FIGURE 106-1. CT after contrast media of 8.5 cm in homogeneous ACC of the left adrenal gland (white arrow) and liver metastases (black arrows).
(Image provided by W. Kenn, Dept. of Radiology, University of Würzburg, Germany.)
Modern MRI of adrenal tumors should include T1- and T2-weighted images plus chemical shift imaging, which consists of in-phase and out-of-phase imaging. Multiplanar MRI is particularly suited to separating adrenal masses from surrounding structures like liver, spleen, pancreas, and kidney. ACCs typically present isointense to liver on T1-weighted images and show an increase in intensity in T2-weighted sequences. Heterogeneity of signals is noted both on T1-weighted and T2-weighted images due to necrosis or hemorrhage. Enhancement after gadolinium is typical, and washout is slow. Modern MRI technology can differentiate benign from malignant adrenal lesions with a sensitivity of 81% to 89% and a specificity between 92% and 99%.41,48,49 However, the optimum MRI method (T1/T2 relaxation time, chemical shift, fast low angle shot, in vivo proton MR spectroscopy, etc.) for diagnosis of ACC remains a matter of controversy.37,50,51
In the past, adrenal scintigraphy with 131I-6β-iodomethyl-norcholesterol (NP59) has been used to characterize adrenal lesions.52 Benign hypersecretory adenomas show enhanced tracer uptake. However, NP 59 scintigraphy is time consuming and associated with a high radiation dose. In contrast, FDG-PET may be highly valuable in patients with suspected ACC. High uptake of 18F-FDG demonstrates increased glucose metabolism, and with few exceptions indicates malignancy. Thus, FDG-PET may be highly valuable for evaluation adrenal masses that are indeterminate by both CT and MRI. However, some benign adenomas or pheochromocytomas also show uptake of FDG.41,50,53 Imaging with FDG-PET has the additional advantage of simultaneously detecting metastases at other sites, but metastatic lesions <1 cm (particularly in the lung) are not easily detected by FDG-PET,54,55 indicating that PET cannot substitute for CT imaging.
None of the imaging methods mentioned can reliably differentiate ACC from a metastasis of other origin or a pheochromocytoma. In this context, a new method for adrenal imaging is promising: 11C-metomidate PET.56,57 Metomidate specifically binds to adrenal 11β-hydroxylase and aldosterone synthase, so uptake indicates the adrenocortical origin of an adrenal lesion. A potentially more widely available tracer is 123I-iodometomidate for SPECT imaging.58
Prior to surgery, high-resolution CT of chest and abdomen should be performed to detect frequent lung and liver metastases. A bone scan is only required if the patient complains of bone pain (see Table 106-1). Fine-needle biopsy of suspected ACC is rarely justified and is associated with the risk of needle-track metastasis. In our view, a biopsy should only be performed if the tumor cannot be removed surgically, and medical therapy needs to be based on clear pathologic evidence.
HISTOPATHOLOGY
The pathologic diagnosis of ACC may be difficult because of the lack of clear-cut morphologic criteria,59 and in all cases, it is recommended to involve a specialized pathologist.
Weight and size are important criteria for malignancy. Most adenomas have a weight of <50 g, whereas most carcinomas weigh >100 g. The likelihood of an ACC increases steeply with a diameter of more than 6 cm.60
The differential diagnosis of carcinomas and adenomas is based largely on morphologic features. Different diagnostic scores61–63 have been introduced for diagnosis of malignancy. The Weiss system is most widely used and combines nine morphologic parameters, which include three parameters related to structure (description of cytoplasm, diffuse architecture, necrosis), three parameters related to cytology (atypia, atypical mitotic figures, mitotic count), and three related to invasion (veins, sinusoids, and capsule). Further morphologic parameters of importance are broad fibrous bands and hemorrhage. It has been shown that the mitotic index also has prognostic importance.64,65 In addition, periadrenal tissue infiltration and vena cava or renal vein invasion carry prognostic significance.66 Careful assessment of the resection status (R0, R1, R2) is also of great importance in that it may define further treatment strategies. For the same reason, violation of the capsule must be reported.
Important information is also provided by immunohistochemistry. Here, Ki-67 expression can be used both for differentiating benign from malignant tumors and for prognosis in ACC. A cutoff value between adenomas and ACCs varying from 1.5% to 4% has been reported. In patients with ACC, a Ki-67 labeling index of >10% was associated with poor survival in the German ACC registry. Other markers like Melan A, D11, inhibin α, and SF-1 are helpful to define the adrenocortical origin of the tumor, whereas ACC is typically negative for chromogranin A and S100.59,60,67,68 A number of new markers (LOH at 17p13, IGF-2 overexpression, cyclin E, matrix metalloproteinase-2 [mmp-2], telomerase activity, topoisomerase IIα, and N-cadherin) have been used to separate benign from malignant adrenal lesions. However, currently none of these markers has gained widespread acceptance.
Staging
In 2004, for the first time, a staging system was published by the Union International Contre Cancer (UICC) and the World Health Organization69 (Table 106-2). Stages I and II describe localized tumors 5 cm or smaller and larger than 5 cm, respectively. Locally invasive tumors or tumors with regional lymph node metastases are classified as stage III, and stage IV consists of tumors invading adjacent organs or presenting with distant metastases. Unfortunately, this staging system, which is largely based on the Macfarlane classification as modified by Sullivan, showed limited prognostic power.66 Thus, a revised TNM classification was proposed by ENSAT (see Table 106-2). In this staging system, stage III is defined by tumor infiltration in surrounding tissue or in adjacent organs, thrombus in vena cava/renal vein, or positive lymph nodes, whereas stage IV is defined by the presence of distant metastasis. The ENSAT staging system provides a powerful prognostic tool, predicting both disease-free and disease-specific survival in patients with ACC (Fig. 106-2).
Table 106-2. Staging Systems for Adrenocortical Carcinoma Proposed by the UICC 2004 and ENSAT 200866,69
| Stage | UICC/WHO 2004 | ENSAT 2008 |
|---|---|---|
| I | T1, N0, M0 | T1, N0, M0 |
| II | T2, N0, M0 | T2, N0, M0 |
| III | T1-2, N1, M0 T3, N0, M0 | T1-2, N1, M0 T3-4, N0-1, M0 |
| IV | T1-4, N0-1, M1 T3, N1, M0 T4, N0-1, M0 | T1-4, N0-1, M1 |
M0, No distant metastases; M1, presence of distant metastasis; N0, no positive lymph nodes; N1, positive lymph node(s); T1, tumor ≤ 5cm; T2, tumor > 5cm; T3, tumor infiltration in surrounding tissue; T4, tumor invasion in adjacent organs (ENSAT: also venous thrombus in vena cava/renal vein).
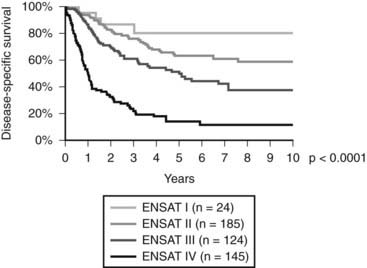
FIGURE 106-2. Disease-specific survival according to tumor stage (ENSAT Classification; see Table 106-2).
(Data derived from the German ACC Registry, September 2008.)
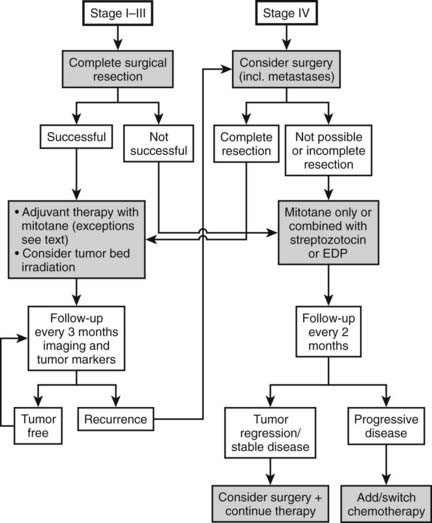
Therapy (Fig. 106-3)
SURGERY
Today most patients are diagnosed in stages I to III (see Fig. 106-2). In these stages, complete removal is the single most important therapeutic measure and offers the best chance for cure. All available data indicate that surgery should be performed by a specialized surgeon aware of the pitfalls of surgery for ACC (violation of the capsule, spilling). An R0 resection (resection margins microscopically disease free) is of utmost importance for long-term prognosis. Often surgery needs to be extensive, with en bloc resection of invaded organs. Spillage during surgery is paramount to an upgrade to stage IV66 and poor prognosis. The presence of a thrombus is compatible with complete resection but occasionally necessitates cardiac bypass technique.70–72 The use of laparoscopic adrenalectomy for ACC is debated. A higher risk of tumor spillage and local recurrences has been reported.73–75 However, these reports suffer from a referral bias, and inferiority of laparoscopic surgery for ACC has never been demonstrated. While most experts still favor open surgery for ACC, an analysis from the German ACC registry suggests that the long-term outcome in terms of disease-free survival and overall survival is not significantly different between the use of laparoscopic adrenalectomy or open surgery in tumors <10 cm. Probably more important than this technical aspect is the expert status of the surgeon.
Surgery for local recurrence or isolated metastases is frequently used and has been associated in retrospective series with improved survival. However, the value of surgery for metastases always requires careful consideration. In our experience, achieving an R0 resection with removal of metastatic disease leads to improved well-being of the patient, although the disease almost always recurs. As an alternative to surgery, radiofrequency thermal ablation can be used for metastatic lesions smaller than 5 cm.
Tumor debulking in stage IV (metastatic disease) is usually not indicated. In individual cases, it may be used to control hormone excess.
RADIATION THERAPY
The high rate of local relapse after surgery with curative intent suggests that adjuvant tumor-bed irradiation may have therapeutic potential by preventing local relapse, which often precedes metastatic tumor spread. This view is supported by a small study indicating that adjuvant radiotherapy of the tumor bed reduces the rate of local recurrences.76 Although more data are needed to better define the role of radiation therapy in an adjuvant setting, it may be justified to use adjuvant radiotherapy in selected patients. We recommend radiation therapy of the tumor bed in patients with histologically incomplete (R1) resection. In patients with macroscopically visible residual tumor, a second surgical approach by an expert surgeon should be considered first. Another group of patients that may benefit from adjuvant radiotherapy are patients with advanced locoregional disease (stage III), no evidence of distant metastases, and no evidence of residual disease after surgery. A standard fractionation scheme with single doses of 1.8 to 2.0 Gy on 5 days per week for 5 to 6 weeks is recommended. Total doses should not fall below a minimum of 40 Gy and ideally should reach 50 to 60 Gy.
Palliative radiotherapy in symptomatic metastatic lesions is well established for other tumor entities and has also been used in patients with ACC, in particular for bone metastases. Palliative radiotherapy with limited doses may also be effective in unresectable abdominal recurrences causing pain or vascular or intestinal obstruction. For optimum results of radiotherapy in ACC, an experienced radiotherapist using modern treatment concepts with CT planning, high-voltage radiation, and multiple fields is a prerequisite.
MEDICAL THERAPY: MITOTANE
Mitotane (1,1 dichloro-2[o-chlorophenyl]-2-[p-chloro-phenyl]ethane, o,p′-DDD) is an adrenolytic compound with specific adrenocortical activity that was introduced in the treatment of ACC more than 40 years ago by Bergenstal et al.77 Its precise mechanism of action is not fully understood.78 Its therapeutic activity depends on metabolic activation in adrenocortical cells. Mitotane is hydroxylated in the mitochondria and transformed into an acylchloride. The activated metabolites may directly bind to macromolecules, thereby inhibiting their activity and inducing oxidative damage through production of free radicals. Impairment of adrenal steroidogenesis has been reported, and evidence for inhibition of 11β-hydroxylase activity has been demonstrated.
The clinical efficacy of mitotane has long been disputed, and only in 2004 was it approved for advanced ACC by the European Medicine Agency (EMEA). However, there is clear evidence that mitotane can induce significant tumor regression in about 25% of cases with metastasized ACC and achieve control of hypercortisolism in the majority of patients.78 Complete responses have been described, and long-term survival is seen in the occasional patient. The use of mitotane in an adjuvant setting has been a matter of debate. However, a large retrospective analysis has indicated that adjuvant mitotane holds great potential to prolong disease-free survival and overall survival in ACC.79 Different from many previous studies, in this investigation, selection bias was largely avoided.
Mitotane is given as tablets (Lysodren; HRA Pharma Paris, Bristol-Myers Squibb New York). Monitoring mitotane blood concentrations is essential for providing optimal efficacy and reducing toxicity. Treatment aims at mitotane concentrations between 14 and 20 mg/L for antitumor response. Higher concentrations may lead to unacceptable toxicity, whereas a response is unlikely at a concentration <14 mg/L. Thus, mitotane has a narrow therapeutic window. However, some patients may respond to lower mitotane concentrations. Owing to the long half-life of mitotane, target levels are often achieved only after weeks to months.80 This time can be shortened by using high-dose regimens in the early phase of therapy81: treatment is initiated with 1.5 g/day and rapidly increased to 5 to 6 g/day until target concentrations have been reached. Most side effects are related to mitotane plasma concentrations, but gastrointestinal side effects like diarrhea seem to be more related to the daily dose. During long-term treatment, the dose of mitotane can usually be reduced, and many patients need only 2 to 3 g/day during long-term therapy. Adverse effects of mitotane treatment are manifold and common.78 The most troubling side effects involve the central nervous system and include ataxia, confusion, tiredness, and dizziness. In addition, dose-limited gastrointestinal side effects such as nausea, vomiting, anorexia, and diarrhea may occur. Treatment with 5-hydroxytryptamine blockers and loperamide, respectively, may be useful. Because of the long half-life of mitotane, blood levels increase slowly, and adverse effects usually become apparent over time, even if the dose has remained unchanged. In case of significant side effects, drug treatment is interrupted for days or a week, and treatment is restarted with a lower dose. Ingestion of mitotane together with a lipid-rich drink or meal may enhance resorption and increase drug concentrations. Owing to its adrenolytic activity, mitotane treatment induces adrenal insufficiency. Furthermore, mitotane also increases the metabolic clearance of glucocorticoids and increases the concentration of cortisol-binding globulin (CBG).82 High-dose glucocorticoid replacement (50 to 80 mg hydrocortisone daily) is needed. Insufficient glucocorticoid replacement enhances mitotane intolerance. Aldosterone secretion is less often affected, since mitotane primarily acts on the zona fasciculata and the zona reticularis. However, aldosterone secretion should be monitored, and replacement with fludrocortisone may become necessary with long-term use of mitotane. In addition, mitotane increases sex hormone–binding globulin82 and often leads to low free-testosterone concentrations in males. With long-term therapy, free–thyroid hormone concentrations decline in the presence of low to low-normal TSH, suggesting secondary hypothyroidism. Mitotane’s estrogenic activity may facilitate the development of gynecomastia.
Changes in hepatic gamma-glutamyl transaminase levels are so frequent that their absence questions patient compliance. However, in some cases, serious hepatotoxicity and even liver failure have been observed. Mitotane prolongs bleeding time, and it is advisable to stop mitotane for a minimum of 1 week prior to major surgery. High LDL-cholesterol and/or triglyceride concentrations are regularly observed, and treatment with a statin may be justified.
Despite the plethora of adverse effects of mitotane, most patients can be managed with acceptable toxicity in the long term.80,83 This is particularly important in an adjuvant setting, because treatment must be given for a minimum of 2 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree