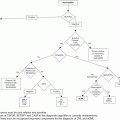
Diagnostic algorithm for pediatric thrombocytosis. TIBC, total iron-binding capacity; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CBC, complete blood count; MPN, myeloproliferative neoplasm; CML, chronic myeloid leukemia.
Secondary erythrocytosis is defined as a situation in which the hematocrit is elevated due to normal hematopoietic precursor cells responding appropriately to external stimuli. Such a finding can be routine amongst adults but is uncommon in the pediatric population. It is most often associated with cyanotic cardiac disease,12 inappropriate erythropoietin production, renal tumors, or atypical hemoglobinopathies, often with increased O2 affinity.13 Again, given the extreme rarity of true PV in the pediatric population, an extensive search for secondary causes ought to be performed.
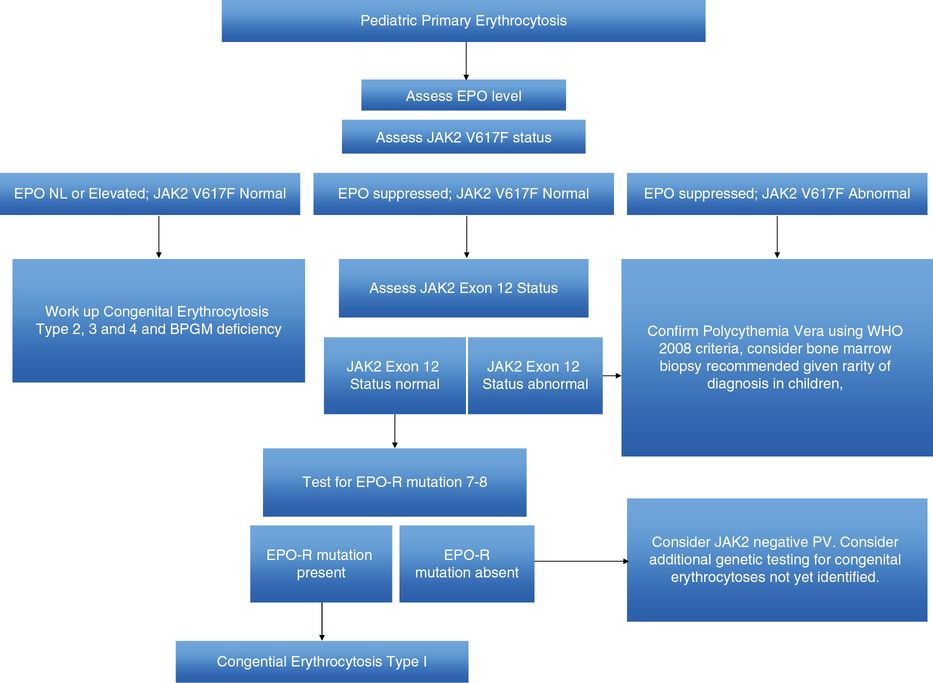
We have included, in Table 16.2, a possible differential diagnosis of secondary erythrocytosis in the pediatric population. As is the case with patients being worked up for secondary thrombocytosis, the diagnosis begins with a full history and physical to exclude the known causes of a secondary erythrocytosis. Patients with persistent erythrocytosis should have their peripheral oxygen saturation measured as well as their serum erythropoietin level and examination of their peripheral blood smear. See Figure 16.2 for a diagnostic algorithm for children with erythrocytosis.
| Acquired | |
| Alterations in arterial oxygen saturation | Cyanotic heart disease, carboxyhemoglobin, pulmonary disease |
| Elevations in erythropoietin | Nephroblastoma, Wilms tumor, other renal tumors, hepatic tumors, central nervous system tumors, endocrine tumors |
| Renal abnormalities | Renal tubular acidosis, renal disease |
| Congenital | |
| Hemoglobinopathies | Variants with high oxygen affinities |
| 2,3-bisphosphoglycerate deficiency | |
| Defective O2 sensing | |
| Familial syndromes | Family erythrocytosis type 2 (VHL); familial erythrocytosis type 3 (EGLN1), familial erythrocytosis type 4 (EPAS1) |
Myelofibrosis in children can be seen as a consequence of acute leukemia, Hodgkin’s lymphoma, or due to metastasis from solid tumors.14 A variety of other etiologies have been illuminated in case reports and small series.15–18 Connective tissue disorders, for example, like ulcerative colitis or Still’s disease, can occasionally manifest with fibrotic marrow findings. These typically reverse with disease treatment. There are also a number of case studies published on the association between vitamin A deficiency and myelofibrosis.18,19 While no hard data exist, it is likely that secondary myelofibrosis is a more common clinical entity than the myelofibrosis of PMF (Table 16.3).
| Acquired | |
| Hematologic malignancy | Acute myeloid leukemia, acute lymphoblastic leukemia, Hodgkin’s lymphoma, hairy cell leukemia, systemic mastocytosis, chronic myeloid leukemia |
| Solid tumor malignancy | Neuroblastoma, rhabdomyosarcoma, carcinomas |
| Central nervous system tumors, endocrine tumors | |
| Renal abnormalities | Renal osteodystrophy |
| Connective tissue disorders | Granulomatous disease, systemic lupus erythematosus, systemic sclerosis |
| Endocrine disease | Hypoparathyroidism, hyperparathyroidism, vitamin D deficiency |
| Other | Langerhans cell histiocytosis |
Step 2: exclude congenital conditions
Once reactive conditions have been eliminated as causative, the clinician should turn their attention to excluding the congenital polycythemic syndromes – which can manifest with thrombocythemia, erythrocytosis, or neutrophilia.20 The most important first step in this clinical assessment is a thorough family history. Typically, these hereditary polycythemic conditions are inherited syndromes that affect a single blood cell lineage and are transmitted via Mendelian inheritance. Penetrance in these conditions is high and they exhibit polyclonal hematopoiesis. They should be considered as a separate entity from the primary MPNs, although these latter conditions can be associated with a genetic predisposition in rare instances.20–22
Though uncommon, forms of hereditary thrombocythemia have been described in multiple families.23–26 These conditions are most often associated with mutations in the thrombopoietin gene or the gene for its receptor (myeloproliferative leukemia virus oncogene (MPL)). A full review of these conditions is beyond the scope of this chapter, but several have been published.20,22,24 Relevant to this discussion, however, is that it should be noted that the clinical course of hereditary thrombocythemias is not typically benign. Individuals can suffer from venous thrombotic events, major vasomotor events, arteriovascular events, and hemorrhage at rates comparable with ET.22,25
Familial or congenital polycythemias refer to rare heritable disorders where the red cell mass is increased. The phenotype can arise from two broad categories: the first group is attributed to a progenitor cell defect and a gain-of-function mutation in the erythropoietin receptor (EPOR). The individuals have a defect in their hematopoietic progenitor cells and a subnormal serum erythropoietin level. These abnormalities are autosomal dominant and variants have been reported in a number of families.20,27–29 The second category of heritable disorders resulting in an elevated red cell mass occurs in conjunction with inappropriately normal or elevated serum erythropoietin levels and can be due to mutations in the von Hippel–Lindau (VHL) gene, mutations in the pathways involving hypoxia-inducible factor, or a consequence of tissue hypoxia caused by hemoglobin variants with increased oxygen affinity or defective biphosphoglycerate mutase.5,20,30–32 For the clinician, excluding these congenital syndromes begins with a complete family history and may require genetic testing done after informed consent and counseling with the families and involving a specialized laboratory.
Step 3: diagnostic pitfalls in the pediatric population
Essential thrombocythemia
Among the challenges of diagnosing these conditions in the pediatric population is that the genetic markers which occur commonly in adults are less frequently manifest in children. For example, in adults with ET, the JAK2 V617F mutation is determined to be present in approximately 50% of cases, the MPL mutation in up to 10%, and the mutation of the calreticulin (CALR) gene is found in 74–80% of patients with JAK2-unmutated disease.33–37 Fewer children than adults with ET are JAK2 mutant-positive.2,38,39 A Japanese study looked at 6 patients with sporadic ET and found that 50% had the JAK2 V617F mutation.40 All carried the PRV-1 mutation. An Italian group published a series of 18 pediatric ET patients and demonstrated that 38% were JAK2-positive.2,41 A follow-up by that same group looked at 64 patients who underwent a robust testing panel for Philadelphia-negative MPN. Thirty-four children were diagnosed with sporadic thrombocythemia, and 47.5% harbored the JAK2 V617F mutation. The CALR mutation, which has been shown to be positive in most JAK2-negative adult patients with ET, does not appear to occur at the same frequency in JAK2-negative pediatric patients.42–45
Polycythemia vera
The V617F mutation or the exon 12 mutation is found in >99% of adult patients who carry a diagnosis of PV. The reliability of this test is one reason why, in adults, the measurement of red cell mass has become increasingly infrequent as a diagnostic tool. In children with an acquired primary erythrocytosis, the clinical and biochemical presentation does not differ significantly from that in younger adult patients.46 However, several series demonstrate that the JAK2 V617F mutation is less likely to be positive. In one investigation, where 8 pediatric PV patients were analyzed, just 3 were positive for the JAK2 V617F mutation, though the mutations in exon 12 were not tested.2 In a second publication, where 11 pediatric patients were analyzed, the JAK2 V617F mutation was detected in only 3 patients. The patients were, however, found to have a high red blood cell mass, histologic criteria for PV, and/or the presence of erythropoietin-independent erythroid colony growth.47
The conclusions of most authors is that, while mutation testing of JAK2V617F, CALR, and MPL can rule in an MPN, the absence of such a molecular marker cannot be taken as evidence that the disease is ruled out. Rather, the diagnostic conclusion needs to be made with the whole of the available evidence, including family history, clinical presentation, and bone marrow results. Several diagnostic algorithms have been proposed in cases of pediatric erythrocytosis and thrombocytosis.5 Figures 16.1 and 16.2 are a summation of these proposed diagnostic tools, with the caveat that, as these conditions are very rare, medical evidence to support this kind of algorithmic decision making is lacking.
Step 4: triggers to initiate treatment
Once a child is diagnosed with an MPN, the question becomes whether or not treatment, especially in the form of cytoreductive therapy, is necessary. As in all diseases, the initiation of a treatment or intervention requires an understanding of the risk of observation alone and the risk associated with the intervention. In adults, current risk stratification systems are in place based on the natural history of the disease in that population, rare as it is.36,48,49 Such systems help limit drug exposure to patients in whom thrombohemorrhagic or transformational events are most likely. Long-term data on the pediatric population are so scanty that such standardized risk systems are not available. However, there is general consensus that, as in the adult population, prior life-threatening arterial or venous embolism constitutes high-risk disease and should trigger cytoreductive therapy after appropriate consent discussion.1
Essential thrombocythemia
With regard to ET, there seems to be a growing consensus that children may be somewhat less likely than adults to suffer thrombotic events related to their disease. This is in contrast to one, early, oft-cited review, which collated 36 published cases of pediatric ET from 1966 through 1997.50 The authors reported a high incidence of complications (42%) either at diagnosis or thereafter. As the researchers acknowledge, this finding is likely based on the fact that their data were drawn from published cases rather than a case series or institutional registry. Four deaths, including 2 from transformation to acute myeloid leukemia (AML), were logged. Both AML cases occurred in patients who had received either radioactive phosphorus or a combination of thiotepa/busulfan.
However, authors of several later studies have argued that children are at lower risk than their adult counterparts of experiencing serious clinical sequelae. In an Italian cohort of 50 heterogeneously treated patients with either ET or hereditary thrombocythemia, 3 patients experienced a thrombotic event after a median follow-up of more than 132 months.47 No patients developed acute leukemia, and while some patients developed splenomegaly and reticulin fibrosis, none transformed to myelofibrosis. In a series of 34 patients with ET published by Italian researchers, patients did have symptoms or organomegaly at diagnosis but none presented with either bleeding or thrombotic episodes at initial manifestation of the disease.47 In a separate series of 18 ET patients under the age of 18, children had a significantly lower incidence of thrombosis than adults.41
In the pediatric patient who has asymptomatic primary ET, observation alone is a reasonable approach.1 In this low-risk cohort, antiplatelet therapy can be initiated if there are significant microcirculatory symptoms, such as migraine headaches, erythromelalgia, or pruritus. A concern of using aspirin in children stems from its possible association with Reye’s syndrome, a rare but potentially devastating disorder of hepatic encephalopathy which occurs in individuals under the age of about 15.
In the teens and young adult years, some patients may acquire risk for arterial thrombotic disease – in particular if they suffer from diabetes or hypertension or if they take up smoking (a practice which should be vigorously discouraged). In patients with ET, once such conditions are present, aspirin as primary prevention of arterial clot should be considered and the risk–benefit relationship discussed with the patient and parents. Antiplatelet agents are administered in the treatment of a large number of adult diseases. However, in childhood there is a lack of large interventional trials and more uncertainty involving the effects of the use of antiplatelet drugs in children. Such a lack of data has led to greater uncertainty, and a less extensive use of these drugs.
While there are no evidence-based risk stratification models, some pediatric patients with ET will require cytoreductive therapy as a last resort.48 The indications for cytoreduction include a venous or arterial embolic event, the finding of an acquired von Willebrand syndrome due to a very high platelet count, or very severe microcirculatory symptoms unresponsive to aspirin therapy.1,51 Unfortunately, in the rare pediatric patient there are few data to guide treatment choices.
Polycythemia vera
Many similar concerns arise in the decision making on risk stratification in PV. Again, central in the care of pediatric patients is the employment of cytoreductive agents only when deemed clearly necessary.
PV in younger individuals may be asymptomatic. However, given the low risk associated with venesection as an intervention, this remains a recommendation for even asymptomatic individuals and may play an important role in preventing vascular events.1 The goal of venesection is a hematocrit below 45%, based on evidence from the Cyto-PV study in adult patients.52,53 In this trial, a total of 365 patients were randomly assigned to either a more aggressive treatment arm with phlebotomy, hydroxyurea, or both, with a hematocrit target of less than 45% (low-hematocrit group) or a second group which received less aggressive therapy for a hematocrit target of 45–50% (high-hematocrit group). Results demonstrated an increased rate of death from cardiovascular causes or thrombotic events in the high-hematocrit group and an improvement in the risk for venous or arterial clotting incidences in the low-hematocrit group. This study was performed in a typical population of PV patients, with an average age in both groups of about 60 years. However, it does provide a target goal, even in children, for venesection procedures. Some physicians aim for a lower hematocrit level in female patients – approximately 43%.
There is no evidence-based guidance for the determination of aspirin therapy in children with asymptomatic PV. Again, the predominant concern of aspirin use in children is the possible association with Reye’s syndrome. However, given the effective prophylactic action of aspirin in preventing clotting events, and given the devastating impact of cardiovascular and cerebrovascular events, many clinicians would strongly consider prophylactic aspirin therapy (81–162 mg daily) in teenagers with documented PV and who, on the basis of age, have outgrown their risk for Reye’s syndrome.1,46
In a symptomatic patient, venesection and aspirin may be especially effective in the treatment of erythromelalgia, pruritus, or other vasomotor symptoms. If unresponsive, then cytoreductive therapy may be considered, again as a last resort.48
Review of published cases lends credence to the concept that certainly, even in children, the disease can be life-threatening. A review of 36 reported cases of pediatric PV published in 2009 determined that a quarter of patients developed severe thrombotic complications and 3 patients died from disease-related complications. About half of the patients were suffering from other symptoms, probably related to PV.46 Life-threatening arterial or venous embolic event is an urgent treatment trigger. Among the clots that are prevalent in the pediatric population is hepatic veins clots, or Budd–Chiari syndrome.54–56 Notably, patients may present with Budd–Chiari and not have obvious abnormalities in their peripheral counts due to enlargement of their spleen or occult gastrointestinal losses. However, any clotting event in the splanchnic system in a child or young adult should prompt a workup for a primary MPN. The Budd–Chiari syndrome has long been known to be associated with MPNs, including PV. And the JAK2 V617F mutation has been found in both the endothelial and hematopoietic cells of patients.57 In adult patients, at least, the JAK2 mutation can be found in about 45% of patients with Budd–Chiari and 34% of patients with PV.51 However, in children, the absence of a mutation does not exclude MPNs. Other complications that can occur in children with MPNs include cerebral venous thrombosis, stroke, and hemorrhage of the gastrointestinal tract or other organ.
Step 5: the choice of cytoreductive therapy
In the literature, a wide range of medications have been used in children and young adults, including treatment with aspirin, phlebotomy, hydroxyurea, interferon, and alkylating agents such as pipobroman, busulfan, and melphalan.50 There are sufficient data on the leukemogenecity of these latter therapies that they should be avoided in the treatment of young patients. For example, in a study of adult patients treated between 1975 and 2000, 70 patients under the age of 50 and with PV were followed for a median time of 14 years.58 About three-quarters were treated with pipobroman. The risk of developing acute leukemia and myelofibrosis at 20 years was 15% and 10%, respectively. On the other hand, a similar-length study of 30 younger adults treated only with phlebotomy, aspirin, and/or hydroxyurea did not demonstrate a high risk for thrombosis, secondary leukemia, or myelofibrosis.59
In patients where therapy is warranted, problems can arise with either of the first-line choices: hydroxyurea or interferon-alpha. Hydroxyurea is the most common medication initially employed, and is the recommendation of the UK guidelines on treatment of adults and children.1,36,49 The oral medication is dosed initially at about 15 mg/kg/day and will often lower the platelet count over the course of the first week of administration. There remains concern over any marginal increase in risk of leukemic transformation in patients taking hydroxyurea, but this is not supported by available evidence.36 The risk of leukemia from among patients treated only with hydroxyurea does not appear to be significantly different from the risk in untreated patients.60 A nested case-control study of more than 11,000 MPN patients demonstrated that the risk of AML was similar to that of controls.61 However, there are data to support that, when hydroxyurea is used concomitantly or in sequence with another cytotoxic agent like busulfan or pipobroman, the risk for transformation is certainly above that seen in untreated patients.62–64
Initially, patients should be seen twice monthly to ensure that the dose of hydroxyurea is acceptable. Once on a stable dose, visit frequency can be moderated, with the understanding that side effects do happen with the drug, including cutaneous toxicities, skin ulcerations, oral ulcerations, and aberrations in red cell production, including macrocytosis.
A second agent that is both feasible and has data supporting its use is interferon-alpha, either in the traditional form or in the pegylated form. The pegylated form is designed to allow the drug to be administered less frequently. The literature includes more than 500 individuals with MPNs treated with pegylated interferon.65 Studies on interferon-alpha, including the use of pegylated interferon-alpha, have been performed in adults,65–69 but no rigorous studies have been published on the pediatric population. Retrospective, non-randomized studies demonstrate complete hematologic remissions with this medication, a decline in JAK2 V617F allele burden, and a diminished thrombosis rate.70 In pediatrics, the bulk of the literature on interferon appears in the form of case reports. As is oft repeated in the published literature, there are insufficient data to recommend a specific agent in children, and the choice should be individually tailored.1,36,48
Adverse effects of interferon-alpha, including flu-like symptoms, changes in mood, and autoimmune phenomena, should be a careful part of the consent discussion with patients and their parents. In adults, there are no data linking interferon therapy leukemogenesis and it is considered to be relatively safe to use in pregnancy.
The effects on fertility of both medications need to be considered in this younger population. As mentioned above, during pregnancy, if there is a need for treatment, then interferon-alpha is the appropriate option. A systemic review looking at more than 60 cases of pregnancy occurring in the setting of interferon exposure has been published.71 With all the caveats of a non-randomized, observational study, the results suggest that the drug is not associated with an increase in the risk of major malformation, miscarriage, stillbirth, or preterm delivery. In fact, the authors postulate that interferon-alpha may have a protective effect against pregnancy loss in cases of ET, a finding that supports the overall antithrombotic effect of the medication in this condition. Notably, however, some authors have noted that there is a detrimental effect of this medication on fertility, at least in females.72,73
Gonadal suppression, resulting in amenorrhea or azoospermia, may occur in patients taking cytoreductive agents. Data on hydroxyurea can be found in the literature on sickle-cell disease. While there are scarce data on humans, the Center for the Evaluation of Risks to Human Reproduction published a report in 2007 on the reproductive risks of hydroxyurea. While there were no conclusive data on the effects of the medication, the authors did conclude that the drug can cause developmental toxicity in rodents exposed in utero to hydroxyurea.74 There does appear to be consensus that the medication can also impair spermatogenesis in human males.73
In summary, cytoreductive agents in either PV or ET should be used only as a last resort in the pediatric population. In low-risk individuals with PV, i.e., asymptomatic with no history of arterial or venous clotting events, patients should have their target hematocrit below 45% and, in the absence of risk for Reye’s syndrome, should receive aspirin. ET patients should be on low-dose aspirin if they have microcirculatory symptoms or cardiovascular risk factors and do not have a risk for Reye’s syndrome. High-risk patients, i.e., with prior clotting events, unmanageable symptoms, or dangerously elevated blood counts, can be considered for cytoreduction. Some authors consider hydroxyurea the optimal first choice, whereas others would advocate for interferon in a young person.
Special consideration: myelofibrosis in children and the question of transplantation
As stated earlier, PMF in children is exceedingly rare, with only a handful of cases reported in the literature. One case series, published by hematopathologists at Texas Children’s Hospital, constitutes the largest collection of patient data.6 A total of 19 patients were identified as having idiopathic myelofibrosis; 5 cases underwent spontaneous resolution; no cases developed transformation to AML. Nine patients were transplanted for their condition and 4 of those transplanted subsequently died.
A study of 3 patients published by pediatricians at Vanderbilt University confirmed that spontaneous resolution of disease can occur. They cite complete resolution of marrow fibrosis and cytopenias in one of these cases, though only after several years. 7 This publication also includes a full review of the published cases of myelofibrosis in children, which illustrates the heterogeneity of treatment approaches. Stem cell transplantation has certainly been one of the approaches employed, and there are more than a dozen reports of transplantation for pediatric myelofibrosis in the literature.6,7,75,76
However, given that there are also a number of reports of spontaneous resolution of disease, and given that mortality following transplantation is not uncommon, the decision to transplant should be one taken only as a last resort and for grave disease. In the 2012 UK guidelines for the diagnosis and management of myelofibrosis, the authors recommend a conservative approach in pediatric cases of PMF. They emphasize the critical importance of verifying the diagnosis and ruling out conditions like autoimmune disorders, secondary myelofibrosis like rickets, and hypocellular myelodysplastic syndrome. Transplantation, according to the guideline, “should be restricted to patients for whom all other diagnoses have been excluded, particularly for those with intractable symptomatic cytopenias, or a rising blast count.”77 There is very limited experience in children with ruxolitinib, the inhibitor of the JAK-STAT pathway which has been proven beneficial in myelofibrosis in adults. A phase I study of the medication was performed studying the side effects and best dose of the medication in patients younger than 21 years with relapsed or refractory solid tumor, leukemia, or myeloproliferative disease.78
Case 1: part 2
The patient and her mother return 2 weeks later. In that time, you have repeated a complete blood count and the platelet count remains elevated at 623 × 1,000/μL. Iron studies do not demonstrate iron deficiency. Testing for the JAK2 V617F mutation is performed and is negative. You discuss that the patient may have a primary bone marrow disorder. Referral to a hematologist is made and a bone marrow biopsy is performed. Abnormalities are noted in the megakaryocytes with clustering and some abnormal forms. Karyotype is normal on cytogenetics, without evidence for the BCR/ABL translocation. CALR mutation analysis is performed on the peripheral blood and a frameshift mutation is detected in exon 9 of the CALR gene. The patient is told that she has ET. She and her mother ask what risks she faces and what her preferred method of birth control should be.
Gender-specific issues in myeloproliferative neoplasms
Excluding the conditions of pregnancy and fertility – which are addressed in Chapter 17 in this textbook – there are unlikely to be instances when the treatment of a patient with MPN varies according to gender. However, the astute clinician should be aware of several differences in incidence, presentation, and in the variant symptoms that have been documented in female patients.
Incidence of MPNs in women
Of the all the MPNs, PV has been reported to be the most common, with estimated incidence rates of around 2.8 per 100,000 persons per year.79 However, a recent meta-analysis reported a pooled annual incidence rate of 0.67 per 100,000, which was similar to reported European epidemiological data.80,81 Traditionally, it has been observed that more men than women develop PV, with the male-to-female ratio at approximately 1.2 to 1.81 However, this has also come into question, with new data showing that the crude annual incidence between males and females did not significantly differ.80 ET has an estimated annual incidence rate of 0.21–2.43 patients per 100,000. It should be noted that ET, despite differences in incidence geographically, appears to have a slightly higher incidence rate among women (range 0.64–2.00 per 100,000 per year) than men (range 0.40–2.00 per 100,000 per year).81 More recent, pooled data showed a crude annual incidence rate of 2.12 per 100,000 in females, compared to 1.44 per 100,000 in males.81 The least common of the MPNs is MF, with an annual incidence rate ranging from 0.22 to 0.99 per 100,000. MF does have a slight male predominance, with a male incidence rate of 0.59 per 100,000 versus 0.30 per 100,000 for female. Now that there are widely accepted diagnostic criteria for all of the MPNs, true gender predominance may become more apparent.
Clinical presentation
Among the other interesting results that have been derived from epidemiologic studies is that gender does appear to influence JAK2 V167F allele burden. One such study, published in 2010, utilized samples from 272 patients with MPNs collected at a single institution.82,83 Researchers found that women had significantly lower allele burdens than men. The fact that women have a lower allele burden and are less likely than their male counterparts to have hypertension or hypercholesterolemia led to hypotheses that there are gender differences in clinical presentations. As a follow-up, a large database was investigated to derive differences in presentation between the genders.84 Data from the European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study showed that history of venous thrombosis was more common among women with PV, compared to men. In addition, women were older at the time of diagnosis, and were more likely then men to have venous embolic events and microcirculatory disturbances while men were more likely to have peripheral arterial disease and myocardial infarction. This study also demonstrated the striking finding observed that splanchnic venous thrombosis occurred most typically in young women; in their database, 6 cases of Budd–Chiari syndrome were observed, 5 of which occurred in females. This finding is concordant with similar studies that point to the higher rates of abdominal vein thrombosis in females versus males. For example, in a 2006 study out of the Mayo Clinic, it was observed that, of 460 patients with ET, 19 cases of abdominal vein thrombosis were documented, 17 of which were in females. Another noteworthy finding was that the average age of these females was 38 years, compared to age 56 years in those females without abdominal vein thrombosis.85 Moreover, there was a significantly higher conversion rate into myelofibrosis/acute leukemia as well as shorter median survival in the females with abdominal vein thrombosis compared to those age-matched without abdominal vein thrombosis. In another cohort observation, when comparing those who presented with vascular events, 60% of the women presented with abdominal vein thrombosis versus 14% of the men.82 There is also a strong female predominance with presentation of cerebral venous thrombosis (CVT). In a retrospective cohort of two large databases in which 706 patients were identified with CVT, 27 patients were found to have an MPN. In one of the databases analyzed, 7 out of the 9 patients with CVT were female.86 This database also demonstrated a higher frequency of CVT among those with PV (5 of 9) than those with ET (3 of 9) or PMF (1 of 9). In an earlier retrospective study, where there were a total of 10 patients with PNM identified with CVT, and although half were female, 4 out of the 5 were diagnosed with ET.87
A high level of suspicion for an underlying MPN needs to be kept in mind for any young person presenting with an abdominal vein thrombosis or a CVT. In particular, young women, who may be prescribed hormonal birth control, likely have synergistic risks for these unusual clots. Of particular importance is that peripheral counts may be in the normal range, in these patients, with the polycythemia masked by splenomegaly, hepatomegaly, or occult gastrointestinal bleeding. The recommendations for the workup of splanchnic venous thrombosis now include recommendations to rule out MPNs as causative.88
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree