FIGURE 129-1. Menstrual cycle length in relation to age. The median and 5th and 95th percentiles are indicated. Note the marked variation in length of the menstrual cycle at the extremes of reproductive life.
(Data from Treloar AE, Boynton RE, Behn BG, et al: Variation of the human menstrual cycle through reproductive life, Int J Fertil 12:77–126, 1967.)
Anovulation is also common at the other end of the reproductive spectrum, and considerable variations in menstrual pattern can be observed.5 The decline in estradiol secretion that characterizes menopause, in fact, occurs at a relatively late stage of the endocrine transition. Prior to this, anovulatory cycles may occur, often with very high estradiol concentrations. In any case, if a woman experiences menstrual disruption after the age of 45 years with associated symptoms of hypoestrogenism, it is likely that she has entered her menopausal transition, and detailed endocrine evaluation is unnecessary.
Physiology of the Menstrual Cycle
Clinical problems of menstruation occur as a result of abnormalities in the physiologic function of the HPO axis or the uterus. Full details of the functions of these endocrine systems are contained elsewhere in this textbook. For an understanding of the pathophysiology discussed here, a brief summary of the normal menstrual cycle follows, highlighting the areas where clinical disorders can arise.
The ovary is formed in utero when primordial germ cells migrate from the yolk sac to the gonadal ridge before proliferating and differentiating into approximately 6 million primordial oogonia by 16 to 20 weeks’ gestation6; this is followed by progression of meiosis as far as arrest at the diplotene stage. From this point onward, rapid loss of oocytes occurs through a process of follicle recruitment, and by the time of birth, the number has dropped to approximately 2 million. After recruitment, most follicles become atretic, and only a minority go on to undergo further development.7 Control of follicle recruitment is unclear.7 A number of genes, including anti-Müllerian hormone, c-kit, and kit ligand, have been implicated, and the step of initial follicle recruitment appears to be largely hormone independent. Follicle-stimulating hormone (FSH) is an essential requirement for the later stages of antrum formation in rodents8 but does not appear to be an essential factor in women.
Abnormalities in early follicle development will result in the clinical picture of primary ovarian failure. This may occur as a result of abnormal germ cell development (as seen in 46,XY gonadal dysgenesis), accelerated oocyte loss (as seen in Turner’s syndrome), failure of follicle recruitment (as seen in autoimmune disorders), or insensitivity to FSH (as seen in “resistant ovary syndrome”).
From a clinical perspective, the critical point of the ovarian cycle is the process of follicle selection, whereby a rise in FSH stimulates a single antral follicle (in a normal unstimulated menstrual cycle) to be selected as the dominant follicle and acquire a number of distinctive features.9 Granulosa cells proliferate rapidly with increased synthesis of aromatase and inhibin α subunit. Downregulation of granulosa cell androgen receptors occurs, thus removing the atretic effect of thecal cell androgens on the follicle.10 Finally, luteinizing hormone (LH) receptors are expressed in granulosa cells, giving these cells the capacity to respond to both LH and FSH through a common cyclic adenosine monophosphate (cAMP)-dependent pathway. Abnormalities in follicle selection account for most cases of chronic anovulation, including the more extreme form, polycystic ovary syndrome. Follicle selection is also the step suppressed by hormonal inhibitors of ovulation such as the oral contraceptive pill and is the point at which different doses of gonadotropins can be administered to achieve monofollicular development for ovulation induction or multifollicular development for in vitro fertilization treatment.
Following growth of the dominant follicle, rising concentrations of estradiol and progesterone evoke a surge of LH from the pituitary gland. LH acts on the ovary to stimulate a group of processes constituting ovulation, including rupture of the follicle, nuclear and cytoplasmic maturation of the oocyte, cumulus expansion, and luteinization of the follicle.11 The corpus luteum that forms at the time of ovulation functions for 14 days before luteolysis sets in through a mechanism that remains unclear in women.12 In women, unlike in some other species, the corpus luteum secretes both estradiol and inhibin A, resulting in suppression of FSH until the time of the luteal-follicular shift at luteolysis.12 Although the LH surge can be manipulated readily for purposes of contraception or fertility treatment, pathologic disorders of this step are not commonly seen. The actions of LH on the ovary may be affected, however, by medical treatments such as nonsteroidal anti-inflammatory drugs.
During the first half of the menstrual cycle, the endometrium proliferates under the influence of estrogen. Thereafter, under the influence of progesterone, the stroma and the epithelium undergo a precisely timed transition to a highly differentiated glandular structure with the capacity to support implantation through glycogen secretion.13 Where pregnancy occurs, progesterone continues to maintain synthesis of the prostaglandin-metabolizing enzyme prostaglandin dehydrogenase and to suppress inflammatory actions within the endometrium.14 In contrast, during the nonpregnant menstrual cycle, declining progesterone secretion results in a fall in prostaglandin dehydrogenase synthesis and activation of the cytokine network, leading to an increase in local prostaglandin concentrations and an influx of leukocytes to the endometrium. This initiates the irreversible stage of menstruation with an increase in tissue edema, local vasospasm, and hypoxia in a process that involves tissue metalloproteinase activation and finally results in sloughing of the tissues and menstruation.
Clinical abnormalities of endometrial function are common and include failure of the endometrium to proliferate due to the actions of androgenic forms of the oral contraceptive; excessive bleeding during menstruation, due to excessive prostaglandin concentrations; and breakthrough bleeding, due to abnormalities of angiogenesis.
Amenorrhea
HISTORY AND EXAMINATION
Amenorrhea is defined as the absence of periods for longer than 6 months (secondary amenorrhea) or the absence of periods at age 16 (primary amenorrhea); oligomenorrhea is bleeding that occurs at intervals between 35 days and 6 months.
History should include coverage of the following points:
One of the most important parts of assessment of the amenorrheic woman, particularly the young amenorrheic woman, is a sensitive and empathetic discussion with the young woman. It often may be appropriate to include her mother in this discussion. This discussion should include consideration of the future consequences of the diagnoses under consideration. What outcome does she anticipate from her investigation? Is she frightened of “abnormality” by comparison with her peers? Is she looking to achieve pregnancy at this stage, or simply to reach a diagnosis? In any case, the patient will need the sympathetic support of her physician throughout the process of diagnosis to explain fully and accurately the nature of the problem and the possible outcomes. In some instances, particularly with emotionally traumatic diagnoses such as intersex variations, formal supportive counseling also will be required.
The extent of the examination to be performed should reflect the circumstances of the patient. Measurement of height and weight and calculation of body mass index are essential parts of the assessment. The general examination should include breast examination with careful check of the nipples for galactorrhea, as well as abdominal examination. The external genitalia should be examined carefully to check for genital development, and the clitoris assessed for possible clitoromegaly. This examination will enable pubertal stage to be identified and will allow early identification of major degrees of hirsutism and pelvic masses secondary to hematocolpos from low outflow obstruction (see later discussion). Internal pelvic examination often can be traumatic, particularly for a young adolescent, and its role is less clear in this era of high-resolution pelvic imaging. If in doubt, this aspect of the examination can be deferred safely until after the initial investigation has identified the general nature of the problem.
INITIAL INVESTIGATION OF AMENORRHEA AND IDENTIFICATION OF THE GENERAL CAUSE
The investigations shown in Fig. 129-2 normally should be performed during the initial assessment of amenorrhea.
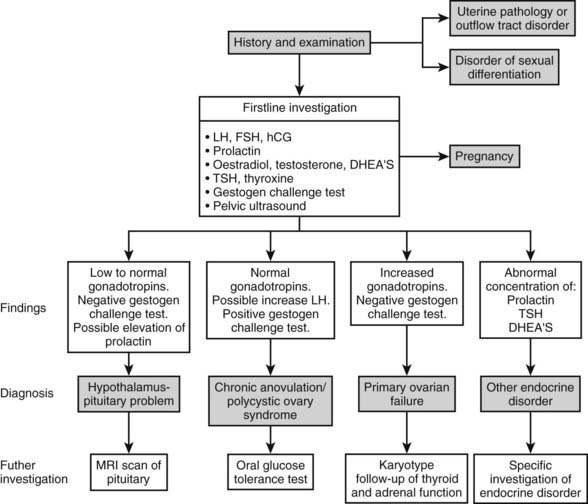
FIGURE 129-2. Algorithm for investigation of women with amenorrhea. DHEA-S, Dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin; LH, luteinizing hormone; MRI, magnetic resonance imaging; TSH, thyroid-stimulating hormone.
Gonadotropin concentrations, particularly FSH, are pivotal to allocation of the case to the diagnostic categories shown in Fig. 129-2, and human chorionic gonadotropin (hCG) is important in excluding pregnancy. Measurements of estradiol and testosterone are similarly important, as despite the undoubted value of the progestogen challenge test (see later), it still is useful to measure the circulating concentrations of these two steroids. Measurement of thyroid-stimulating hormone (TSH), prolactin, and dehydroepiandrosterone (DHEAS) is important in making early identification of thyroid, pituitary, and adrenal disease, respectively. Although a range of complex dynamic tests of function of the HPO axis have been used previously, their use now is largely restricted to research, with little application in everyday clinical practice.15
Measurement of the estradiol concentration is helpful in distinguishing conditions characterized by low to absent estrogen production from those characterized by ongoing estrogen production, such as chronic anovulation and uterine outflow abnormalities. The estradiol concentration can fluctuate significantly, however, and overlap between these conditions is not uncommon. It is for this reason that the progestogen challenge test is a particularly valuable investigation. This is normally performed by administering intramuscular progesterone (100 mg) or a pure progestogenic preparation such as medroxyprogesterone acetate (10 mg) daily for 10 days. Within a few days of cessation of the progestogen, a significant vaginal bleed (a positive progestogen challenge test) or an absent or very scanty bleed (a negative progestogen challenge test) will occur. This test enables conditions with low estrogen production or a genital tract disorder to be distinguished from conditions causing amenorrhea in which some estrogen production and a normal genital tract are found, such as chronic anovulation or polycystic ovary syndrome.
Pelvic ultrasound similarly is a very helpful initial investigation. This can be performed through the abdominal route when the woman is young or has never had sexual intercourse. However, the vaginal route provides more information and is preferable when the woman is older and has had sexual intercourse previously. The pelvic ultrasound will establish the presence and general structure of the uterus and will enable the ovaries to be assessed for a polycystic appearance (see later). The thickness or lack of thickness of the endometrium is a useful guide to the degree of estrogenization that is present. When the initial assessment suggests a genital outflow disorder, a magnetic resonance imaging (MRI) scan is likely to be a more appropriate method of establishing the final diagnosis.4
On the basis of history, examination, and initial investigation, it usually is possible to identify the general cause of the amenorrhea, as shown in Fig. 129-2. The initial classification also will allow a high level of prediction of future fertility, as in most forms of amenorrhea, pregnancy is a likely outcome of treatment (Fig. 129-3). By contrast, in hypergonadotropic amenorrhea, the prospects for pregnancy are very limited. In the past, great emphasis was placed on the distinction between primary and secondary amenorrhea. However, although some conditions may be associated specifically with primary or secondary amenorrhea, nowadays it is recognized that there is a significant overlap, and that the distinction between primary and secondary amenorrhea is not particularly helpful in reaching the final diagnosis. Thus, the remainder of this chapter is presented according to the primary pathology involved.
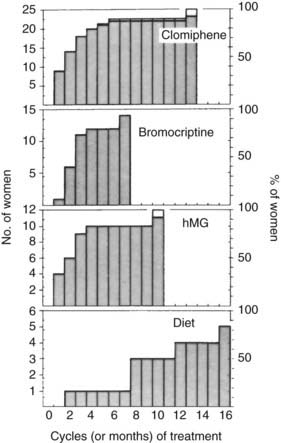
FIGURE 129-3. Time to conception: conceptions after treatment of amenorrheic women with clomiphene, human menopausal gonadotropin (hMG), bromocriptine, or diet. Selection of therapy was based on the cause of the amenorrhea.
(Data from Hull MGR, Savage PE, Jacobs HS: Investigation and treatment of amenorrhea resulting in normal fertility, BMJ 1:1257–1261, 1979.)
Physiologic Cause
Pregnancy and lactation are two common physiologic causes of amenorrhea. Early signs of pregnancy are easy to overlook, and all women in the reproductive age group who have missed a period should be assumed to be pregnant until it is proved otherwise. Ovulation and pregnancy can occur without resumption of menstruation after lactation or after recovery from hypothalamic suppression associated with weight loss. Measurement of hCG in blood or urine can exclude pregnancy.
DISORDERS OF THE HYPOTHALAMUS-PITUITARY
Failure of the hypothalamus and/or anterior pituitary is a much more common cause of amenorrhea than is ovarian failure. A disturbance in ovarian function also occurs if the feedback loops are interrupted by steroids or gonadotropins extrinsic to the HPO axis. In the absence of clearly localized destruction, injury, or disease (e.g., pituitary infarction, as in Sheehan’s syndrome), it often is difficult to distinguish between disorders of the hypothalamus and of the anterior pituitary. Therefore, it is convenient to regard the hypothalamus and the pituitary as a single unit, and to classify the conditions accordingly. Disorders of the hypothalamus-pituitary that result in amenorrhea are shown in Table 129-1.
Table 129-1. Causes of Amenorrhea Due to Disorders of the Hypothalamus-Pituitary
The characteristic feature of amenorrhea resulting from hypothalamic-pituitary disorder is a reduction in the pulsatile secretion of LH.16,17 Although basal levels of LH and FSH may be within the range of the normal follicular phase of the cycle, careful analysis of the pattern of pulsatile secretion of gonadotropins demonstrates a reduction in the frequency of pulses of LH and FSH (Fig. 129-4). Hypothalamic-pituitary disorders also are characterized by loss of normal responses to physiologic stimuli, such as estrogen provocation (Fig. 129-5). These effects occur in most disorders of the hypothalamus-pituitary, including disorders of prolactin secretion, and result in a continuum ranging from a hypogonadotropic state resembling infancy to minor abnormalities of cycle control, as represented by the formation of an inadequate corpus luteum. Although the degree of inactivity of the hypothalamic-pituitary unit can be tested directly by measuring the pattern of gonadotropin secretion, in practice it is much more convenient to assess estrogenic status. In the absence of adequate gonadotropic stimulation, the ovaries secrete minimal quantities of estradiol, levels of which are very low (<50 pg/mL).
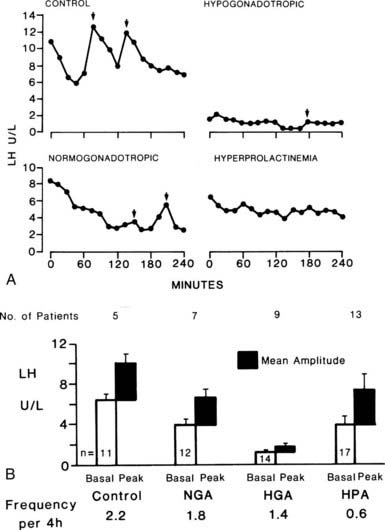
FIGURE 129-4. Luteinizing hormone (LH) secretion in relation to diagnosis. A, Patterns of concentration of LH in the serum of patients with secondary amenorrhea. Note the absence of pulsatile release of LH in hyperprolactinemic patients, although the basal level is within the normal range. B, Mean basal level and pulse amplitude and frequency in women with amorrhea. HGA, Hypogonadotropic; HPA, hyperprolactinemic; NGA, normogonadotropic.
(Courtesy CW Vaughan Williams and DT Baird.)
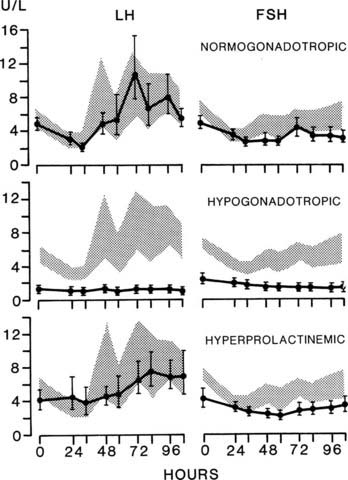
FIGURE 129-5. Loss of luteinizing hormone (LH) response. Estrogen provocation test in women with secondary amenorrhea. The range of normal control women in the early follicular phase of the cycle is indicated by the shaded area. Note the absence of a secondary rise in LH concentration in the hypogonadotropic women. FSH, Follicle-stimulating hormone.
(Data from Vaughan Williams CW, McNeilly AS, Baird DT: The effects of chronic treatment with LHRH on gonadotrophin secretion and pituitary responsiveness to LHRH in women with secondary hypogonadism, Clin Endocrinol 19:9–19, 1983.)
Hyperprolactinemia Including Prolactin-Secreting Tumors
Hyperprolactinemia normally presents with reasonably short-onset secondary amenorrhea, and prolactin measurements above the normal range (>20 ng/mL, Friesen Standard; 360 mU/L, International Standard) are common. Mild elevations are of little clinical significance, and because of the sensitivity of prolactin to even trivial stress and effects such as venipuncture, it is important to ensure that any elevations observed are consistent. A mild elevation in prolactin concentration may be seen in amenorrhea due to polycystic ovary syndrome, presumably caused by the unopposed estrogens that stimulate growth of the lactotrophs. The other clinical features, particularly the hypoestrogenism, should enable amenorrhea due to primary hyperprolactinemia to be distinguished from polycystic ovarian syndrome.
Prolactin has a physiologic role in lactogenesis; thus, galactorrhea is an unsurprising symptom of pathologic hyperprolactinemia. The association of amenorrhea with galactorrhea has been described previously under a number of eponyms,18 such as Chiari-Frommel (postpartum), Argonz-Del Costello (without a relationship to pregnancy), and Forbes-Albright (galactorrhea with a pituitary tumor). The relationship with galactorrhea is complex, however. Only about half of amenorrheic women with hyperprolactinemia have demonstrable galactorrhea. Conversely, only about half the women with galactorrhea have raised levels of prolactin, and where galactorrhea is present in the absence of amenorrhea, the prolactin concentration usually is normal.19 Galactorrhea also may be present in hypothyroid women who have normal levels of prolactin; thus it is likely that other factors, such as thyroxine, may affect the sensitivity of the breast to prolactin.
The amenorrhea of hyperprolactinemia is likely to result from a direct central effect of prolactin, as characteristic disturbances of LH pulsatility can be demonstrated in women with hyperprolactinemia (Fig. 129-6),20 and selective enucleation of a prolactin-secreting tumor results in restoration of normal secretion of gonadotropins and cyclic ovarian activity. It has been reported that naloxone administration increases the frequency of LH pulses, thus suggesting the involvement of endogenous opioids in the amenorrhea of hyperprolactinemia.21 This finding is not consistent, however, even in women without a prolactin-secreting adenoma. A direct effect on the gonad is possible and has been suggested on the basis of the evident actions of prolactin in a range of transgenic and knockout mice.22 Prolactin actions vary between species, however, and normal ovulation can be induced in untreated hyperprolactinemic women following treatment with pulsatile gonadotropin-releasing hormone (GnRH) alone.23 Although this does not preclude a direct gonadal effect of prolactin, the significance is likely to be limited.
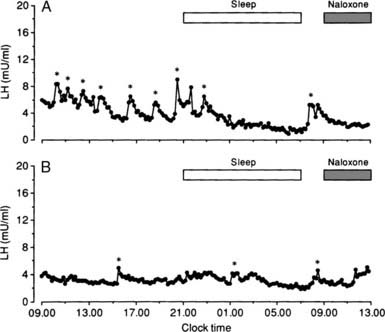
FIGURE 129-6. The 24-hour secretory pattern of luteinizing hormone (LH) in two women with amenorrhea caused by hyperprolactinemia and during 4-hour infusions with naloxone (1.6 mg/hr). Statistically significant LH pulses are indicated by an asterisk. Note the reduced number of LH pulses in both women.
(Data from Tay CCK, Glasier AF, Illingworth PJ, et al: Abnormal 24 hour pattern of pulsatile luteinising hormone secretion and response to naloxone in women with hyperprolactinaemic amenorrhea, Clin Endocrinol 39:599–606, 1993.)
Several conditions, including hypothyroidism, stress, and use of major tranquilizers, can result in hyperprolactinemia. The first step in investigation is to exclude a prolactin-secreting tumor of the pituitary. MRI of the sella turcica is now the investigation of choice, and tumors can be identified in about 50% of women with hyperprolactinemia (see Chapter 18). Most of these tumors are microadenomas (<10 mm diameter) and do not need specific treatment other than for symptomatic relief or for treatment of infertility. In the remainder, it is assumed that a tumor is too small to be identified, or that a defect in the hypothalamic production of dopamine is leading to hypertrophy of the lactotrophs. The latter occurs physiologically during pregnancy and is important in maintaining the increased secretion of prolactin during lactation. In hypothyroidism, excessive secretion of thyrotropin-releasing hormone has been suggested as being responsible for the increased secretion of prolactin from lactotrophs, but this theory has not been proved.
The clinical features and the detailed investigation of patients with pituitary tumors are dealt with elsewhere. The degree of hypogonadism in hyperprolactinemic women can vary from gross evidence of estrogenic deficiency to a minor disturbance in the regulation of ovarian function. Many women with amenorrhea associated with hyperprolactinemia complain of dyspareunia associated with vaginal dryness and estrogen deficiency. Although single measurements of the basal concentration of gonadotropins can be within the normal range, the concentration of estradiol may be less than 50 pg/mL, and no bleeding is induced in response to progestogen challenge. Others may have spontaneous episodes of vaginal bleeding that reflect estrogen concentrations in the range of 50 to 200 pg/mL in association with follicular activity. In these women, LH pulses are present, although at a reduced frequency.20
Treatment of a hyperprolactinemic woman with amenorrhea depends on whether she wants to become pregnant, the presence and size of a pituitary tumor, and the presence of associated symptoms such as galactorrhea. If tumor diameter exceeds 1 cm, it normally should be treated before the patient becomes pregnant.24 If untreated, these women have a small but definite risk for further enlargement of the tumor during pregnancy, with subsequent pressure on the optic chiasma. Many of these tumors may be enucleated safely by the transsphenoidal route with minimal morbidity and preservation of function of the anterior pituitary.25 The operation is not totally without risk, however, and is curative in only about 60% to 80% of patients.
Even in the presence of significant tumors, medical treatment with a dopamine agonist is the first line of treatment.25 Dopamine agonists interact with dopamine receptors of the lactotrophs and inhibit the secretion of prolactin. Cabergoline is now the treatment of first choice, with a higher remission rate and a significantly lower level of side effects than are seen with bromocriptine.22 Concerns have been raised about an observed association with chronic pleuropulmonary, pericardial, and retroperitoneal fibrosis, but the implications of this for clinical practice are not clear.26 Using a twice-weekly dosage with a total weekly dosage of up to 3 mg, cabergoline reduces the concentration of prolactin and produces a coincidental rise in the frequency of LH pulses. Although menses usually return within 3 months, the first few cycles may be anovulatory or may have a short and/or inadequate luteal phase. Normal ovarian cyclicity is restored within 6 months in most cases, and the fertility rates are satisfactory.
Usually, it is recommended that a dopamine agonist should be stopped as soon as pregnancy is diagnosed, although no conclusive evidence of teratogenicity has been found. Some authorities recommend use of bromocriptine for women trying to conceive on the basis that cabergoline is a newer preparation, but it is not clear that bromocriptine is less likely to be teratogenic than cabergoline. In the rare instance of tumor enlargement in pregnancy, a dopamine agonist can be restarted safely during pregnancy, and this usually results in prompt shrinkage of the tumor. Evidence that permanent remission sometimes can be achieved with cabergoline is increasing,22 and in the absence of a significant tumor, it is reasonable to have a trial cessation of therapy after 1 to 2 years.
It is not necessary to use a dopamine agonist to treat hyperprolactinemic women with no symptoms, no tumor, and no desire to conceive. Moreover, not all hyperprolactinemic women are deficient in estrogen, and restoration of cyclic ovarian function may provoke menstrual symptoms that require treatment. However, if a dopamine agonist is not given, some form of therapy is required to treat the osteoporosis in estrogen-deficient women. If a dopamine agonist is given to those who are sexually active, it is necessary to provide some form of contraception (avoiding the combined pill) to prevent unwanted pregnancy. The long-term consequences of repeated ovarian cycles (breast and endometrial cancer) may well outweigh the risks for osteoporosis. The relative health risks and benefits of treatment versus no treatment must be weighed carefully in each case. Whatever the choice, the woman should be informed of the possible long-term risks and should be monitored at intervals of 6 to 12 months.
Pituitary Disease
Rare pituitary tumors may cause amenorrhea without significant prolactin secretion through compression of the portal tract. An MRI scan thus is important in all otherwise undiagnosed women with amenorrhea of hypothalamic-pituitary origin. Hypogonadotropic amenorrhea is also likely in all cases of clinical hypopituitarism, whether it is due to previous surgery, Sheehan’s syndrome, or other pituitary disease. Measurement of other pituitary hormones will clarify this.
Hypothalamic Amenorrhea
Women with hypothalamic amenorrhea are characterized by progestogen-negative amenorrhea in the presence of low or normal gonadotropins and the absence of any demonstrable pathology such as hyperprolactinemia, tumors, or pituitary disease.
Eugonadotropic hypothalamic amenorrhea is commonly related to metabolic disorders subsequent to stress, weight loss, and extremes of exercise. In particular, hypothalamic amenorrhea is an important feature of serious psychological disease such as anorexia nervosa and bulimia. It thus is very important to ensure that the underlying psychological disease is adequately treated before the amenorrhea is managed, and that body weight has returned to normal before any endocrine therapy is started. Ovulation induction in women significantly below ideal body weight may result in a pregnancy complicated by intrauterine growth retardation, with significant health consequences for the child.
As was discussed earlier, amenorrhea in the presence of apparently normal gonadotropin concentrations results from changes in the normal pattern of GnRH pulsatility. Several metabolic changes and endocrine changes that occur in women who are underweight or are strenuously exercising can contribute to this. The principle of a critical body weight above which menstruation returns is generally accepted and appears to be approximately 15% below ideal body weight.27 Considerable interindividual variation is seen in this model, however, for reasons that have not been apparent until recently.
Greater understanding has emerged since the identification of leptin, a small protein hormone secreted by adipocytes with a critical role in the regulation of appetite and metabolism.28,29 It now is clear that reduced body weight is associated with reduced secretion of leptin, and that a consequently reduced leptin stimulus to the GnRH pulse generator may play a role in amenorrhea associated with significant weight loss.29 In addition, replacement of leptin has been found to reverse hypothalamic amenorrhea.30 Exercise is associated with metabolic changes, in particular, increases in corticotropin-releasing hormone (CRH) and β-endorphin concentrations in the hypothalamus that may inhibit GnRH pulsatility.31 In some trials, restoration of normal LH pulsatility and ovulation has been observed in response to treatment with opioid inhibitors such as naltrexone,32 although the effects of opioid inhibitors have not been consistent.
Idiopathic Hypogonadotropic Hypogonadism
Idiopathic hypogonadotropic hypogonadism (IHH) has been defined as GnRH deficiency in the absence of a hypothalamic or pituitary lesion.33 GnRH deficiency (with a correspondingly low gonadotropin concentration) accounts for only a small proportion of women whose amenorrhea is due to hypothalamic-pituitary dysfunction. Patients with anosmia previously were considered to form the separate group defined as Kallmann’s syndrome,34 a condition that occurs five to seven times more commonly in men than in women, in whom it is seen only rarely. However, it is now clear that there is considerable overlap between Kallman’s syndrome and IHH, and both entities have been described in the same family.33 GnRH deficiency has been associated with several other genetic defects, in addition to mutations in the classic KAL1 gene of Kallman’s syndrome and the adhesion molecule, anosmin-1. Other genes cited in IHH include the DAX-1 gene, the GnRH receptor gene, and others such as FGFR1, GPR54, PROK2, PROKR2, FGF8, CHD7, TAC3, and TAC3R,35 although no defects have been identified in the GnRH gene itself.33 The classic X-linked inheritance of Kallman’s syndrome appears to represent only a small number of cases, and autosomal dominant or autosomal recessive modes of inheritance are possible.36 Even among the carefully defined group of women with IHH, however, the incidence of identifiable genetic abnormalities is low, and it is not appropriate to search routinely for them in clinical practice.36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








