The cells of the thyroid anlage simultaneously express four transcription factors: Hhex,Nkx2-1/Ttf-1, Pax8 and Foxe1.79 This unique molecular signature hallmarks these cells, which can be defined as the precursors of the TFCs.
The genetic program directing the specification of thyroid precursor cells is rather obscure. Any genes relevant in the patterning process of the foregut, such as Nodal, members of the Gata family, or Sox genes could play a role in thyroid specification. As yet, there have been no reports of either cell-fate mapping studies or genetically modified mice clarifying the early steps of the thyroid specification. Actually, mouse embryos carrying targeted inactivation of genes involved in foregut patterning usually show developmental arrest at stages that preclude the assessment of the thyroid anlage. In zebrafish, Bon and Gata5, two transcription factors that are downstream effectors of Nodal activity, seem to be specifically involved in the commitment of thyroid fate. Indeed, in the absence of these factors, endodermal cells form a reduced gut tissue but do not contribute to the thyroid.80
The study of the developing thyroid in mice has shown that at E8.5, the earliest identifiable thyroid anlage appears in close apposition to the aortic sac, the cardiac region that gives rise to the embryonic heart outflow and pharyngeal arteries. This observation suggests that short-range inductive signals from cardiac mesoderm or from the endothelial lining of the aortic sac could have an inductive role to specify undifferentiated endodermal cells towards a thyroid fate. In mice, alterations in the foregut have been observed as a consequence of an impaired heart development81; furthermore, it has been reported that signals from cardiac tissue adjacent to the endodermal layer are crucial in the early stages of liver, pancreas, and lung development.82–84
Data obtained from zebrafish are proving invaluable for the study of thyroid specification. The close association between thyroid anlage and aortic sac is maintained also in zebrafish.85 In this species it has been demonstrated that in the absence of the basic Helix-loop-helix (bHLH) transcription factor Hand2, expressed in the cardiac mesoderm surrounding the site of initiation of thyroid development, the endoderm appears to be normal, but thyroid precursor cells are not present. Experiments strongly suggest both that Hand2 has a non–cell-autonomous role in thyroid specification and that FGF proteins participate in this pathway.85 Interestingly, in mice it has been proved that FGF signals are required for correct thyroid development, but the role of these factors in thyroid specification has never been demonstrated.86
Consistent with the hypothesis of a role of cardiac tissue in thyroid development are the findings that cardiac malformations represent the most frequent birth defects associated with thyroid dysgenesis in humans,87 and DiGeorge syndrome is characterized by both congenital heart defects and an increased risk of congenital hypothyroidism.88
EARLY STAGES OF THYROID MORPHOGENESIS: BUDDING, MIGRATION, AND LOBULATION OF THE THYROID PRIMORDIUM
After the specification stage, the developing thyroid undergoes very rapid changes in its appearance. In mice, by E8.5 the thyroid anlage first appears as a multilayered epithelium. Shortly after, it forms a bud that, evaginating from the floor of the pharynx, invades the surrounding mesenchyma as an endodermal extroflexion close to the aortic sac. In these early stages of morphogenesis, the expansion of the thyroid primordium does not seem to be due to the proliferation of cells of the primitive thyroid anlage; indeed, these cells show a very low cell proliferation rate as compared to that of the cells of the surrounding endoderm. Other cells from the pharyngeal endoderm could be recruited into the developing thyroid, thus contributing to the increase of the number of thyroid progenitor cells.89 At E10.5, the thyroid bud has a flask-shaped structure still connected to the floor of the pharynx by a thin cord, the thyroglossal duct, a transient narrow channel. One day later, the thyroid bud is visible as a caplike shape that has migrated caudally into the mesenchyme, losing all connections with the floor of the pharynx (see Fig. 72-4). At this stage, the developing thyroid begins to expand laterally, the first step of the process that eventually leads to the formation of the two lobes. At E12.5, the thyroid primordium appears as an elongated structure, extended laterally, in contact with the third pharyngeal arch arteries that will participate in the formation of the definitive carotid vessels.
By E13, the thyroid bud continues its downward relocation into the mesenchyme and approaches the ultimobranchial bodies that are accomplishing their ventro-caudal migration from the fourth pharyngeal pouch. By E13.5, the developing thyroid, already formed by rudimental lobes connected by a thin central portion, reaches its definitive pretracheal position where it merges with the ultimobranchial bodies containing the precursors of C cells derived from the neural crest.78 Once the gland reaches its final location, the two rudimentary paratracheal lobes expand, and by E15 and 16, the thyroid gland assumes its definitive shape (see Fig. 72-4). In the last stages of thyroid organogenesis, the gland increases further in size, probably due to the high proliferation of TFCs.
In humans, as described in mice, at an early stage of embryonic life, the thyroid anlage appears as a bud invading the mesenchyma at E26; in a few days it appears as a migrating primordium, connected to the pharynx by the thyroglossal duct that disappears around E37. At this stage, the thyroid bud acquires a bilobed shape. The developing thyroid merges with the ultimobranchial bodies by the sixth week and reaches its final position in front of the trachea around the seventh week.90
The translocation of TFC precursors to reach the sublaryngeal position is a process that lasts for almost 4 days in the mouse embryo and almost 4 weeks in the human embryo. Budding and translocation from the gut tube is a developmental process shared by many endoderm-derived organs.91 In the case of the thyroid, since the definitive location is rather distant from the site of primitive specification, this process mostly involves active migration of the precursors, even if other morphogenetic events occurring in the neck region and in the mouth92 could contribute to the definitive location of the thyroid. The molecular mechanisms involved in the movement of the thyroid primordium are still a matter of debate. In many processes of embryogenesis, such as gastrulation, neural-crest migration, and heart formation, cell migration is involved. However, in these processes, migrating cells lose the epithelial phenotype and acquire mesenchymal features.93 This phenomenon, called epithelial-mesenchymal transition, is hallmarked by an increased expression of N-cadherin and the down-regulation of E-cadherin. In contrast, TFC precursors seem to use a different and yet unidentified pathway to move, because they maintain their epithelial phenotype throughout the entire translocation process and never acquire a mesenchymal identity.94 The expression of transcription factors such as either Hhex or Pax8 or Nkx2-1/Ttf-1 is not sufficient for thyroid migration, while Foxe1 plays a crucial role because the presence of this factor in the thyroid bud is required to allow the cells to move.79,95
The genetic basis of the formation of two symmetrical lateral lobes (lobulation) is beginning to be understood. When the lobulation process begins at E11.5, the developing thyroid is in contact with the third pharyngeal arch arteries that will participate in the formation of the definitive carotid vessels located most closely to the mature thyroid lobes. Signals originating from adjacent vessels or factors regulating vasculogenesis could instruct the process of lobulation by non–cell-autonomous mechanisms. The study of animal models seems to confirm this hypothesis. Mice deficient in either Shh (a key regulator of embryogenesis)96 or TBX (a factor regulated by Shh itself)88 display a disturbed morphogenetic patterning of vessels adjacent to the developing thyroid. In these mutated embryos, as a consequence of the absence of caudal pharyngeal arch arteries, the thyroid bud is never in close contact with vessels, and the lobulation process is impaired. The thyroid fails to separate into two distinct lobes, maintaining the shape of a single tissue mass throughout development. In line with these findings is the observation that thyroid dysgenesis is not uncommon in patients affected by DiGeorge syndrome,97 characterized by congenital anomalies of the heart and great vessels. However, the inductive signals from adjacent tissues must interact with events restricted to the thyroid cells to accomplish the lobulation process. Indeed, thyroid hemiagenesis is very frequent in mice double heterozygous for the null allele of Nkx2-1/Ttf-1 and Pax8, genes expressed in thyroid precursor cells and absent in other structures close to the developing thyroid.98
LATE STAGES OF THYROID MORPHOGENESIS: FUNCTIONAL DIFFERENTIATION AND HISTOGENESIS
When the thyroid primordium reaches the sublaryngeal position, TFC precursors accomplish their functional differentiation. Notably, the normal final location of thyroid follicular cells in front of the trachea is not an essential requirement for functional differentiation, since an ectopic, sublingual thyroid expresses thyroglobulin in both human patients99 and mutated mice.95
The functional differentiation of TFCs is hallmarked by the expression of a series of proteins essential for thyroid hormone biosynthesis, such as Tg, TPO, TSHR, NIS, Duox, and pendrin. This program requires almost 3 days, between E14 and E16.5, and results in the differentiation of the thyroid primordium in a functional thyroid gland that is able to produce and release hormones. Tg and Tshr genes are expressed by E14100; TPO and NIS, the two key enzymes involved in the process of Tg iodination appear between E15 and E15.5,101 probably because their expression is absolutely dependent on the pathway activated by the binding of TSH to its receptor, TSHR.101 Duox appears at E15.5102 and finally, thyroxine by E16.5.14
Alongside functional differentiation, the thyroid gland accomplishes its peculiar histologic organization. An inductive role of the stromal component surrounding follicular cells can be hypothesized in histogenesis. Accordingly, follicular cells, when explanted from a developing chick thyroid, can organize a correct histologic pattern in vitro only if co-cultured in the presence of fibroblasts obtained from the capsule of a thyroid gland.103 By E15.5, TFCs start to form small rudimentary follicles, as revealed by the expression of ZO-1, a tight-junction marker. At E16.5, the gland displays an evident follicular organization. The histogenesis is complete in late fetal life between E17 and E18: the thyroid parenchyma is organized into small follicles surrounded by a capillary network, enclosing thyroglobulin in their lumen.89 At birth, the thyroid gland is able to produce and release thyroid hormones, though the regulation of its growth and function by the hypothalamic-pituitary axis is fully active only after birth.104
The molecular mechanisms involved in the differentiation of the human thyroid are not much different from those found in mice. Functional differentiation of TFCs requires almost 3 weeks. It begins after the developing thyroid is located in front of the trachea, at E48, when TFCs express Tg and TSHR; T4 synthesis is detected at the 10th week.90 In humans, the establishment of the characteristic histologic organization lasts several weeks and can be divided into three phases: the precolloid, the beginning colloid, and the follicular growth, which occur at 7 to 10, 10 to 11, and after 11 weeks of gestation, respectively.76 In the precolloid phase, small intracellular canaliculi develop as an accumulation of colloid material. These small canaliculi enlarge, and the colloid organizes itself into extracellular spaces. In the last phase, primary follicles are clearly visible, and the fetal thyroid is able to concentrate iodide and synthesize thyroid hormones. The human thyroid continues to expand until term, and contrary to mice, the hypothalamic-pituitary-thyroid axis starts functioning at mid-gestation.
It is widely accepted that thyroglobulin-producing cells are derived from the endodermal cells of the thyroid anlage. However, the thyroid is assembled from both thyroid anlage and ultimobranchial bodies. Because of this composite origin, the question arises whether the neural crest–derived cells of ultimobranchial bodies (fated to become calcitonin-producing parafollicular cells) could also differentiate towards thyroglobulin-producing cells. In fish, amphibians, and birds, Tg- and calcitonin-producing cells are found in separate gland organs. In addition, lineage studies in zebrafish suggest that ultimobranchial bodies do not contribute to the development of the thyroid, which derives completely from the endodermal cells of the thyroid anlage.105
In mammals, where endodermal cells from thyroid anlage and ultimobranchial bodies merge in the definitive gland, the contribution of the different cell lineages to the TFC population is still controversial. In the past, embryologists considered the ultimobranchial bodies as the lateral anlage of the thyroid, whose cells were fated to differentiate towards the typical follicular cells and become a definitive component of the mature gland.106 This hypothesis is consistent with the report of patients displaying thyroid tissue in the submandibular region and no detectable thyroid tissue in the normal median position.107 Furthermore, structures appearing as colloid-containing follicles have been observed in ultimobranchial bodies which fail to fuse with the thyroid bud (persistent ultimobranchial body).108 Data suggesting that thyroid follicular cells could originate from ultimobranchial bodies are supported by the study of some murine models displaying persistent ultimobranchial bodies.109–112 In mice, the size of the follicular thyroid appears smaller than would be expected if only cells contributed by ultimobranchial bodies were missing. However, it is worth noting that the expression of follicular cell-specific genes (such as Tg or TPO) in the ultimobranchial bodies has not been described. Thus, there is no conclusive evidence that these cells can differentiate in cells producing thyroid hormones.
ULTIMOBRANCHIAL BODIES DEVELOPMENT
In vertebrates, calcitonin-producing cells differentiate from the ultimobranchial body, a structure that derives from the fourth pharyngeal pouch. The ultimobranchial body is a definitive organ in all vertebrates except in placental mammals, where it is an embryonic transient structure fated to join the medial thyroid bud.
By E10 in mice, the fourth pharyngeal pouch is evident for the first time. It appears as a lateral extroflexion of the primitive foregut expressing both the transcription factor Islet1 (Isl)113 and protein gene product (PGP)9.5.114,115 Shortly after, the caudal portion of the pouch grows, and at E11.5 the fourth pharynx-branchial duct is pinched off, forming an ultimobranchial body primordium visible as an ovoid vesicle with a lumen lined by a columnar epithelium identified by the simultaneous presence of Isl1, PGP9.5, and Nkx2-1/Ttf-1.64,114,115 By E11.5 ultimobranchial body primordia migrate, and at E13 they appear as solid clusters of cells in contact with the midline primordium of the thyroid. By E14.5, ultimobranchial body cells begin to disperse within the thyroid parenchyma, and 1 day later only remnants of ultimobranchial bodies can be distinguished in the thyroid gland. C cells complete their differentiation program through the expression of a series of proteins according to a precise temporal pattern: the basic helix-loop-helix transcription factor Mash1 is expressed by E12.5; the neuronal markers TuJ1, CGRP, and somatostatin by E14.5. One day later, the expression of Mash1 disappears, and calcitonin-producing cells can be detected between follicular cells. During the late stages of thyroid morphogenesis, the expression of Isl1 decreases,113 while calcitonin-producing cells gradually increase in number.114,115
In humans, the development of ultimobranchial bodies is similar to that of mice. At E24 the ultimobranchial body primordia appear as an outpouching of the ventral component of the fourth pharyngeal pouch. At this stage, the primordium of parathyroid IV is visible as a dorsal evagination of the same pouch. Some authors describe a transient fifth pouch as the endodermal origin of the ultimobranchial body.116 Probably the shape of the ultimobranchial body anlage itself, which appears as an incomplete pouch, has generated these different interpretations. By E35 the ventral extroflexion is a long-necked flask still attached to the pharynx; a few days later, the ultimobranchial body primordium loses its connection with the pharyngeal cavity, starts its migration, and at E40 reaches the posterior surface of the median thyroid. A connective layer separates these two buds, which display a different histologic organization: the lateral bud is composed of a compact mass of cells, while the median bud is composed of interconnecting sheets of epithelium. Finally, at E55 the ultimobranchial bodies are incorporated with the lateral lobes of the thyroid, and the cells from both structures mix with each other.
The genetic mechanisms that allow the developing thyroid and ultimobranchial body to recognize each other and fuse are beginning to be understood. Ultimobranchial bodies are absent in Splotch mutant mice,117 a strain characterized by an impaired migration of neural crest cells due to a loss-of-function mutation in Pax3, a gene expressed in the migrating neural crest cells.118 The absence of ultimobranchial bodies is also reported in Pax9 null mice.119 Pax9 is expressed in the entire pharyngeal endoderm and has been reported to be involved in the regulation of epithelial-mesenchymal interactions that are crucial for the correct morphogenesis of both teeth119 and thymus.120 Ultimobranchial body defects have been reported in mice carrying mutations in Hox3 paralog genes. In Hoxa-3 null mice,110 the migration of neural crest cells is not impaired and C cells differentiate correctly, but their number is significantly reduced. In many cases, ultimobranchial bodies fail to fuse with the thyroid bud and remain as bilateral vesicles composed exclusively of calcitonin-producing cells (persistent ultimobranchial bodies). The phenotype appears more severe in mice carrying various mutant combinations in Hoxa3 and its paralogs Hoxb3 and Hoxd3.111 These data indicate that Hox3 paralogs do not play a direct role in the migration and differentiation of C cells but control the correct development of the ultimobranchial body and its fusion to the ventral thyroid primordium. Another gene, Eya1, expressed in the pharyngeal arches mesenchyme and in the endoderm pouches, could control the interactions between ultimobranchial bodies and the thyroid primordium. Indeed, mice in which the Eya1 gene has been inactivated show fewer calcitonin-producing cells and persistent ultimobranchial bodies.112 Thus both Hoxa3 and Eya1 seem to control the merging of ultimobranchial bodies and thyroid primordium, a step in thyroid gland organogenesis that only occurs in mammals. Recently it has been demonstrated that Nkx2-1/Ttf-1is required for the survival of ultimobranchial body cells during migration, but it is not necessary for ultimobranchial body formation.64 Nkx2-1/Ttf-1 functions are in part dosage sensitive. Indeed, Nkx2-1/Ttf-1+/− mice display an abnormal fusion of the ultimobranchial bodies with the thyroid diverticulum. Ultimobranchial body cells are incompletely incorporated into the thyroid parenchyma and remain at the dorsal part of the thyroid lobe.64 A phenotype similar to that has been reported in mice defective for Hox3 genes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree