FIGURE 10-1. Schematic representation of the hypothalamic-pituitary-adrenal axis, showing sites of glucocorticoid negative feedback. ACTH, Adrenocorticotropic hormone; CRH, corticotropin-releasing hormone.
History
The many important contributions made to the understanding of ACTH physiology make it difficult to provide a synopsis. However, the following events are some of the major milestones:
Pro-Opiomelanocortin Gene
STRUCTURE OF THE POMC GENE
Humans have a single POMC gene located on the short arm of chromosome 2 at 2p23 (the mouse and pig have two copies of the gene). The structure of the gene is well conserved and has been characterized in humans15–17 as well as in other species.18 The POMC gene consists of three exons interspersed with two large introns (Fig. 10-2). The first exon, which consists of 87 base pairs (bp), contains no coding sequence, and its RNA transcript is thought to act as a leader sequence that binds the ribosome at the start of translation. Exon 2 (152 bp) contains the initiation sequence, a signal sequence that translocates the nascent peptide into the endoplasmic reticulum, and then the N-terminal part of the coding sequence for the POMC peptide. The third exon (835 bp) encodes most of the mature protein, including ACTH,15–17 the termination codon, and the signal for addition of the poly A tail.
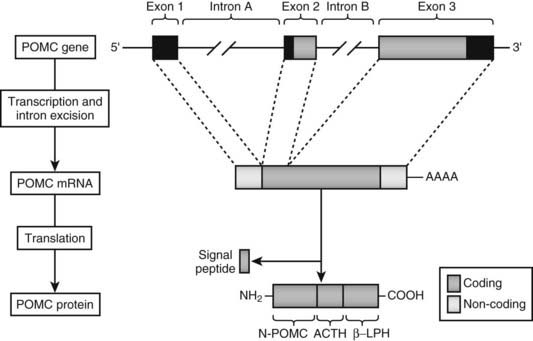
FIGURE 10-2. Genomic structure of human pro-opiomelanocortin (POMC) with the major spliced product and preprohormone. ACTH, Adrenocorticotropic hormone; βLPH, β-lipotropin.
POMC Transcripts
The POMC gene has three RNA transcripts of 1200 (T1), 800 (T2), and 1380 (T3) nucleotides, respectively (Fig. 10-3).
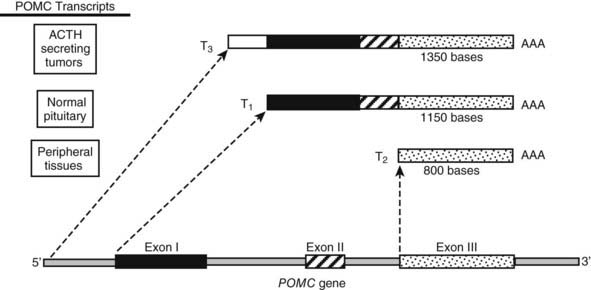
FIGURE 10-3. Tissue-specific transcriptional variants of the human pro-opiomelanocortin (POMC) gene. T1 has high-level expression of POMC in pituitary tissue. T2 has low-level expression in numerous extrapituitary tissues. T3 is present in some extrapituitary tumors causing the ectopic adrenocorticotropic hormone (ACTH) syndrome. AAA, Poly A tail.
The Pituitary Transcript T1
T1 is the mRNA transcript found in corticotrope cells of the anterior pituitary in man and has a size of 1200 nucleotides as detected by Northern blotting.19 In the hypothalamus, the POMC mRNA transcript seems to be identical to the pituitary transcript except for a longer poly A tail.20
The Upstream Transcript T3
The T3 transcript is 1380 nucleotides in size and is presumed to be under the regulation of promoter elements that lie upstream of the pituitary promoter. This transcript produces the same peptide product as T1, because the only relevant translation initiation site is in exon 1. This longer POMC mRNA transcript has been found in normal tissues (e.g., placenta21,22) and has also been associated with abnormal expression of POMC such as that seen in small cell lung carcinoma (SCLC).23
The Downstream Transcript T2
The downstream T2 transcript is an 800-nucleotide RNA transcript that has been shown in humans and rats to arise from transcription initiation at the 5′ end of exon 3.24,25 This finding suggests that regulatory sequences may occur starting from the 3′ end of intron 2.18 This transcript could not give rise to a mature POMC molecule and would lack a signal peptide, so its physiologic role is unclear.26 The smaller POMC transcript is found primarily in a variety of peripheral tissues, which indicates possible differential regulation in these tissues.
Regulatory Sites for Transcription
The POMC promoter has a number of common elements found in other genes that may contribute to regulation of POMC gene transcription (Fig. 10-4). Owing to the lack of availability of a human corticotrope cell line, the mouse-equivalent cell line (AtT20) has been used to assess POMC transcriptional regulation along with transgenic mouse models,27 although some studies have utilized primary human pituitary cells from surgical procedures.28
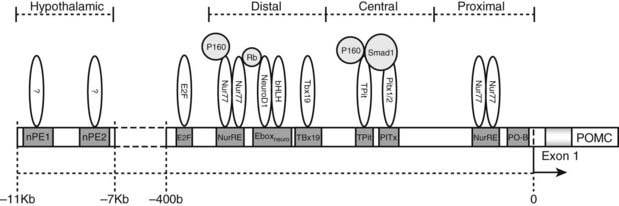
FIGURE 10-4. The promoter region of the pro-opiomelanocortin (POMC) gene. Response elements are shown along with bound transcription factors in association with known coregulators (light grey). bHLH, Basic-helix-loop-helix transcription factors; E2F, transcription factors involved in cell cycle regulation and synthesis of DNA; Ebox, specific DNA sequence that binds heterodimers of Neuro D1 and other bHLH proteins; Neuro D1, neurogenic differentiation 1 transcription factor; nPE, neural pro-opiomelanocortin enhancer; Nur77, also known as nerve growth factor IB (NGFIB), a member of a family of transcription factors involved in cell cycle mediation, inflammation, and apoptosis; NurRE, Nur factor response element; P160, family of coactivators; Pitx, a homeobox family member which is involved in organ development; PO-B, a transcription factor originally described as POMC specific. Rb, Retinoblastoma protein, a tumor suppressor protein; Smad1, a transcriptional modulator that mediates multiple signaling pathways; Tbx19, transcription factors present only in pituitary POMC-expressing cells and involved in the regulation of developmental processes; Tpit, transcription factors involved in regulation of development.
The T1 and T3 mRNA transcripts of POMC are initiated from a TATA box that is located close to the start of exon 1.
The correct spatial, temporal, and hormonal regulation of POMC transcription in the pituitary is conferred by two promoter regions immediately 5′ of exon 1. These regions are between −314 and −276 bp and between −67 and −27 bp in the human POMC gene.27,29,30
Hypothalamic and CNS-specific expression of POMC has been shown to require DNA control elements distal to those required for POMC expression in the pituitary. A 13 kb region immediately 5′ of the POMC gene has been demonstrated to control CNS and hypothalamic expression in transgenic mice.31 A 4-kb distal region of the POMC gene situated −13 to −9 kb in mouse and −11 to −7 kb in human from POMC exon 1 has been shown to contain two neuronal-specific enhancer regions capable of directing POMC expression in the arcuate nucleus of the hypothalamus.12 These two neuronal POMC enhancer regions (nPE1 and nPE2) have been shown by deletion studies to be individually capable of driving POMC transcription in the arcuate nucleus and have also been demonstrated to be inactive in the pituitary, implying that there is a modular independence between the promoter regions used to control pituitary and hypothalamic POMC transcription.12
The expression of the short POMC mRNA transcript (T2) seems to be regulated by “GC box” promoter sequences located in the 3′ end of intron 2.19
EXPRESSION OF THE POMC GENE
Expression in the Pituitary
In humans, expression of POMC is most abundant in the corticotrope cells of the anterior pituitary, and in healthy subjects, these cells are the only ones that express the gene at high levels.32,33 POMC is one of the top 10 most abundant transcripts in the pituitary gland.34 The main POMC mRNA expressed in the pituitary is the T1 transcript with a size of 1200 nt. POMC mRNA is also detected in the intermediate lobe of the pituitary, which is present during fetal life in humans and is found in other species such as the mouse and rat.35–37
Pituitary expression is conferred by the 5′ flanking region of the gene25,27,38–40 (see Fig. 10-4), but there does not appear to be a specific element sufficient to direct high-level transcription, as for example, in the prolactin gene, where the pituitary-specific transcription factor Pit-1 binds to multiple sites to direct transcription. Rather, in the case of the POMC gene, there appears to be a requirement for integrity of the promoter.
The region just 5′ to the start site close to the TATA box confers basal expression and contains the binding sites for PO-B (at −27 nucleotides) and NUR 77 (−67 nucleotides). Further upstream, in the central region of the pituitary promoter, there is a response element which binds the homeobox protein Pitx141 and the related Pitx2.42 During development, Pitx1/2 play an important role in corticotrope development and in the development of the anterior pituitary in general.42–45 Close to the response element which binds Pitx1, there is a binding site for the T box factor, Tpit, which acts in synergy with Pitx1 and is required for expression of the POMC gene and for terminal differentiation of the pituitary corticotrope lineage.46 Tpit functions as an activator of transcription by recruiting SRC/p160 co-activators to its cognate DNA target in the POMC promoter.47
Evidence for the importance of the role of Tpit comes from Tpit-deficient mice, which represent a model of isolated ACTH deficiency, and from humans with Tpit gene mutations which are associated at high frequency with early-onset isolated ACTH deficiency (IAD).48,49 One cause of neonatal death has been identified as congenital IAD associated with mutations of the Tpit gene.50 Different Tpit mutations have been associated with IAD, but all these mutations are likely to manifest functionally through disruption of DNA-protein and protein-protein interactions. In one case, a mutation of the Tpit gene (M86R) has been shown to inhibit the binding of other DNA-bound proteins, ultimately leading to loss of recruitment of the p160 coactivator SRC-2.51
Other evidence for the importance of Tpit relates to bone morphogenic proteins (BMP) 4 and 2, which are signaling molecules associated with early organogenesis and cell differentiation of the pituitary. BMP4 stimulation leads to the recruitment of activated phospho-Smad1 by the POMC promoter through “tethered” interactions with Pitx1 and Tpit, thus reducing their transcriptional activity and resulting in repression of POMC transcription.52
The distal region of the pituitary promoter cannot confer activity independently of the central region but does contain a binding site (Eboxneuro) for NeuroD/1A acting as a heterodimer with other basic helix-loop-helix (bHLH) factors and synergizing with Pitx.40,53 The Eboxneuro element of the distal pituitary promoter, and a nearby Nur response element (NurRE), are required to modulate expression of POMC throughout pituitary development.54 A member of the T-box gene family, Tbx19, a pituitary development–specific transcription factor, has been shown to have a putative binding site at −310 nt, close to the Eboxneuro site. However, this protein may also exert POMC transcriptional effects synergistically with other pituitary-specific transcription factors.55
Expression in Other Tissues
POMC is also expressed, but at a much lower level, in other tissues such as the arcuate nucleus of the hypothalamus, skin, testis, ovary, placenta, duodenum, liver, kidney, adrenal medulla, lung, thymus, heart, and lymphocytes.14,19,22,25,56–59 Extrapituitary POMC mRNA is frequently expressed as the 1200 nt T1 transcript (similar to that in the pituitary), for example in the hypothalamus, skin, and placenta. However, POMC mRNA from extracranial tissues can also have a preponderance of the shorter 800 nt T3 transcript.58,60,61 As indicated earlier, this shorter T2 transcript could not give rise to mature POMC and would lack a signal sequence, so its physiologic role is unclear. It may be that low-level expression of longer POMC transcripts (T1 and T3) account for POMC transcription and expression even when there is high expression of the short transcript, for example as demonstrated in the testes.20,60,62
POMC expression and regulation in the skin is now well established.14,63,64 Current evidence would suggest that POMC transcription in the skin is regulated by the region immediately 5′ of exon 1 in keratinocytes64 and melanocytes.65
Expression in Pituitary Tumors
In corticotrope adenomas that give rise to pituitary-dependent Cushing’s disease, POMC is the most highly expressed gene,66 similar to that in the normal pituitary.34,67 The loss of retinoblastoma tumor-suppressor protein (Rb) expression has been linked to pituitary corticotrope tumor progression.68 It has been shown that Rb is a transcriptional activator of POMC by bridging between NeuroD and Nurr77 and potentiating interactions between Nurr77 and the p160 co-activator SRC-2.69,70
Expression in Nonpituitary Tumors
Tumors giving rise to the ectopic ACTH syndrome produce an mRNA transcript of 1200 bp, similar to that found in the pituitary, and approximately 20% of tumors express a larger transcript of 1400 to 1500 bp.67 This larger transcript seems to be under the regulation of a promoter region located at −392 and −432 bp relative to the conventional start site.21,71–73 Analysis of this domain in the human small cell lung carcinoma cell line DMS-79 showed that it binds the E2F family of trans-acting factors.74
The expression of this promoter in the ectopic ACTH syndrome suggests loss of the tight tissue-specific expression. This promoter is embedded within a CpG island which has been shown to be unmethylated in a number of tumors giving rise to the ectopic ACTH syndrome and in the POMC-expressing small cell lung carcinoma cell line, DMS-79.75 In contrast, the CpG island was methylated in normal nonexpressing tissues.27
Recently it has been shown that hypomethylation of the POMC promoter in thymic carcinoid tumors correlates with POMC overexpression and the ectopic ACTH syndrome. The region of the POMC promoter that underwent change in methylation status was shown to correspond to the E2F binding region of the POMC promoter.76
REGULATION OF POMC GENE EXPRESSION
Regulation of the POMC Gene in the Pituitary
Numerous factors are known to regulate POMC gene expression in the pituitary, but perhaps the most important are CRH and glucocorticoids (Fig. 10-5). Expression of the POMC gene appears to be predominantly controlled at the level of gene transcription.77
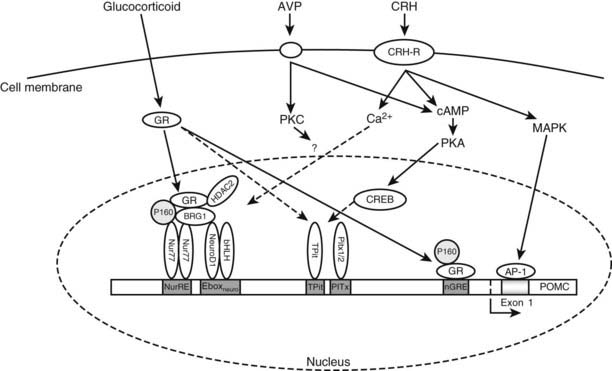
FIGURE 10-5. Intracellular signaling pathways regulating transcription of the pro-opiomelanocortin (POMC) gene. Through its receptor, corticotropin-releasing hormone (CRH) induces cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA) and thereby phosphorylation of cAMP response element–binding protein (CREB). CRH also activates mitogen-activated protein kinase (MAPK) pathways, which ultimately induce activator protein-1 (AP-1) binding to an exon 1 response element. Arginine-vasopressin (AVP) activates cAMP and/or protein kinase C (PKC) pathways, which may also feed into this pathway. Glucocorticoids acting through the glucocorticoid receptor (GR) can repress transcription through two cooperative binding sites.
Corticotropin-Releasing Hormone Stimulation of the POMC Gene
CRH binds transmembrane receptors on corticotrope cells and stimulates cyclic adenosine monophosphate (cAMP) production and activation of protein kinase A (PKA)78 (see Fig. 10-5). CRH effects on POMC transcription do not require de novo protein synthesis.79 There is no cAMP response element (CRE) in the promoter region of the POMC immediately 5′ of exon 1, but two DNA elements have been identified that appear capable of conferring CRH responsiveness to the gene. One element at −171 to −160 nt upstream from the transcription start site binds a protein termed the CRH response element–binding protein.80,81 The second element reported to be CRH responsive is found in the noncoding exon 1 of the rat POMC gene.81,82 This element in exon 1 (+41/+47) shares close homology with a consensus activator protein-1 (AP-1) transcription factor–binding site and binds recombinant AP-1 protein and cAMP response element–binding protein (CREB) in a sequence-specific manner.82,83
CRH causes activation of mitogen-activated protein kinase (MAPK) and induction of the DNA-binding activity of AP-1 in the mouse pituitary corticotrope cell line, AtT20.78,84 In addition, the POMC exon 1 element confers both phorbol ester and CRH responsiveness to a heterologous promoter.84 Therefore, there is considerable evidence for a physiologic role of the MAPK/AP-1 cascade in mediating some actions of CRH.81,85,86
In AtT-20 corticotropes, CRH, and cAMP induce Nur77 expression, and POMC transcription is activated through the NurRE site by protein kinase A (PKA) and calcium-dependent and calcium-independent mechanisms.78 The NGFI-B (Nur77) subfamily of orphan nuclear receptors (NRs), which also includes Nurr1 and NOR1, bind the NurRE as either homo- or heterodimers formed between subfamily members. Nur factors behave as endpoint effectors of the PKA signaling pathway acting through dimers and AF-1-dependent recruitment of co-activators, such as TIF2.79
Tpit/PitxRE also mediates CRH-induced activation of POMC gene expression in a calcium-dependent manner. Clearly Tpit/PitxRE is an important element by which both CRH and Gcs regulate the POMC gene expression.87
Glucocorticoid Inhibition of the POMC Gene
Glucocorticoids are known to decrease ACTH levels, mainly as a result of inhibition of POMC transcription, as highlighted in the GR knockout mouse, where there is an increase in POMC expression in corticotropes,88 although they also act at the level of translation89 and antagonize actions of CRH90 (see Fig. 10-5). Considerable evidence indicates that glucocorticoids suppress transcription of the POMC gene.91–95
Glucocorticoids enter the cell, where they bind glucocorticoid receptors complexed to heat shock proteins in the cytoplasm. This results in the translocation of the ligand-bound receptor to the nucleus, where it recruits co-regulator proteins and acts as a transcription factor, binding (usually as a dimer with another glucocorticoid receptor) to the promoter region of a gene in order to regulate gene expression. This process is modulated by the presence of tissue-specific co-regulators.96
In the pituitary corticotrope, the glucocorticoid receptor mediates inhibition of the POMC gene. In the rat POMC gene, there are four sites through which glucocorticoid action is mediated, although only those at −63 and between −480 and −320 are needed in vivo.97 This latter glucocorticoid-regulated element is required to interact for the full glucocorticoid repression of pituitary POMC expression to be manifested. The −63 negative glucocorticoid-regulated element overlaps the putative COUP (chicken albumin upstream promoter) box98 and the proximal Nur response element.99 It has been suggested that the inhibitory effect of glucocorticoids on POMC transcription may occur by displacement of a stimulatory factor such as Nurr 77.99,100 In the mouse corticotrope cell line, AtT20, the Nurr77-mediated actions of CRH are antagonized by glucocorticoids. The mechanism of glucocorticoid transcriptional repression through this region of the POMC promoter involves the co-repressor HDAC2 and the Swi/Snf chromatin-remodeling protein Brg1 in the modulation of the recruitment of GR to the Nur77-bound NurRE site.13,101 The loss of nuclear expression of Brg1 or HDAC2 has been associated with 50% of glucocorticoid-resistant human and dog corticotrope adenomas.13
The importance of co-regulators in glucocorticoid receptor-mediated regulation of POMC transcription is demonstrated by the inhibition of pituitary POMC transcript levels in mice with a deletion of SRC-1, a glucocorticoid receptor co-activator.102 However, this inhibition of POMC may be through SRC-1 interaction with other transcription factors that affect POMC transcription.
Stimulation of the POMC Gene by Arginine Vasopressin
A number of other hypothalamic factors act on the pituitary corticotrope to influence POMC expression; however, their modes of action are less well defined. In particular, arginine vasopressin (AVP) augments the effect of CRH and can act independently, though rather weakly, to stimulate POMC expression.32,103–105 AVP acts on corticotropes via V1b receptors, resulting in the activation of the protein kinase C pathway and leading to a “cross-talk” interaction with the cAMP/protein kinase A pathway activated by binding of CRH to CRH1 receptors.106
Leukemia Inhibitory Factor Stimulation of the POMC Gene
A number of lines of evidence point to intrapituitary factors as important modulators of corticotrope function. One such factor is the proinflammatory cytokine, leukemia inhibitory factor (LIF). This factor has been shown to stimulate the POMC gene through STAT-3 at a response element that overlaps with the −166-nucleotide CRH response element, although this site does not directly bind STAT transcription factors.85,107,108 However, a functional STAT1-3 binding site was identified in the distal region of the POMC promoter,108 and this region has been shown to mediate LIF-CRH synergy through a mechanism involving synergy with the NurRE.109
Recently an interaction between LIF and glucocorticoids has been demonstrated that reduces the repressive properties of GR on POMC expression. This may occur by the loss of a co-repressor from GR tethered to Nurr77 at the NurRE.110 Other modulators of LIF effects on POMC transcription include CCAAT/enhancer–binding protein β (C/EBPβ) and glial cell–derived neurotrophic factor (GDNF)-inducible factor (GIF).111
Regulation of the POMC Gene in Other Tissues
POMC expression can occur in a wide range of nonpituitary tissues, as described earlier, although in many of these extrapituitary tissues, the shorter POMC transcript predominates, leading to extremely low levels of protein.19 However, there are several tissues where there is significant POMC expression, and subtle differences occur in regulation compared to the pituitary.
Brain
POMC is expressed mainly in the arcuate nucleus of the hypothalamus, although it is also expressed at lower levels in the hippocampus and cortex.112,113 In the arcuate nucleus, POMC plays a major role in regulating food intake and energy balance.114 Its importance is highlighted by children with mutations in the POMC gene who are obese.115 POMC is regulated by leptin, insulin, and glucose to generate an anorexigenic effect116,117 (Fig. 10-6). Fasting in rats causes a decrease in both POMC and ACTH peptides.118
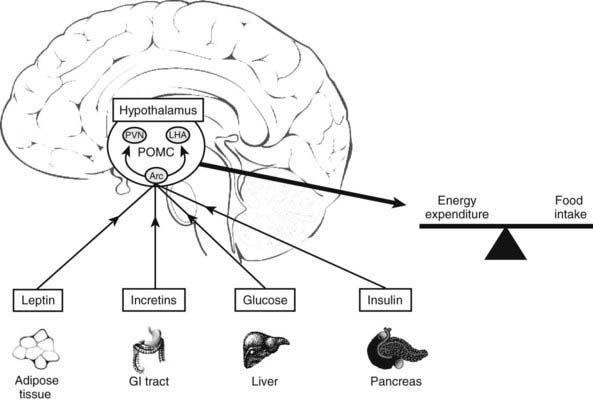
FIGURE 10-6. Factors that influence POMC expression in the arcuate nucleus (Arc) of the hypothalamus. PVN, Paraventricular nucleus; LHA, lateral hypothalamus.
Surprisingly, POMC expression is up-regulated by glucocorticoids in the hypothalamus. Adrenalectomized rats have a marked decrease in POMC mRNA, an effect that is completely reversed by glucocorticoid treatment.119,120
Skin
POMC is expressed in several components of the skin, including melanocytes, keratinocytes,121,122 and dermal microvascular endothelial cells123 (Fig. 10-7). The expression of POMC in the skin is regulated by both glucocorticoids and CRH but also by ultraviolet radiation.
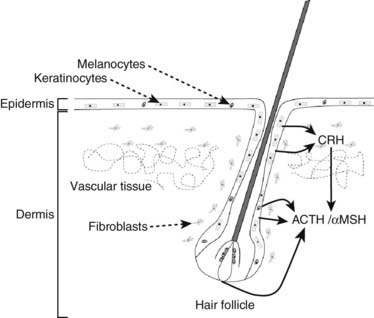
FIGURE 10-7. Components of the hypothalamic-pituitary axis present in the skin. Corticotropin-releasing hormone (CRH) released from skin and hair follicle cells can induce the secretion of adrenocorticotropic hormone (ACTH) and alpha melanocyte-stimulating hormone (αMSH) elsewhere in the skin.
The effect of glucocorticoids on POMC expression has been shown in mouse skin, where a down-regulation has been demonstrated which is coupled to hair follicle cycling.14,124 CRH expressed in skin cells up-regulates local POMC expression,14,64 and this is inhibited by glucocorticoids.125 POMC expression in the skin is increased by ultraviolet light,65,126 which also induces CRH production in human melanocytes, with subsequent stimulation of the CRH signaling pathway resulting in POMC expression.65 UV induction of POMC expression in mouse skin can be directly controlled by p53, and the mouse POMC promoter is stimulated by p53 in response to UV,127 although p53 is not the main or sole regulator of POMC expression.128
Regulation of the POMC Gene in Tumors
Corticotropin-Releasing Hormone Regulation of the POMC Gene in Tumors
In general, CRH stimulates POMC expression only in pituitary corticotrope tumors, but a few exceptions occur in ectopic tumors.132–134 However, it is likely that the increased ACTH stimulation of glucocorticoids will result in inhibition of the CRH gene. Therefore CRH may not be relevant in POMC-expressing tumors.
Glucocorticoid Regulation of the POMC Gene in Tumors
In pituitary corticotrope cells, expression of the POMC gene is repressed by glucocorticoids, and in pituitary corticotrope tumors, glucocorticoids are able to repress ACTH secretion. In contrast, in extrapituitary tumors, ACTH is characteristically resistant to glucocorticoids.135 This concept is the basis of the high-dose dexamethasone suppression test used to distinguish pituitary from ectopic sources of ACTH in Cushing’s syndrome. Most extrapituitary tumors are resistant to glucocorticoid inhibition of POMC expression, and this suggests that the mechanism of this glucocorticoid resistance is of importance.
A panel of human small cell lung carcinoma cell lines have been established as models of the ectopic ACTH syndrome.33,136 These cell lines express the POMC gene, and the glucocorticoid receptor has been found to be present, although at low levels.18,33,137 Significantly, all cell lines studied are resistant to glucocorticoid suppression. To determine whether glucocorticoid signaling was functional, a synthetic, glucocorticoid-responsive gene linked to a reporter gene was transfected into the cells. In contrast to the brisk induction of expression seen in control pituitary cells, none of the human small cell lung carcinoma cells responded to either natural or synthetic glucocorticoids.137 Thus resistance of the POMC gene to glucocorticoids is part of a global resistance of these malignant cells to glucocorticoid action. Expression of high concentrations of wild-type glucocorticoid receptor in the cells was found to be sufficient to restore glucocorticoid signaling.137 In two of the cell lines, mutations in the endogenous glucocorticoid receptor appeared to be the cause of the resistance,136,138 and in another cell line, altered co-regulator expression and recruitment by the glucocorticoid receptor was the cause of resistance.139
Because glucocorticoids can inhibit proliferation in some cell types and induce differentiation in others, it is possible that evasion of glucocorticoid signaling confers a survival advantage to the malignant cells. Indeed, when high levels of a wild-type glucocorticoid receptor were expressed in one of the resistant cell lines, it led to cell death by apoptosis,139a this suggests that the glucocorticoid resistance does confer a survival advantage, and because it also disinhibits POMC expression and ACTH secretion, these become biomarkers of a malignant phenotype.
Adrenocorticotropic Hormone and Related Peptides
STRUCTURE AND PROCESSING OF POMC AND RELATED PEPTIDES
Many bioactive peptides are synthesized from large precursor molecules, and a number of techniques have been used to elucidate the structures of these peptides. Studies have used pulse chase analysis whereby labeled amino acids are incubated with cells to detect the labeled precursors and the peptides derived from them. Subsequently, sequence analysis and cDNA cloning have been important approaches to determine peptide structures. Discovery of the structure and biosynthesis of POMC and ACTH-related peptides and the differences between species is reviewed extensively by Eipper and Mains.140
POMC
In 1973, high-molecular-weight forms of ACTH were identified in human plasma,141 mouse pituitary cells,142,143 and human tumors,144 which led to predictions of the presence of a precursors of ACTH.
Expression of the POMC gene leads to synthesis of the preprohormone POMC. This protein undergoes proteolytic cleavage at dibasic amino acid residues, which generates a series of small molecules, including ACTH140 (Fig. 10-8). Processing of POMC to its constituent peptides varies in a tissue-specific fashion, in that both the nature of the processing and the degree of processing varies in different tissues. This results in different groups of peptides being secreted from different tissues, although the exact ratios of the constituent peptides and precursors are still not fully understood.
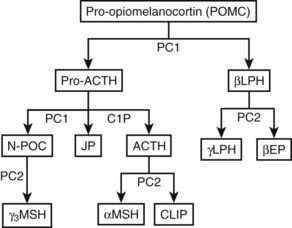
FIGURE 10-8. Processing of pro-opiomelanocortin (POMC). POMC is cleaved into pro-adrenocorticotropic hormone (pro-ACTH) and β-lipotropin (βLPH). Further processing of pro-ACTH yields ACTH, joining peptide, and N-pro-opiomelanocortin (N-POC), all of which are found in human plasma. Cleavage to smaller fragments occurs in a tissue- and species-specific manner. Shaded boxes represent peptides found in the human circulation. CLIP, Corticotropin-like intermediate lobe peptide; EP, endorphin; JP, joining peptide; LPH, lipotropin; MSH, melanocyte-stimulating hormone; PC, prohormone convertase.
ACTH
The ACTH peptide consists of 39 amino acids, is a single polypeptide chain, and has a molecular weight of 4.5 kD145 (see Fig. 10-8). The N-terminal 12 amino acids are highly conserved between species, thus reflecting the importance of this region for biological activity. In comparison with the human sequence, ACTH in other mammals has only one or two substitutions, which are in the region of amino acids 24 to 39. In birds, amphibians, and fish, although the N-terminal sequence is conserved, the ACTH sequence is more variable, particularly between amino acids 24 and 39. The melanocyte-stimulating hormone (MSH) sequence His-Phe-Arg-Trp is found at ACTH 6–9 and is termed α-MSH. Identical sequences are present in β-lipotropin (as β-MSH) and N-pro-opiomelanocortin (N-POC) (as γ-MSH). Given that these three forms of MSH bind different melanocortin receptors, it is thought that the surrounding amino acids influence their specific activity.
α-Melanocyte-Stimulating Hormone
α-MSH consists of ACTH 1–13 and is derived from ACTH 1–39 by proteolysis at the C-terminal, which is followed by C-terminal amidation and N-terminal acetylation. α-MSH is produced predominantly by melanotrope cells in the intermediate lobe of the pituitary, particularly in species such as the rat and mouse. The adult human pituitary does not have a distinct intermediate lobe, and therefore this is not a source of α-MSH in humans. In addition, α-MSH is not thought to be produced in the anterior lobe. Therefore, it is not clear whether α-MSH circulates in humans under normal circumstances.146,147
CLIP
Corticotropin-like intermediate lobe peptide (CLIP) consists of ACTH 18–39 and is produced during the cleavage that generates α-MSH. Because this process occurs primarily in the intermediate lobe of the pituitary, which is not present in humans, CLIP is not thought to circulate in humans under normal circumstances.
N-Pro-opiomelanocortin
Also called N-pro-opiocortin, N-POC (see Fig. 10-8) comes from the N-terminal sequence of POMC, and in humans, it is a 76–amino acid peptide with an MSH sequence in the mid-region.148 The peptide has a tryptophan residue at the N terminus and two disulfide bridges linking cysteines 2 to 24 and 8 to 20, which are thought to be important for the sorting signal that directs POMC to the regulated pathway.149 N-POC can also undergo N-glycosylation at Asn65 and O-glycosylation at Thr45.
γ-Melanocyte-Stimulating Hormone
γ1-MSH is found at position 51 to 62 of human N-POC and has sequence homology with α-MSH. There are C-terminally extended forms of γ1-MSH, which are called γ2-MSH (51–63) and γ3-MSH (51–76).
Joining Peptide
Joining peptide is found between N-POC and ACTH and is a 30–amino acid peptide, amidated at the C terminus. It was isolated from human pituitaries in 1981148 and has been shown to circulate in humans in the form of homodimers.150
β-Lipotropin
β-Lipotropin lies at the C terminus of POMC and can be cleaved to γ-lipotropin (which contains the β-MSH sequence at its C terminus) and β-endorphin (see Fig. 10-8). In the human anterior pituitary, cleavage appears to be limited, inasmuch as the main form of this peptide in the human circulation is β-lipotropin with very little β-endorphin.151,152
β-Endorphin
This 31–amino acid peptide contains the sequence for met-enkephalin as the first five amino acids at its N terminus. β-Endorphin can undergo N-acetylation, which is thought to be a tissue-specific effect, and C-terminally truncated peptides have been found, such as α-endorphin (β-endorphin 1–16), γ-endorphin (β-endorphin 1–17), and δ-endorphin (β-endorphin 1–27).
THE PROCESSING PATHWAY AND PROCESSING ENZYMES
Processing
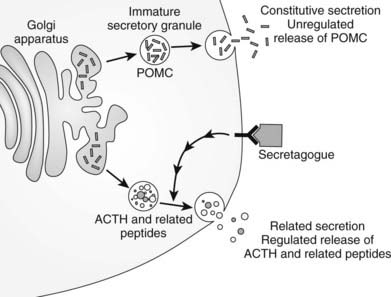
After translation of POMC mRNA into peptide, a series of processing stages are needed for release of the constituent peptides.112,114,130,153 The N-terminal signal sequence involved in movement of the peptide into the endoplasmic reticulum is no longer required and is removed at an early phase of posttranslational modification. Subsequently, POMC undergoes glycosylation and phosphorylation in the Golgi apparatus before transport to secretory vesicles, where it undergoes cleavage into its constituent peptides. The ACTH-related peptides are stored in dense core secretory granules and released from the cell on stimulation (e.g., by CRH), as in the stress response (Fig. 10-9). The prohormone, POMC, is found in the human circulation154 and could be released from the constitutive pathway perhaps as an “overflow” mechanism.
N-Glycosylation and Phosphorylation
These events occur in the Golgi apparatus before cleavage of the peptides. γ-MSH has the sequence Asn-X-Ser, which can be glycosylated on the Asn residue, and in mouse POMC, N-glycosylation of the CLIP sequence can occur. Some evidence indicates phosphorylation of serine 31 in ACTH, although the significance of this finding is unclear.155
Processing Enzymes
POMC is cleaved to its constituent peptides by limited proteolysis at pairs of basic amino acids, primarily Lys-Arg and Arg-Arg. The mammalian convertases responsible for this endoproteolytic cleavage are precursor converting enzymes from the subtilisin/Kex2 serine proteases, which include furin, a protease known to cleave peptides in the constitutive pathway of secretion.156 Prohormone convertase 1, or PC1 (also called PC3),157 cleaves POMC preferentially at pairs of basic residues and produces ACTH, β-lipotropin, N-POC, and joining peptide in the anterior pituitary. Although cleavage can begin in the Golgi apparatus, it continues in secretory vesicles. PC1 can be regulated in a manner similar to POMC in that PC1 mRNA is up-regulated by CRH and decreased by glucocorticoids in mouse AtT20 cells.158
ACTH 1–39 can be further cleaved by PC2 to produce ACTH 1–17 and CLIP. PC2 cleaves at different pairs of basic residues to PC1 to produce the smaller peptides159 (see Fig. 10-8). PC2 is not expressed in the human anterior pituitary but is found in the neurointermediate lobe, hypothalamus, and skin. This selective expression explains the tissue-specific presence of the MSH peptides and β-endorphin. PC2 can only cleave peptides within secretory vesicles, and therefore the MSH peptides are only produced intracellularly.
Cleavage by PC2 generates ACTH 1–17, which then has amino acids removed from the C terminus by carboxypeptidase E to produce ACTH–113. Subsequently, α-amidation is catalyzed by peptidylglycine α-amidating monoxygenase, an enzyme that has multiple molecular forms, and acetylation occurs by the action of specific acetyltransferases.112 These posttranslational modifications yield α-MSH (see Fig. 10-8).
Given that POMC can be released intact from cells, then cleavage by extracellular peptidases is theoretically possible in some circumstances. This phenomenon has been recently demonstrated by the extracellular peptidases angiotensin-converting enzyme and neprilysin derived from dermal microvascular endothelial cells.160
PROCESSING IN DIFFERENT TISSUES
POMC processing varies depending on the species and the tissue. Although POMC is expressed primarily in the pituitary, POMC mRNA has been detected in many extrapituitary tissues.130 However, such detection does not provide evidence that the peptides are synthesized or secreted there. POMC derived from the pituitary and placenta has been shown to be released into maternal blood.129,154 Whether the POMC peptides produced in other extrapituitary tissues reach the circulation is debatable, and it is more likely that they act in a paracrine role.
Anterior Pituitary
In the human anterior pituitary, POMC is cleaved to give pro-ACTH, which is then cleaved to ACTH, N-POC, and joining peptide (see Fig. 10-8). Interestingly, the ACTH precursors, POMC and pro-ACTH, are found in the human circulation with ACTH, N-POC, joining peptide, and β-lipotropin.154 That very little β-endorphin appears to be present indicates that processing of β-lipotropin is minimal (Fig. 10-10). However, reports can be confounded by the fact that in some β-endorphin assays, the antibodies also detect β-lipotropin. In the rat and sheep anterior pituitary, some ACTH is also processed to des-acetyl-α-MSH and α-MSH.112
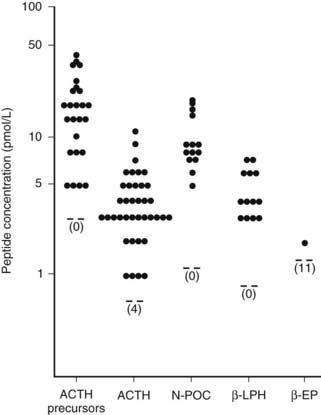
FIGURE 10-10. Concentrations of adrenocorticotropic hormone (ACTH) precursors and derived peptides in the circulation of normal subjects. EP, Endorphin; LPH, lipotropin; N-POC, N-pro-opiomelanocortin.
(From Gibson S, Crosby SR, Stewart MF et al: Differential release of pro-opiomelanocortin-derived peptides from the human pituitary: Evidence from a panel of two-site immunoradiometric assays. J Clin Endocrinol Metab 78:835–841, 1994.)
Studies in the mouse pituitary tumor cell line, AtT20, suggest that cleavage is sequential, starting with the C terminus of ACTH.140 However, the same pair of basic amino acids is found between ACTH and β-lipotropin, joining peptide and ACTH, ACTH 1–16 and ACTH 17–39, and γ-lipotropin and β-endorphin. Therefore, the adjacent amino acids and peptide folding must influence the sequential processing.
Intermediate Lobe
In the rodent intermediate lobe, POMC is found in melanotrope cells and undergoes more comprehensive processing to give the smaller fragments α-, β-, and γ-MSH, CLIP, and β-endorphin. The protease responsible for this cleavage is PC2. After endopeptidase cleavage, ACTH 1–17 is further modified by an exopeptidase that removes amino acids from the C terminus to give ACTH 1–13. Subsequently, ACTH 1–13 undergoes N-acetylation and C-terminal amidation.
Hypothalamus
POMC is produced primarily in the neurons of the hypothalamic arcuate nucleus where the peptides are central to regulation of food intake and energy balance114 (see Fig. 10-6). POMC is also expressed in the median eminence and ventromedial border of the third ventricle and much smaller amounts in the tractus solitarius. Processing is different from the anterior pituitary, in that smaller peptides characteristic of the neurointermediate lobe are produced.161 However, most of the studies are limited to the rat hypothalamus. In these extracts, high-performance liquid chromatographic separation of peptides suggests that ACTH is processed to CLIP and that des-acetyl-α-MSH is detected rather than α-MSH, thus indicating that N-terminal acetylation is limited. β-Endorphin 1–31 is found in the rat hypothalamus, again suggesting more extensive processing.114,112,162 PC1 and PC2 expression in the hypothalamus are increased by leptin and further regulated by the transcription factor Nescient Helix-Loop-Helix (Nhlh)-2, suggesting possible physiologic control of POMC peptide function at the level of peptide processing.163
POMC has also been detected in cerebrospinal fluid (CSF), although whether it originates from the pituitary or hypothalamus is uncertain. Evidence from changes in precursors and ACTH in rat CSF in relation to food intake and obesity suggest that they originate from the hypothalamus.118 In human CSF, the POMC precursor peptide has been shown to occur at high concentrations and predominates over ACTH when molar ratios are compared.164 However, several of the POMC peptides can be detected in CSF.165,166
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree