FIGURE 148-1. Maternal serum levels of unconjugated estrone (E1), estradiol (E2), and estriol (E3) from the last menstrual flow through a conceptual cycle to term pregnancy. The points represent mean steroid hormone levels compiled from four separate studies. LH, Luteinizing hormone.
(Data from Buster JE, Abraham GE: The applications of steroid hormone radioimmunoassays to clinical obstetrics, Obstet Gynecol 46:489–499, 1975. Reprinted with permission from the American College of Obstetricians and Gynecologists.)
Placental estrogen, progesterone, lactogen, and possibly the GH variant also function as counterregulatory hormones with respect to insulin and cause insulin resistance and accelerated lipolysis. These phenomena are dealt with fully in Chapter 145.
The placenta produces enzymes that may affect normal endocrine function. Placental vasopressinase is so potent that the production rate of vasopressin in normal individuals must increase substantially to maintain normal levels. In patients with mild, subclinical diabetes insipidus (DI), the accelerated metabolism of vasopressin may make this subclinical condition manifest.
This chapter reviews the changes in normal physiology that occur during pregnancy and result in altered hormone levels that may create diagnostic difficulties when attempting to evaluate endocrine disorders in pregnant patients. Specific aspects of pregnancy as it interacts with intermediary metabolism and diabetes mellitus are dealt with in other chapters (see also Chapters 144 and 146).
Pituitary
PROLACTIN
Changes in Prolactin Physiology During Pregnancy
Estrogens produced by the placenta stimulate lactotroph DNA synthesis and mitotic activity, PRL mRNA levels, and PRL synthesis.10,11 Progesterone has also been shown to stimulate PRL secretion.12 During pregnancy, a progressive rise occurs in serum PRL levels13 (Fig. 148-2) that parallels an increase in the size and number of pituitary lactotroph cells.14,15 By term, PRL levels may be increased 10-fold to levels over 200 ng/mL.15 These elevated PRL levels found at term prepare the breast for lactation. Thus, the finding of amenorrhea associated with hyperprolactinemia could well be due to pregnancy and not due to pathologic hyperprolactinemia.
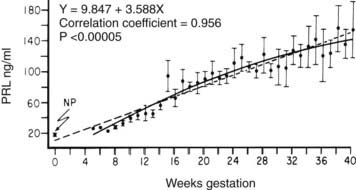
FIGURE 148-2. Mean ± SEM (n = 4) serum prolactin (PRL) concentrations measured serially at weekly intervals as a function of the duration of gestation. The dashed line represents the linear regression, and the solid line represents second-order regression. NP, Nonpregnant PRL value.
(Data from Rigg LA, Lein A, Yen SCC: Pattern of increase in circulating prolactin levels during human gestation, Am J Obstet Gynecol 129:454–456, 1977.)
It should be noted that PRL identical to pituitary PRL is also produced by the human decidua during pregnancy and causes high amniotic-fluid PRL levels.16 This decidual PRL is not present in maternal blood, however, and its physiologic role may be limited to regulation of water and ion transport across extraembryonic membranes.16 The placenta makes another hormone called placental lactogen (also known as chorionic somatomammotropin) that has lactogenic activity but is more structurally related to GH than to PRL and also does not contribute to blood PRL levels during pregnancy.16
Prolactinomas
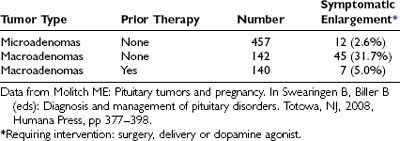
The normal pituitary enlarges during pregnancy, predominantly as a result of hyperplasia of the PRL-producing lactotroph cells.14,15 A magnetic resonance imaging (MRI) scan performed during pregnancy often reveals an enlarged pituitary because of this hyperplasia, but careful review of the scan shows no evidence of a pituitary tumor.17 The finding of an enlarged sella during pregnancy, however, may not be due only to normal lactotroph hyperplasia; it could also be due to the stimulatory effect of pregnancy on a preexisting prolactinoma (Fig. 148-3). In a series of cases collected from the literature, comprising 739 patients with prolactinomas who became pregnant, it was found that the risk of significant tumor enlargement was 2.6% for microadenomas, 31.7% for macroadenomas that had not been subjected to prior treatment by surgery or irradiation, and 5.0% for macroadenomas that had been subjected to prior surgery and/or irradiation (Table 148-2).18
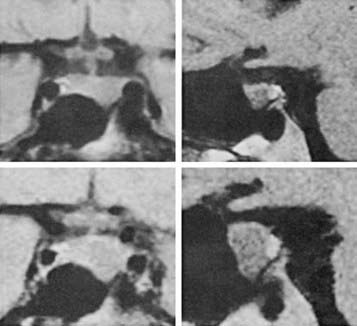
FIGURE 148-3. Magnetic resonance imaging scans with coronal (left) and sagittal (right) views demonstrating enlargement of a prolactin-secreting macroadenoma (above) before pregnancy and (below) in the third trimester of pregnancy. The patient had been complaining of increasing headaches.
Lymphocytic Hypophysitis
Pituitary enlargement during pregnancy may also be due to lymphocytic hypophysitis.19–21 Lymphocytic hypophysitis is characterized by massive infiltration of the pituitary by lymphocytes and plasma cells, with destruction of the normal parenchyma.19 The disorder is thought to have an autoimmune basis.19 Most cases occur in association with pregnancy, and women are seen during pregnancy or postpartum, either with symptoms of varying degrees of hypopituitarism or with symptoms related to the mass lesion, such as headaches or visual-field defects. Mild hyperprolactinemia and DI may also be found. On a computed tomography (CT) scan or MRI, a sellar mass is found that may extend in an extrasellar fashion and cause visual-field defects. The condition is usually confused with a pituitary tumor and in fact cannot be distinguished from a tumor except by biopsy. By virtue of the hypopituitarism that it produces, lymphocytic hypophysitis can also be clinically confused with Sheehan’s syndrome postpartum, except that these women have no history of obstetric hemorrhage.21 The diagnosis of lymphocytic hypophysitis should be entertained in women with symptoms of hypopituitarism and/or mass lesions of the sella during pregnancy or postpartum, especially in the absence of a history of obstetric hemorrhage. Evaluation of pituitary function is warranted, as well as CT or MRI. If PRL levels are only modestly elevated (<150 ng/mL) in the presence of a large mass, the diagnosis is unlikely to be an enlarging prolactinoma and more likely to be hypophysitis or a nonsecreting tumor.20,21 Furthermore, there seems to be a particular predilection for loss of ACTH secretion, and deaths have occurred when this has gone untreated.21
GROWTH HORMONE
Changes in Growth Hormone Physiology During Pregnancy
In the second half of pregnancy, pituitary GH secretion falls, and a GH variant made by the syncytiotrophoblastic epithelium of the placenta increases in the circulation22,23 (Fig. 148-4). The episodic secretion of pituitary GH is replaced by constant, nonpulsatile secretion of this variant at the rather high levels of 10 to 20 ng/mL.23,24 Production of the placental variant is accompanied by decreased production of normal pituitary GH, presumably because of negative-feedback effects. Evidence in favor of this feedback effect of placental GH on pituitary GH secretion includes the fact that in patients with acromegaly who have autonomous GH secretion and who become pregnant, both forms of GH persist in the blood throughout pregnancy.25
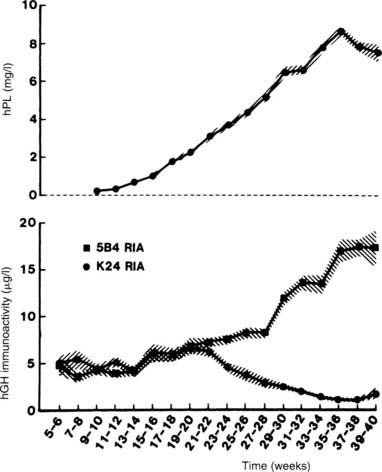
FIGURE 148-4. Mean (±SD) serum growth hormone (GH) and placental lactogen levels in women throughout pregnancy. The 5B4 radioimmunoassay (RIA) antibody is specific for the GH placental variant, whereas the K24 RIA antibody is specific for pituitary GH. Note the rise in serum levels of the GH placental variant coinciding with the fall in serum levels of pituitary GH.
(Data from Frankenne F, Closset J, Gomez F et al: The physiology of growth hormones [GHs] in pregnant women and partial characterization of the placental GH variant, J Clin Endocrinol Metab 66:1171–1180, 1978.
The GH variant differs from pituitary GH by 13 amino acids and has similar somatogenic but less lactogenic bioactivity than pituitary GH does,26 a reflection of differences in binding to somatogenic versus lactogenic GH receptors.27 Most reports have demonstrated considerably elevated insulin-like growth factor 1 (IGF-1) levels during pregnancy, commensurate with the elevated GH variant levels.28–30 The GH variant also has activity similar to that of normal pituitary GH with respect to carbohydrate and lipid metabolism.31
The physiologic significance of the change in the source of circulating GH during pregnancy is not known. Although animal studies suggest that placental GH promotes placental growth and maturation, placental nutrient transport, and possibly fetal growth, such functions in humans have not yet been established.32 However, the elevated levels of the placental variant with elevated levels of IGF-1 may account for the coarsening of features occasionally seen in some pregnant women, and such women may be suspected of having acromegaly. It is not clear whether these elevated placental GH variant levels contribute to the insulin resistance of pregnancy. Lack of the GH variant does not affect fetal growth,33 but simultaneous loss of HPL and the GH variant has been reported to cause severe intrauterine growth retardation but an otherwise normal baby.34
Acromegaly
Standard immunoassays for GH cannot usually distinguish between normal pituitary GH and the placental GH variant and may consequently give misleading results with respect to assessment of pituitary GH secretion during the latter half of pregnancy. Basal levels of the variant are considerably higher than normal nonpregnant GH levels and may therefore erroneously indicate excessive pituitary GH secretion. Special assays that recognize specific epitopes on the two hormones23,24 are necessary to distinguish normal from placental GH. When such specific assays are not available, it may be necessary to wait until delivery to assess pituitary GH secretion accurately, because placental GH variant is undetectable within 24 hours.25 However, two differences between placental GH variant secretion and pituitary GH secretion in acromegaly may allow a distinction to be made during pregnancy: (1) pituitary GH secretion in acromegaly is highly pulsatile, with 13 to 19 pulses per 24 hours,35,36 whereas secretion of GH variant in pregnancy is nonpulsatile24; and (2) in acromegaly, about 70% of patients have a GH response to thyrotropin-releasing hormone (TRH),37 whereas the placental GH variant does not respond to TRH.25 However, at present, TRH is no longer available for testing in the United States.
Actual reports of pregnancies in acromegalic patients are uncommon,18,25,38–41 perhaps because about 40% of such patients are hyperprolactinemic.18 Indeed, correction of the hyperprolactinemia with bromocriptine may be necessary to permit ovulation and conception in these patients.38 It is possible that in a patient in whom acromegaly is initially diagnosed during pregnancy, the finding of hyperprolactinemia may be due to the stimulated production of PRL by normal lactotrophs and not to tumor production. Only two patients with tumors secreting GH have been reported to have enlargement of their tumors, with a resultant visual-field defect in one during pregnancy18,39; neither tumor co-secreted PRL.
VASOPRESSIN
Changes in Vasopressin Physiology During Pregnancy
The primary regulator of vasopressin secretion is the osmolality of plasma, which is sensed by osmoreceptors (see Chapter 21). The osmotic threshold for vasopressin release in nonpregnant subjects is about 280 to 285 mOsm/kg, with a normal range of 275 to 290 mOsm/kg. Thirst is generally stimulated at a plasma osmolality level about 5 mOsm/kg above that for vasopressin release, a level near which maximum concentration of urine will already have been achieved.
Pregnancy is associated with lowering of the “osmostat,” the set-point for serum osmolality, by about 10 mOsm/kg.6 The decline in plasma osmolality begins by the time of the first missed menstrual period and gradually decreases until the 10th week of gestation, after which little further change occurs42 (Fig. 148-5). Pregnant women experience thirst and release vasopressin at lower levels of serum osmolality than do nonpregnant individuals to maintain this lower osmolality43 (Fig. 148-6). A water load will suppress vasopressin secretion appropriately and result in dilute urine and excretion of this water load, thereby maintaining this lower osmolality.43,44 This reset osmostat results in lowering of serum sodium by about 4 to 5 mEq/mL.42,44 The physiologic basis for this reset osmostat is not clear. Experiments in humans, however, have shown that hCG injected into nonpregnant women lowers their osmostat by 5 mOsm/kg.45 Furthermore, a case has been described in which a woman with a hydatidiform mole had a lower osmostat that returned to normal only when hCG levels in the serum finally became undetectable 40 days after evacuation of the mole.45 Evidence from rat studies and from human studies in which pregnant women were immersed in water to expand the central blood volume suggests that a decreased “effective” blood volume does not play a role in this resetting of the osmostat.44
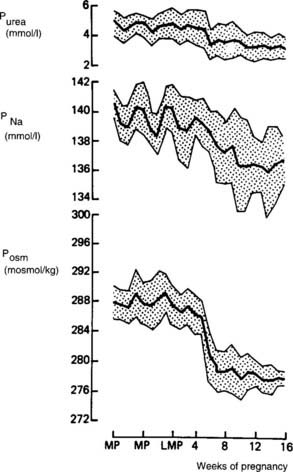
FIGURE 148-5. Mean values (±SD) for plasma urea (Purea), sodium (PNa), and osmolality (Posm) measured at weekly intervals from before conception to the first trimester in nine women with a successful obstetric outcome. LMP, Last menstrual period; MP, menstrual period.
(Data from Davison JM, Vallotton MB, Lindheimer MD: Plasma osmolality and urinary concentration and dilution during and after pregnancy: Evidence that lateral recumbency inhibits maximal urinary concentrating ability, Br J Obstet Gynaecol 88:472–479, 1981.)
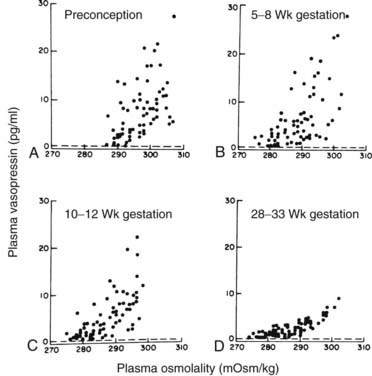
FIGURE 148-6. Relationship of plasma arginine vasopressin to plasma osmolality during a 2-hour hypertonic saline infusion in eight women studied serially before and during pregnancy. Each point is an individual plasma determination. Dashed lines indicate the lower limit of detection for arginine vasopressin.
(Data from Lindheimer MD, Barron WM, Davison JM: Osmoregulation of thirst and vasopressin release in pregnancy, Am J Physiol 257:F159–F169, 1989.)
The placenta produces vasopressinase, a cystine aminopeptidase that rapidly inactivates vasopressin. Vasopressinase levels increase 1000-fold between the 4th and 38th weeks of gestation.46 How much this increase in vasopressinase activity contributes to the increased clearance of endogenous vasopressin during pregnancy is not clear, however.46 The placenta itself is also able to metabolize vasopressin.47 Overall, the combination of increased vasopressinase levels and increased placental metabolism of vasopressin results in a twofold to fourfold increased metabolic clearance rate for vasopressin.46,47
Diabetes Insipidus
In a patient with preexisting DI, this increased metabolism of vasopressin during pregnancy results in worsening of the DI.6,48,49 Thus, patients with mild DI treated with either increased fluids or chlorpropamide will probably experience considerable worsening. Women being treated with pitressin tannate in oil or lysine vasopressin spray may also experience such worsening. The vasopressin analog 1-deamino-8-d-arginine-vasopressin (DDAVP, or desmopressin acetate) is not affected by vasopressinase, and a number of women have been treated quite satisfactorily with this medication.48,50,51 Rarely, asymptomatic women will experience symptomatic DI only during pregnancy.50,52,53
When polyuria or polydipsia develops in a pregnant patient, the finding of lower than expected serum sodium levels should not, therefore, exclude the diagnosis of DI.6,44,48 Testing for DI (see Chapter 21) in a pregnant woman should be performed with the woman in the sitting position, because the lateral recumbent position results in an inhibition of maximal urinary concentrating ability.44 The increased metabolism of vasopressin also limits the rise in plasma vasopressin levels during testing, especially in the last trimester.43,45 Therefore, the levels of vasopressin and osmolality used in nomograms for nonpregnant patients cannot be used for pregnant ones, but an extrapolation from Figure 148-6 can be used. When obtained for vasopressin measurement during pregnancy, blood should be drawn into syringes containing orthophenanthroline (0.1 mL in a 10-mL syringe) to inactivate vasopressinase activity and then rapidly transferred to chilled heparinized tubes, centrifuged in the cold, and extracted within 3 hours.43,46
One recent important finding in women with transient DI of pregnancy is the simultaneous occurrence of acute fatty liver of pregnancy in some patients. Kennedy and colleagues reported six such cases seen at 35 to 39 weeks of gestation, with nausea, vomiting, and polyuria; preeclampsia was diagnosed in four.52 In four cases, polyuria developed before delivery, and in two it developed postpartum. All six had complete resolution of their DI and liver abnormalities by the fourth week postpartum. Three additional cases of transient DI of pregnancy associated with mild transaminase abnormalities were reported by Hamai et al.54 Other cases have been reported in patients with the HELLP (hemolysis, elevated liver-enzyme levels, low platelets) syndrome.55 It has been hypothesized that the acute liver dysfunction in these patients reduces the degradation of vasopressinase, thereby increasing vasopressinase levels even more than usual with yet greater clearance of vasopressin.52 Thus, women in whom DI develops very late in gestation should also be screened for liver function abnormalities.
Syndrome of Inappropriate Antidiuretic Hormone Secretion
As mentioned earlier, the normal serum sodium is reset about 5 mEq/L lower during pregnancy. However, true hyponatremia due to the syndrome of inappropriate antidiuretic hormone (SIADH) secretion has been reported in a small number of cases with preeclampsia, but the mechanism is not clear.56
Thyroid
THYROID HORMONES AND THYROID REGULATION
Changes in Normal Thyroid Physiology During Pregnancy
A number of changes occur in the thyroid and in levels of thyroid hormones during pregnancy.57 It is widely believed that a goiter commonly develops during pregnancy. This belief stems from uncontrolled early observations and the fact that most such studies were done in iodine-deficient regions. In iodine-replete areas, no increase in the frequency of goiters is noted during pregnancy.58 In a study of Belgian women with borderline iodine status, thyroid volume was found to increase about 18% during pregnancy,59 a change that would not be clinically apparent. Of 309 pregnant adolescents evaluated by Long and colleagues, 18 were found to have goiters: 2 had Graves’ disease and were hyperthyroid; 3 had Hashimoto’s thyroiditis, 1 being hyperthyroid; and 4 had subacute thyroiditis, 1 being hyperthyroid.60 The other 9 with goiters were thought to have simple nontoxic goiters. Thus, although thyroid volume does increase slightly during pregnancy, perhaps because of relative iodine deficiency and/or the stimulatory effect of hCG (see discussion to follow), the presence of a palpable goiter in iodine-replete areas indicates significant disease in about 50% of patients and should always be evaluated. In one-third of patients found to have goiters, an increase in size of 17% to 55% may be expected over the course of gestation.59
Renal clearance of iodine is increased because of the increased GFR that occurs with pregnancy. When iodine intake is marginal, this increased loss may result in iodine deficiency.57 Even in the absence of hypothyroidism, the total body iodine pool becomes decreased, and radioactive iodine uptake is therefore increased.61 Radioactive iodine is, of course, contraindicated during pregnancy because the placenta is freely permeable to iodine, and the fetal thyroid is 20 to 50 times more avid for iodine than the maternal thyroid.62 The tracer dose used in these studies is so small, however, that it is not sufficient to cause concern if given inadvertently during pregnancy.62 The currently recommended intake of iodine is 150 to 250 µg of iodide per day, and the iodine in iodized salt and prenatal vitamins (usually 150 µg) usually prevents iodine deficiency.63–65 Excessive iodine ingestion (>500 µg/day) is to be avoided, however, because it crosses the placenta and may cause neonatal goiter.65
The fetal thyroid and the fetal hypothalamic-pituitary-thyroid axis develop independently of maternal thyroid status (see Chapter 145). At 11 to 12 weeks’ gestation, the fetal thyroid begins to concentrate iodine. In addition to iodine, the placenta is freely permeable to medications used to treat hyperthyroidism, such as propylthiouracil, methimazole, and propranolol; triiodothyronine (T3) and TSH cross the placenta only minimally.57 Thyroxine (T4) crosses the placenta in somewhat larger (although still small) amounts, and late in gestation, large doses are able to ameliorate the effects of hypothyroidism in infants with congenital hypothyroidism caused by enzyme defects or agenesis of the thyroid.57,66
Bioassayable thyroid-stimulating activity in serum is increased in the first trimester as a result of the intrinsic thyroid-stimulating activity of hCG.57,67,68 The hCG may cause increased T4 and T3 levels, as well as a transient hyperthyroidism associated with hyperemesis gravidarum (see discussion to follow). The markedly elevated levels of hCG seen in trophoblastic neoplasms may also cause hyperthyroidism.69 A human chorionic thyrotropin with little thyroid-stimulating activity may also be made by the placenta, but it probably plays no physiologic role.57
Alterations in Thyroid Function Tests During Pregnancy
T4 is about 75% bound to TBG, 10% to 15% bound to albumin, and 10% to 15% bound to transthyretin (prealbumin); only about 0.04% is unbound or “free.” The increased estrogens of the placenta result in increased TBG levels in the blood because of increased production by hepatocytes,70 as well as decreased degradation from altered glycosylation.71 The increased TBG levels result in increased bound T4 measurements, beginning by 4 to 6 weeks of gestation57,72 (Fig. 148-7). The metabolic activity of the hormones correlates best with free hormone levels; free T4 levels have been reported to be unchanged, increased, or decreased during pregnancy, although they usually remain within the normal range57,73 (see Fig. 148-7). These differences may relate to relative iodine deficiencies in the populations studied57 and the stage of pregnancy that is being assessed.57,73 Free T4 levels are minimally elevated or in the high normal range in the first trimester and then fall back toward baseline and even below baseline, in parallel with changes in hCG57 (Fig. 148-8). T3 is bound to TBG with somewhat less affinity than T4 is, but the same increase in total T3 measurements is seen as with T4 during pregnancy.57,73
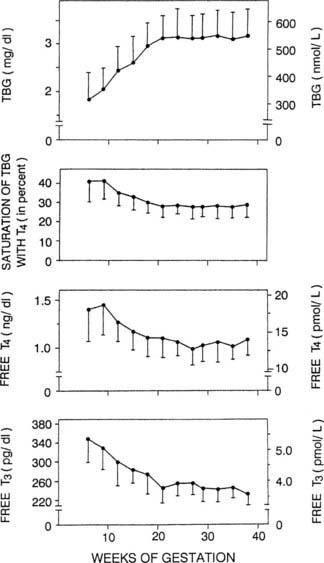
FIGURE 148-7. Data collected in 606 normal pregnancies in Brussels are illustrated, showing the progressive rise in serum thyroxine-binding globulin (TBG) during the first part of gestation, accompanied by a progressive decrease in the free T4 index (saturation level of TBG by T4), and free T4 and T3 concentrations. Brussels being in an area with a restricted iodine intake, the quantitative reduction in free hormone concentrations observed in the second part of gestation is more pronounced than in areas without iodine deficiency. T3, Triiodothyronine; T4, thyroxine.
(Data from Glinoer D: Thyroid regulation during pregnancy. In Delange F, Dunn JT, Glinoer D [eds]: Iodine deficiency in Europe: a continuing concern. NATO ASI Series (vol 241). New York, 1993, Plenum, pp 181–190.
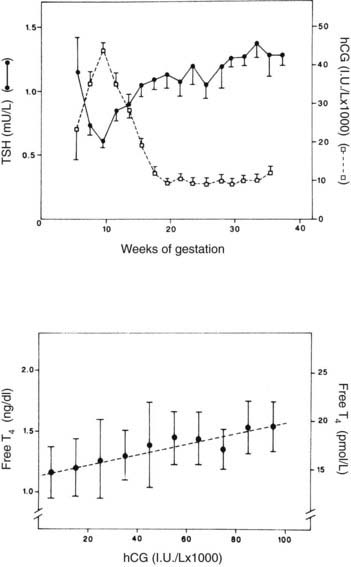
FIGURE 148-8. Upper graph, serum thyroid-stimulating hormone (TSH) and human chorionic gonadotropin (hCG) as a function of gestational age in 606 healthy pregnant women. Between 8 and 14 weeks’ gestation, the changes in hCG and TSH levels are mirror images of each other, and there is a significant negative correlation between the individual TSH and hCG levels (P < 0.001). Each point gives the mean value (±SE) of individual determinations pooled for 2 weeks. Lower graph, scattergram of free thyroxine (T4) levels in relation to hCG concentrations in the first half of gestation. Each point represents the mean (±1 SD) free T4 values, determined between 6 and 20 weeks, plotted for 10,000 IU/L increments in hCG. The dashed line indicates the linear regression curve (P < 0.001).
(Data from Glinoer D, DeNayer P, Bourdoux P et al: Regulation of maternal thyroid during pregnancy, J Clin Endocrinol Metab 71:276–287, 1990.
TSH levels are minimally decreased during the first trimester of pregnancy (see Fig. 148-8), possibly because of the increased free T4 levels.57,72 In a recent study of 9562 pregnant women by Lambert-Messerlian and colleagues, the median and 95% confidence limits for TSH for the first and second trimesters were 1.05 (95% CI 0.13 to 3.07) and 1.23 (0.36 to 3.00) mU/L, and similar values for free T4 were 1.10 (0.85 to 1.38) and 1.01 (0.77 to 1.26) ng/dL, using the Immulite 200 methodology.74 Free T4 levels may actually fall and TSH levels rise in the last trimester in those with significant iodine deficiency.59
T4 turnover is increased during pregnancy,73 and in patients receiving thyroxine replacement, TSH levels should be checked each trimester to determine whether the dose needs to be increased.73 In one study, 75% of patients needed to increase their thyroxine doses during pregnancy75 (Fig. 148-9).
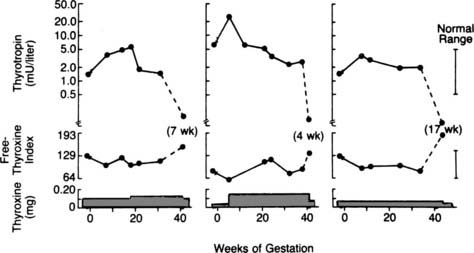
FIGURE 148-9. Representative patterns of changes in thyroid function and thyroxine dose during pregnancy in women with hypothyroidism. The left-hand and middle panels show the results for two women who required an increased dose of thyroxine during pregnancy. The right-hand panel shows the results for a woman whose thyroxine dose was not changed during pregnancy. The normal ranges for serum thyrotropin and the free thyroxine index are indicated by the vertical bars on the right side of the figure. The discontinuity marks indicate the beginning and end of pregnancy. The dashed line represents the change between the last gestational and the first postpartum values (the number of weeks postpartum is noted in parentheses).
(Data from Mandel SJ, Larsen PR, Seely EW, Brent EA: Increased need for thyroxine during pregnancy in women with primary hypothyroidism, N Engl J Med 323:91–96, 1990;
HYPERTHYROIDISM
Because of the hyperdynamic state of pregnancy, the clinical diagnosis of hyperthyroidism may prove difficult. The features of tachycardia, warm skin, systolic flow murmurs, and heat intolerance are common to both. Even the finding of a goiter may not be specific. Weight loss, a marked tachycardia, eye signs, and a bruit over the thyroid are more suggestive of hyperthyroidism.65,73 Infiltrative dermopathy and ophthalmopathy are specific for Graves’ disease but do not indicate the degree of hyperthyroidism.
The changes in thyroid hormones already discussed may cause further diagnostic difficulty. Because of the elevation of TBG in blood, total T4 and T3 levels are elevated. Free T4 levels may also be mildly elevated in some assays early in the first trimester in normal pregnancy, and TSH levels may be suppressed to below normal because of the high levels of free T4 and hCG (see previous discussion).57,65,73 TSH will not be very low with these newer assays in pregnancy, as in typical hyperthyroidism, however, which makes TSH the critical assessment when other levels are borderline.65 It is important to make the diagnosis of hyperthyroidism accurately and carefully because untreated hyperthyroidism clearly has adverse consequences on fetal outcome.65,73
Most cases of hyperthyroidism in pregnancy are due to Graves’ disease and to antibodies that stimulate the TSH receptor.65,73,76,77 These antibodies cross the placenta and may cause neonatal hyperthyroidism in 2% to 10% of women with active disease during pregnancy.65,77 TSH-receptor antibodies should be measured in the last trimester. If levels are high, the neonate should be followed carefully for the development of hyperthyroidism.65,76,77 Rarely, these antibodies may cause intrauterine fetal hyperthyroidism.65 Fetal goiter can develop from fetal hyperthyroidism, or the thionamides used to treat the mother may cross the placenta and cause fetal hypothyroidism. Some centers have advocated screening the susceptible developing fetus with transvaginal fetal thyroid ultrasounds,78 measurement of thyroid hormone levels in amniotic fluid samples,79 and even umbilical blood sampling,80 but these approaches have not yet gained widespread acceptance.
Hyperthyroidism and Hyperemesis Gravidarum
Nausea and vomiting may occasionally be the predominant symptoms in patients with hyperthyroidism. Conversely, the severe nausea and vomiting that sometimes occur in the first trimester of pregnancy, known as hyperemesis gravidarum, may be associated with biochemical hyperthyroidism.65,73 Elevated T4 and T3 levels may be found transiently in more than a third of subjects with hyperemesis gravidarum, but only about 10% to 20% of such patients manifest clinical thyrotoxicosis.65,81–83 The increased thyroid hormone levels are due to stimulation by hCG.65,82 On the other hand, hCG levels overlap considerably with those of normal individuals, so some authors have postulated that in these particular cases associated with transient hyperthyroidism, the hCG may have some structural variation resulting in increased biological activity.84 The link between the elevated hCG and vomiting may be the hCG-induced increase in estradiol.85 A very rare cause of recurrent gestational hyperthyroidism occurring in families has been found to be a missense mutation in the extracellular domain of the TSH receptor. This receptor was more sensitive than the wild-type receptor to hCG and occurred with normal rather than elevated levels of hCG.86
In most patients, free T4 levels fall to normal over a period of 6 to 133 days (mean, 35.1 days) while still pregnant,82 generally paralleling the fall in hCG levels (Fig. 148-10). Therefore, patients with hyperemesis and elevated free T4 and T3 levels present a considerable diagnostic dilemma. Most patients with Graves’ disease will have some symptoms that predate the pregnancy, whereas most patients with hyperthyroidism associated with hyperemesis have no goiter and generally no eye findings.65,83 Because of the increased maternal and fetal morbidity associated with untreated hyperthyroidism, it appears to be safest to administer antithyroid drugs to any woman with symptomatic hyperthyroidism or to those in whom severely elevated (>50% over pregnancy-adjusted “normal”) thyroid hormone levels do not spontaneously revert to normal over a period of 1 to 3 weeks, and in whom TSH levels are suppressed below detectability in a highly sensitive assay.65 Careful follow-up and adjustment of medications may allow withdrawal of antithyroid medication in those with transient hormonal abnormalities.
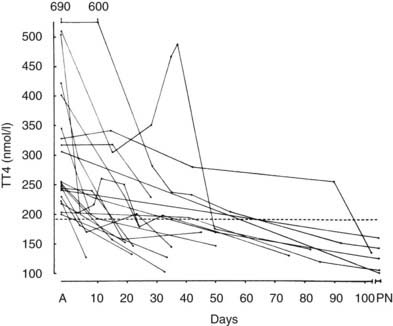
FIGURE 148-10. Changes in plasma total thyroxine concentration in 20 subjects with hyperemesis on admission (A) and during the course of their pregnancy. The interrupted line represents the mean (±SD) value seen in normal pregnancy. PN, Postnatal.
(Data from Swaminathan R, Chin RK, Lao TTH et al: Thyroid function in hyperemesis gravidarum, Acta Endocrinol (Copenh) 120:155–160, 1989.)
HYPOTHYROIDISM
The clinical picture of spontaneous hypothyroidism developing during pregnancy may be confusing because of the tendency of pregnancy to cause tachycardia, fatigue, warm skin, and heat intolerance. Other typical findings of hypothyroidism should gradually become apparent, however, such as muscle cramps, excessive fatigue, dry skin, and so forth.65,73 The elevated TBG levels may cause total T4 levels to be normal, but free T4 levels will be low and TSH levels will be elevated. As noted earlier, previously established replacement doses of thyroxine may need to be increased75 because of the increased clearance of thyroxine during pregnancy. This has been shown to be critically important; even subclinical degrees of maternal hypothyroidism have been recently shown to be associated with impairment of subsequent infant intellectual development.87,88 Because these effects may occur even with subclinical hypothyroidism in the first trimester, routine screening of all pregnant women at their first prenatal visit has been advocated by many but not all.65,89 Treatment with thyroxine is advocated for women with TSH levels above the normal range, even when free T4 levels are normal, to prevent adverse outcomes.65 However, in a recent study of 10,990 pregnant patients, those with normal free T4 levels but elevated TSH levels (>4.28 mU/L in the first trimester or >3.93 mU/L in the second trimester) had no adverse outcomes.90 In this study, those with normal TSH levels using this cutoff but low free T4 also had no consistent pattern of adverse outcomes.90 This latter finding was confirmed in a another study of 233 of 17,298 women who had normal TSH levels (<3.0 mU/L) but low levels of free T4.91
A number of studies have demonstrated an association between autoimmune thyroid disease and increased rates of infertility and miscarriage even when thyroid hormone and TSH levels are normal.65,92 One controlled trial showed that women with positive antiperoxidase antibodies who were treated with thyroxine had lower miscarriage and premature delivery rates than did untreated women, and these adverse effect rates were similar to those without antibodies.92a The untreated antibody-positive group had a gradual rise of TSH levels from 1.7 to 3.5 mU/L, whereas the treated antibody-positive group maintained TSH levels < 2.0 mU/L, similar to the antibody-negative group.93 These findings remained to be confirmed.
Adrenal Cortex
GLUCOCORTICOSTEROIDS AND GLUCOCORTICOSTEROID REGULATION
Changes in Adrenal Physiology During Pregnancy
Cortisol levels increase progressively over the course of gestation, with a two- to threefold increase by term93–97 (Fig. 148-11). Most of the elevation in cortisol levels is due to the estrogen-induced increase in cortisol-binding globulin (transcortin) levels,93,95–97 but the biologically active “free” fraction in serum is elevated threefold as well.93,96–98 This increase is reflected in two- to threefold elevations in urinary free cortisol levels.93,99,100 The increased CBG results in a prolonged cortisol half-life in plasma, but the cortisol production rate is also increased.101,102 Urinary 17-hydroxycorticosteroid levels are decreased, however, because of a decrease in the excretion of cortisol-tetrahydro metabolites.101,102 Cortisol can cross the placenta, and the major direction of transfer is from mother to fetus.103 However, the presence in the placenta and in many fetal tissues of high concentrations of the enzyme, type 2 11β-hydroxysteroid dehydrogenase, which converts cortisol to cortisone, protects the fetus from these high maternal levels of cortisol.104,105 Deficiencies in this enzyme, which result in high fetal glucocorticosteroid exposure, have been hypothesized to retard intrauterine fetal growth and may even program the fetus for the subsequent development of hypertension in adult life.93 Dexamethasone is not metabolized by this enzyme and readily crosses the placenta.92
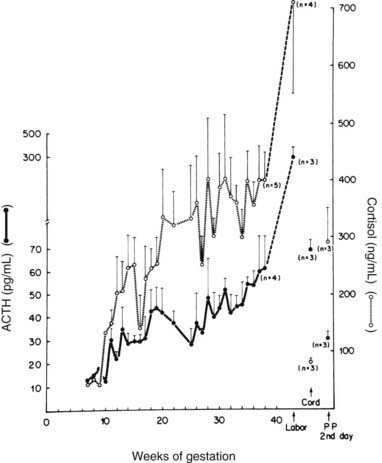
FIGURE 148-11. Plasma concentration of adrenocorticotropic hormone (ACTH) and cortisol during normal pregnancy. Blood samples were obtained weekly at 8 to 9 am from five normal pregnant women and from three women during labor and on the second postpartum day. In addition, umbilical cord plasma was obtained from the newborn infants of three of these subjects. The mean plasma concentrations for ACTH are denoted by the solid circles, whereas plasma cortisol levels are denoted by open circles. The vertical bars correspond to the magnitude of the standard error of the mean.
(Data from Carr BR, Parker CR Jr, Madden JD et al: Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy, Am J Obstet Gynecol 139:416–422, 1981.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree