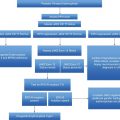
Manage scenarios associated with increased risk, e.g., surgery, pregnancy
The most important direct way of assessing compliance is through patient interview.2 Other measures, such as direct observation of medication intake, have been employed but the importance of the consultation remains paramount. Therefore, physicians have to gradually modify their role from sole decision makers to expert advisers and the patient’s perspective should be taken into account. During consultation, adequate time should be dedicated for the patient to comfortably express expectations, concerns, and questions. Through active listening the physician can assess the patient’s level of understanding, include the patient in decision making, and identify reasons for poor adherence.
Case 2
A patient with longstanding PV has recently turned 65 years old. The patient has always maintained a PCV <45% with venesections, although recently his white cell count reached 25 × 109/L, and his platelet count has always remained above 600 × 109/L. He has no history of thrombosis or hemorrhage, no disease-related symptoms, and his spleen size is normal. He is a smoker, with no other cardiovascular risk factors. The patient has been receiving low-dose aspirin since the time of diagnosis, 10 years ago. What is the appropriate management for this patient?
The aims of current therapy for PV and ET patients are to lower thrombotic risk and to avoid recurrence of thrombosis and hemorrhage, to minimize the risk of disease transformation to post-ET/PV MF and leukemia, and to alleviate symptoms, whilst avoiding if possible iatrogenic harm. Some patient subgroups such as pregnant women and children need a tailored approach (as discussed elsewhere in this book). Risk situations such as surgery or a thrombotic or hemorrhagic event also have to be managed; again, these scenarios are discussed elsewhere. This scenario demonstrates the importance of ongoing monitoring and being aware when the patient might change from a low- to high-risk group.
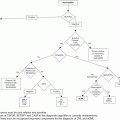
As previously mentioned, the current risk stratification for ET and PV is based on the estimation of thrombotic risk. High-risk patients are aged >60 years or with prior history of thrombosis or hemorrhage or with platelets above 1,500 × 109/L, which can be associated with increased bleeding risk. Classification beyond this is somewhat controversial with regard to intermediate and low risk. In our practice we regard patients aged 40–60 years and lacking any other high-risk factors as belonging to an intermediate-risk group and patients aged <40 years to the low-risk group.
In PV, the target is to maintain a hematocrit of below 45% by means of phlebotomy, irrespective of risk group.5,6 Venesection was established as the mainstay of treatment for PV by the Polycythemia Vera Study Group (PVSG-01) study,7 which compared the overall survival between patients who were venesected and patients who received either 32P or chlorambucil. Patients in the venesection arm had longer overall survival as well as lower risk of leukemic transformation or other non-hematologic malignancies. In the venesection arm an increased risk of thrombosis was observed, predominantly during the first 3 years when the target hematocrit was 52%; the recent Cytoreductive Therapy in PV (Cyto-PV) study demonstrated the importance of controlling the hematocrit strictly to 0.45.8 However, it is also becoming increasingly apparent that controlling the leukocyte count is of importance; indeed, the Cyto-PV study also suggests this, as the leukocyte counts were different between the two arms.9 Control of the platelet count may also be important as it may contribute to reducing thrombotic risk as well as the risk of progression to myelofibrosis. However, there is little consensus as to what the appropriate threshold for initiating treatment based upon platelet count should be.6
As well as monitoring for change in risk group for patients it is important to ensure that the patient receives regular assessment for cardiovascular risk factors such as hypertension, diabetes, and hyperlipidemia, which should be aggressively managed, and patients should be advised to stop smoking. These factors are sometimes left to another clinician – for example, primary or community care – but there may be challenges here as MPNs are rare disorders and the risks of thrombosis with which they are associated may not be recognized by another non-hematologist clinician.
Monitoring of response to disease-specific therapy as well as for its toxicity is also important. This is generally based upon the full blood count, examination for splenomegaly, and the assessment of disease-related symptoms. With regard to symptoms we find that the use of a structured assessment form on a fairly regular basis is helpful both for robust and speedy assessment as well as for patient education. We use the MPN Symptom Assessment Form for this purpose;10 we have not discussed symptom assessment specifically here as this is the subject of a different chapter (see Chapter 6). Bone marrow aspirate and biopsy are indicated when disease transformation is suspected. However, for patients receiving anagrelide, the results of the PT-1 study demonstrated that bone marrow reticulin was increased in the anagrelide arm, so we offer regular bone marrow evaluations at 3-yearly intervals.11 Molecular response including sequential assessment of the JAK2 V617F or CALR allele burden is not routinely monitored, except in clinical trial settings.
Case 3
A 65-year-old patient with newly diagnosed ET was started on HU. After the first 3 months of treatment his blood count normalized and he was without any side effects. He remained on long-term follow-up, having blood count review every 4–6 months. At one review he mentioned that he had developed leg ulcers.
HU is one of the the first-line cytoreductive treatments in both ET and PV. It is an antimetabolite that acts on ribonucleoside diphosphate reductase, an enzyme that catalyzes the reduction of ribonucleosides. Through this action, it slows DNA synthesis and repair, acting primarily on the S-phase of the cell cycle. HU has many side effects: myelosuppression, skin changes (leg ulceration, rashes, alopecia), and gastric disturbances (anorexia, nausea, diarrhea, vomiting). Most of these side effects are mild and reversible. Fever, acute liver function test elevations, pneumonitis, and azoospermia are rare side effects. HU may induce a range of skin changes such as onychodystrophy and melanonychia as well as actinic keratosis and squamous cell carcinoma, but these changes occur after prolonged treatment. It is important to educate patients about these side effects, so that for example they can be more careful with sun exposure, as well as monitor for them on a regular basis.
The European LeukemiaNet has published the definitions of resistance/intolerance to HU therapy.12,13 These are:
For PV: (1) need for phlebotomy to keep hematocrit <45% after 3 months of at least 2 g of HU or (2) uncontrolled myeloproliferation (platelets >400 × 109/L and white blood cells >10 × 109/L) after 3 months of at least 2 g of HU or (3) failure to reduce massive splenomegaly by >50% as measured by palpation or failure to completely relieve symptoms related to splenomegaly after 3 months of at least 2 g of HU or (4) absolute neutrophil count <1.0 × 109/L or platelet count < 100 × 109/L or hemoglobin <10 g/L at the lowest dose of HU required to achieve a complete or partial response or (5) presence of leg ulcers or other unacceptable HU-related non-hematologic toxicities such as mucocutaneous manifestations, gastrointestinal symptoms, pneumonitis, or fever at any dose of HU constitute the criteria for resistance/intolerance.
In ET patients, criteria are as follows: (1) platelet count >600 × 109/L after 3 months of at least 2 g/day of HU (2.5 g/day in patients with a body weight >80 kg) or (2) platelet count >400 × 109/L and white blood cells <2.5 × 109/L at any dose of HU or (3) a platelet count of >400 × 109/L and hemoglobin <10 g/L at any dose of HU or (4) presence of leg ulcers or other unacceptable mucocutaneous manifestations at any dose of HU or (5) HU-related fever.
Patients with HU resistance are at a higher risk of death than others and so are an important group to consider carefully in terms of future management.14,15 The strategy to manage these patients consists of three options: relaxing the treatment target, for example, platelet target to 600 × 109/L; switching therapy to a non-leukemogenic agent such as anagrelide or interferon or using combination therapy; or considering a clinical trial. However, lowering the platelet threshold of 600 × 109/L is not a recommended strategy for patients with recurrent thrombotic events.
Case 3 continued
For our patient HU was stopped and anagrelide was initiated. After the first month of treatment, the patient started complaining of palpitations and diarrhea. His electrocardiogram (ECG) and echocardiogram were normal; however, palpitations were persistent. The patient was advised to stop anagrelide and interferon-alpha (IFN-alpha) was started. IFN was well tolerated and the platelet count remained well controlled. The patient reported only minor headache appearing in the next 2 days after IFN administration, which was alleviated with acetaminophen and gradually improved after the first 3 weeks of treatment.
Anagrelide hydrochloride is considered to be the second choice for reducing the platelet count in ET patients, as has emerged from the PT-1 study.17 It is an orally active quinazinolone derivative that reduces the platelet count in a species-specific manner. Its action as a phosphodiesterase inhibitor is responsible for its major side effects: cardiotoxicity ranging from palpitations to congestive cardiac failure, headaches, diarrhea, and non-cardiac edema. Prior to initiating anagrelide, all patients must undergo a chest X-ray and ECG. An echocardiogram should be performed in patients with a previous heart history or abnormal ECG or increased cardiothoracic ratio on chest X-ray. Patients who complain of palpitations should avoid exercise and caffeine-containing drinks immediately before or after dose.16 If these persist, discontinuation of the drug may be needed. Patients who become more symptomatic should be carefully followed up and further investigated. At high doses anagrelide also inhibits platelet aggregation. The PT-1 study demonstrated that patients on the anagrelide arm had a higher rate of hemorrhagic complications,17 but this was not seen in the ANAHYDRET study, where anagrelide was used alone.18 Therefore, combination of anagrelide and aspirin should be decided depending on the relative risk of thrombosis and hemorrhage of each patient.11
IFN-alpha is the therapy of choice in high-risk men and women contemplating conception and its use in pregnancy has been proven safe.5 Its common side effects include depression, flu-like symptoms, hepatitis, and pneumonitis. Flu-like symptoms can be managed with the administration of acetaminophen prior to IFN administration and every 6 hours after in order to alleviate symptoms. Patients should be closely monitored for signs and symptoms of depression and if psychiatric symptoms persist or worsen, discontinuation may be needed along with psychiatric intervention. IFN has been shown to be useful as a second-line agent for patients who are refractory or intolerant to HU.19,20
As discussed above, those patients who are resistant and, to a lesser extent, those who are intolerant to HU reflect a group of patients with a worse prognosis. These are the patients in whom there is a need for new therapies. Those under consideration include histone deacetylase inhibitors, JAK inhibitors, and others such as telomerase inhibitors; this is discussed in detail in a recent review.21 There are data supporting the ability of some JAK inhibitors to control myeloproliferation in patients with PV and ET. However, beyond this, aspects that are uncertain include whether they prevent thrombosis and affect the probability of accelerated-phase disease such as myelofibrosis (MF) or indeed leukemia. The size and duration of studies required to evaluate these aspects of therapy in ET and PV will be challenging. Two large phase III commercially sponsored studies of ruxolitinib in PV are fully recruited; the results of one of these studies (RESPONSE) were reported at the 2014 American Society of Clinical Oncology and European Hematology Association meetings. Here ruxolitinib was superior to best-available therapy for the composite primary endpoint of 35% spleen size reduction and freedom from venesection. This trial studied a highly selected group of patients who were intolerant/resistant to HU yet had splenomegaly and still required venesection. A confounding aspect is that over 50% of the control arm were treated with HU, perhaps reflecting limited options for these patients. Most interesting was the reduction in thrombosis for ruxolitinib-treated patients. Further updates of these important data are awaited.22 There is one ongoing academic study of ruxolitinib in ET patients who are resistant or refractory to HU.
Some important safety concerns have arisen with regard to JAK inhibition: those of a “withdrawal syndrome,” neurological toxicity, and, lastly, risk of infections. An early report suggested a risk of severe inflammatory syndrome after ruxolitinib withdrawal and that these patients had a poor outcome.23 The COMFORT studies have not reported this as a risk, but a slow taper or consideration of steroid cover when ruxolitinib is withdrawn is suggested.
A second JAK2 inhibitor, fedratinib, has recently been put on full clinical hold due to the development of complications similar to Wernicke’s encephalopathy. Whilst this complication has not been reported with ruxolitinib, it will be important to evaluate other JAK inhibitors for this toxicity.
Meanwhile, histone deacetylase inhibitors such as vorinostat24 and givinostat25 have been used in patients with ET and PV. Toxicities with these agents are variable and for vorinostat in a phase II study led to high discontinuation rate. Givinostat continues to be evaluated. The oral telomerase inhibitor imetelstat was investigated in ET, demonstrating molecular responses but also significant rates of hematological toxicity.26 Further studies of sufficient duration to permit the evaluation of the safety and efficacy of novel agents are certainly needed, and will require long-term follow-up.
Case 4
A 50-year-old patient with longstanding JAK2 V617F-negative ET presented in the outpatient clinic with pancytopenia. On examination, spleen was palpable at 5 cm below left costal margin and liver at 3 cm. His peripheral blood smear revealed leukoerythroblastosis, circulating blasts <1% and teardrop cells; lactate dehydrogenase was elevated. The bone marrow biopsy was diagnostic, demonstrating collagen fibrosis and marked hypocellularity. Megakaryocytes were atypical, appearing in large clusters. CD34-positive cells were less than 10%. Cytogenetic and molecular analysis revealed a normal karyotype 46,XY, and the presence of the MPLW515K exon 10 mutation.
Myelofibrotic and leukemic transformation are well-known complications of ET and PV. Transformation of ET to PV is rarer, but has also been reported. Below we will discuss risk factors and data with regard to these events, focusing particularly upon myelofibrotic transformation.
In a large Spanish study of 195 ET patients with a median follow-up of 7 years, evolution to myelofibrosis with myeloid metaplasia, now termed post-ET MF (PET-MF), was observed in 13 cases and indicated that as time progresses there is an increasing probability of developing PET-MF, with an actuarial probability of 2.7%, 8.3%, and 15.3% at 5, 10, and 15 years, respectively.27 More recently, an Italian study with 605 patients showed that 2.8% developed PET-MF with a 10-year risk of 3.9%.28 Anemia was found to be an independent prognostic factor for PET-MF, whereas no correlation was found between cytotoxic agents and PET-MF. Leukemic transformation in this study had a cumulative risk of 2.6% at 10 years and 5.3% at 15 years.28 The PT-1 study comparing the efficacy of hydroxycarbamide plus low-dose aspirin versus anagrelide plus low-dose aspirin in 809 high-risk ET patients demonstrated that the rate of myelofibrotic transformation was signifantly higher in the anagrelide arm.17 A possible explanation for this finding might be that HU reduces reticulin fibrosis, whereas anagrelide acts by blocking megakaryocyte differentiation; as a result, high levels of profibrotic cytokines may be produced. It is important to note that similar results were not found in the ANAHYDRET study.18 Data from the long-term follow-up study EXELS are awaited. The PT-1 study also suggested that patients’ risk of events such as arterial thrombosis could be discriminated via reticulin grade in bone marrow.29 There has never been a comparative study with IFN, so data are unclear, though it has been suggested that IFN can normalize marrow morphology in early phases of MF: this represents data from small case series.30
Concerning similar data for PV patients in the large prospective European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study of 1,638 patients, acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) was diagnosed in 22 patients after a median of 2.5 years from recruitment and a median of 8.4 years from diagnosis of PV; this figure was similar to other studies.31 However, long-term data from a French PV-patient population enrolled in a study comparing pipobroman with HU suggested higher rates of transformation in the pipobroman-treated group and higher rates overall for leukemia and post-PV myelofibrosis.32 Here median survival was 17 years for the whole cohort, 20.3 years for the HU arm, and 15.4 years for the pipobroman arm (p = 0.008) and differed significantly from that in the general population. At 10, 15, and 20 years, cumulative incidence of AML/MDS was 6.6%, 16.5%, and 24% in the HU arm and 13%, 34%, and 52% in the pipobroman arm (p = 0.004). Cumulative post-PV myelofibrosis incidence at 10, 15, and 20 years according to main treatment received was 15%, 24%, and 32% with HU vs. 5%, 10%, and 21% with pipobroman (p = 0.02). These data and those from the PT-1 study are important, as they suggest that the risk of MF may be impacted upon by treatment. Although at present this does not form part of consideration for treatment choice.
The myelofibrotic process has been attributed to alterations in the cross-talk between hematopoietic and stromal cells, the so-called ‘‘bad seeds in bad soil’’ concept.5 These altered interactions enhance the release of cytokines by the clonal hematopoietic cells and especially by megakaryocytes and monocytes, such as the alpha granule contents, platelet-derived growth factor, platelet factor 4, transforming growth factor-β (TGF-β), basic fibroblastic growth factor, interleukin-1, and calmodulin). Schmitt et al. in 2002 reported that abnormal subcellular location of P-selectin within megakaryocytes correlates with emperipoiesis, thence disrupting megakaryocyte organelles and causing leakage of alpha-granule contents. Monocytes activate following contact with the extracellular matrix and upregulate CD25 expression and subsequently increase the production of TGF-β and interleukin-1 agents which have profibrotic potential.33 The release of hematopoietic, fibrogenic, and angiogenic growth factors activates stromal and endothelial cells and leads to MF, osteosclerosis, and angiogenesis. Reciprocally, these stromal cells, which acquire new properties, form a pathological microenvironment which supports the proliferation of the clone. Consequently, these abnormal stem cells are mobilized to the blood and then migrate to the spleen, resulting in extramedullary hematopoiesis and splenomegaly.
Thiele et al. highlighted the importance of the initial trephine biopsy for distinguishing true ET from prefibrotic MF with thrombocythemia and proposed a histological subdivision into three subgroups of patients (true ET, prefibrotic MF, and early MF), with a loss of life expectancy of 10.9%, 29.6%, and 51.3% respectively.34,35 Megakaryocyte morphology is one of the most important distinctive features. In ET megakaryocytes are scattered, large to giant-formed, and their nuclei are enlarged with deep lobulations, whereas in prefibrotic PMF megakaryocytes appear dysplastic and form clusters. In true ET, no increase in cellularity or in reticulin fibers was detected.11 While the World Health Organization classification includes ‘‘pre-fibrotic/early-stage myelofibrosis,’’36 the diagnosis of MF is not solely based on trephine biopsy but other features such as splenomegaly, leukoerythroblastosis, and constitutional symptoms are required to establish diagnosis. Not all pathologists have agreed with the ease and robustness of discriminating ET from early stages of primary myelofibrosis (PMF), though this must now be combined with clinical features, as discussed above.37,38
The diagnostic criteria for PET-MF and post-PV MF are proposed by an international group of experts.39,40 We use a modified version of these criteria, as shown in Table 9.2. The management of these patients is generally as for PMF, which is discussed elsewhere. Transformation to acute leukemia is diagnosed when the percentage of blasts is consistently 20% and above, either in the blood or bone marrow. Advanced age is found to be an important risk factor for leukemic transformation in both ET and PV and up to 25% of AML occurs in untreated patients according to data from the Swedish cancer registry.41 Leukemic transformation has been observed in previously untreated patients,41 but there are also many studies to support its correlation with cytostatic agents. Leukemogenesis is enhanced by the sequential use of more than one cytotoxic agent. It might be the case that the need for multiple agents may represent a more aggressive disease, but it is also likely that one drug may potentiate the leukemogenic effect of a subsequent drug.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree