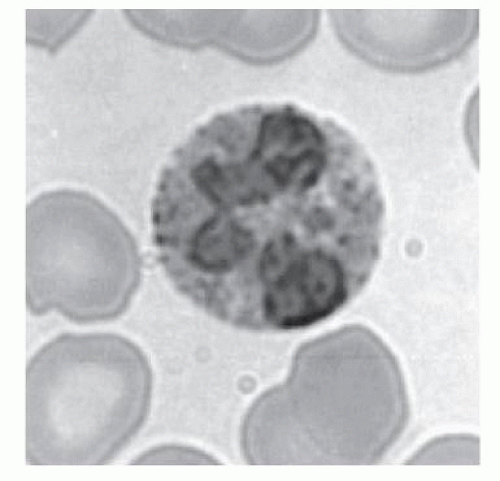
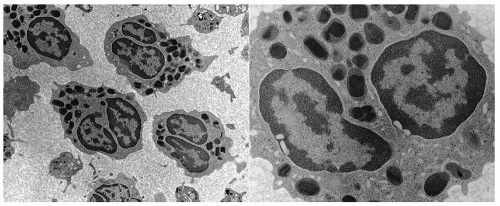
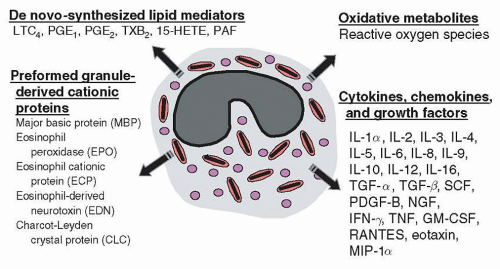
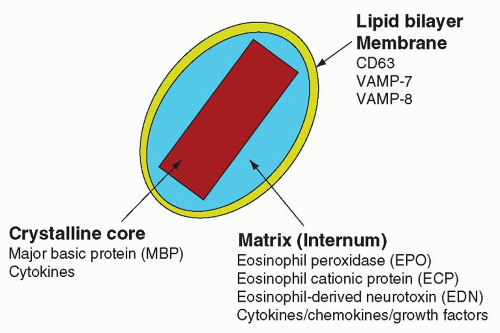
Eosinophil Granule Proteins
Eosinophils contain at least five different populations of phospholipid bilayer membrane-bound granules.
Crystalloid granules: these specialized and unique granules measure between 0.5 and 0.8
µm in diameter, contain crystalline electron-dense cores (internum) surrounded by an electron-lucent matrix, and can take up acidic dyes avidly due to their cationic nature.
8,
9 They are mainly present in mature eosinophils, although coreless granules have been observed in immature eosinophils. These granules contain the bulk of highly charged cationic proteins present in eosinophils, including major basic protein (MBP), eosinophil peroxidase (EPO), eosinophil cationic protein (ECP), and eosinophil-derived neurotoxin (EDN). There are approximately 200 crystalloid granules in each cell. The core is predominantly comprised of crystallized MBP (
Fig. 8.4).
Primary granules: these coreless granules are enriched with Charcot-Leyden crystal protein (CLC) and are present mainly in immature eosinophils, although mature eosinophils have been found to contain primary granules as well. Some authors refer to immature crystalloid granules as primary granules in eosinophil promyelocytes. These measure between 0.1 and 0.5 mm in diameter and are less abundant than crystalloid granules.
Small granules: these granules are free of cores, are less than 0.1 µm in diameter, and contain acid phosphatase, arylsulfatase B, catalase, and cytochrome β558.
Lipid bodies: there are around five lipid bodies per mature eosinophil, the number of which increase in certain eosinophilic disorders, especially in idiopathic hypereosinophilia. Lipid bodies are enriched in arachidonic acid esterified into glycerophospholipids.
Secretory vesicles: eosinophils are densely packed with small secretory vesicles in their cytoplasm. These vesicles appear as dumbbell-shaped structures in cross-sections, and contain albumin, suggesting an endocytotic origin. These structures are also known as microgranules or tubulovesicular structures.
Eosinophil MBP (13.8 kDa) is an arginine-rich 117 amino acid protein that constitutes a significant proportion of total cell protein in human eosinophils (5 to 10 pg/cell). MBP was originally named for its abundance in guinea pig eosinophils, which contain as many as 250 pg/cell, making up 50% of the total cellular protein.
10 The high calculated pI point of MBP (11.4) cannot be measured accurately due to the extremely basic nature of the protein.
11 MBP is initially translated as pro-MBP (23 to 25 kDa)
with a calculated pI of 6 to 6.2 in maturing eosinophils.
12,13 The synthesis of MBP is initiated during the promyelocytic stage of eosinophil development, characterized by the presence of message encoding this protein, in a neutral prepro-form which is later processed to form pro-MBP that is subsequently transported to the immature crystalloid granule and cleaved to form MBP.
12,13 The pro-segment of pro-MBP is postulated to protect the cell from cytotoxic effects of MBP during trafficking of pro-MBP from the Golgi apparatus to the crystalloid granule. Mature MBP undergoes condensation from the periphery of immature crystalloid granules to the internum, where it develops a crystalline core as its concentration is increased.
13,14 Once eosinophils have reached full maturity, MBP is no longer synthesized and messenger RNA encoding MBP disappears from the cell.
13,15MBP has been shown to exert cytotoxic effects on helminthic parasites and certain bacteria.
8 It is also cytotoxic to human cells and especially airway tissues, including bronchial epithelial cells and pneumocytes, by disruption of cell membrane lipid bilayers.
16 Such disruption leads to cell permeabilization and lysis, and leakage of cell contents to the extracellular milieu. Thus, MBP may be at least partly responsible for tissue damage and neural dysfunction associated with eosinophil infiltration into the bronchial mucosa in asthma.
9,17,18 Indeed, airway sections from patients with status asthmaticus exhibit intense MBP-specific immunofluorescence, suggesting that infiltrated eosinophils were fully activated, undergoing extracellular secretion of their contents of MBP.
19 MBP acts on other inflammatory cells, including neutrophils and eosinophils, to induce degranulation and lipid mediator release.
20,21 Parasympathetic ganglia in the airways of patients dying of asthma exhibit eosinophil infiltration, and MBP is an allosteric antagonist for the M2 muscarinic receptor. Loss of M2 receptor function results in airway hyperreactivity.
18 A homolog of MBP has been recently discovered, MBP2, with a calculated pI of 8.7, that possesses similar activities to MBP in cell killing as well as neutrophil and basophil stimulation, but with reduced potency.
22Other eosinophil basic proteins, including EPO, ECP, and EDN, reside in the matrix compartment of the crystalloid granule. EPO is a highly basic (pI of 10.9) heme-containing protein composed of two subunits, a heavy chain of 50 to 57 kDa and a light chain of 11 to 15 kDa. EPO is a haloperoxidase with 68% sequence identity to neutrophil and monocyte-expressed myeloperoxidase, suggesting that a peroxidase multigene family may have developed through gene duplication.
11,23 Eosinophils store approximately 15 pg/cell of EPO, which is important in catalyzing the peroxidative oxidation of halides and pseudohalides, leading to the formation of bactericidal hypohalous acids in reaction with hydrogen peroxide generated during respiratory burst.
24,25,26 Unlike myeloperoxidase, EPO preferentially uses bromide over chloride in its enzyme activity, generating hypobromous acid.
27 EPO in the presence of H
2O
2 and halide (usually bromide) kills a variety of microorganisms, but also inflicts collateral damage on host tissue such as mast cells, and possesses antitumor activity. Binding of EPO to bacterial and parasitic pathogens also enhances their killing by phagocytic cells.
The molecular mass of ECP is 15 kDa, with around 15 pg/cell expressed in human eosinophils. The pI of ECP (10.8) is similar to that of MBP due to a similar arginine-rich sequence. ECP, also known as RNase-3, possesses intrinsic ribonuclease (RNase) activity and is a member of a subfamily of RNase A multigenes, with homology to pancreatic RNase. It is also antiviral, bactericidal, promotes degranulation of mast cells, and is toxic to helminthic parasites.
28,29,30 and 31 The mechanism of action of ECP is thought to involve the formation of pores or channels in the target membrane, which is apparently not dependent on its reversible RNase activity.
32 ECP is also neurotoxic as derived from its ability to elicit the Gordon phenomenon, involving the destruction of nerve cells.
33,34EDN, another member of the RNase A multigene family of 18.5 kDa with approximately a hundredfold higher RNase activity than ECP, is less basic than MBP or ECP with a pI of 8.9 due to a relatively smaller number of arginine residues in its sequence. ECP and EDN share a remarkable sequence homology of 67% at the amino acid level for the pre-form of both proteins, suggesting that evolutionarily, these proteins are derived from the same gene.
35,36 Eosinophils express approximately 10 pg/cell of EDN, but there is marked variation between individuals. EDN also induces the Gordon phenomenon.
33,34 Similar to ECP, EDN also has significant toxicity against viruses, bacteria, and helminths.
31,37 Messenger RNA encoding EPO, ECP, and EDN has been detected in mature eosinophils, suggesting that eosinophils have the capacity to continue to synthesize these proteins.
15The gene family expressing ECP and EDN has among the highest rates of mutation in the primate genome, ranking with those of immunoglobulins, T-cell receptors, and major histocompatibility complex (MHC) classes.
36 These genes effectively comprise a superfamily of RNases expressed in the mammalian genome. Such an extreme rate of mutation suggests that the evolutionary constraints acting on the ECP/EDN superfamily have promoted the acquisition of a specialized antiviral activity. This may be inferred from the high mutation rates of other genes commonly associated with host protection against viral infection. Whether ECP or EDN possess any antiviral activity has yet to be demonstrated, although some studies have indicated that EDN may be a potent antiviral factor in respiratory infections.
31The CLC protein (17.4 kDa) is produced in eosinophils at very high levels (accounting for 10% of the total cellular protein) although its functional role is still obscure. CLC is a hydrophobic protein with lysophospholipase activity and bears a strong sequence homology to the carbohydrate-binding galectin family of proteins. For this reason, CLC protein has been designated galectin-10.
38 CLC is released in large quantities in the tissues in eosinophilic disorders, resulting in the formation of distinct, needle-shaped structures which are colorless and measure 20 to 40
µm in length and 2 to 4
µm across. CLC crystals are abundant in the sputum and feces of patients with severe respiratory and gastrointestinal eosinophilia, which were first observed by Charcot and Robin in 1853.
A list of these and other granule proteins synthesized and stored in eosinophils is presented in
Table 8.1 and published elsewhere.
1,2,39,40 and 41
Eosinophil-derived Cytokines, Chemokines, and Growth Factors
Human eosinophils have been shown to produce at least 30 different cytokines, chemokines, and growth factors (
Table 8.2) with the potential to regulate various immune responses. These cytokines have been identified in eosinophils by detecting mRNA and/or protein using RT-PCR, in situ hybridization, and immunocytochemical staining.
42,43,44,45 In addition, picogram amounts of cytokines, chemokines, and growth factors were measured in supernatants of stimulated eosinophils.
43,46 These cytokines are likely to act in an autocrine, paracrine, or juxtacrine manner, thereby regulating local inflammatory events. Studies have demonstrated that the production of eosinophil-activating cytokines (e.g., IL-3 and GM-CSF) by eosinophils may be important in prolonging the survival of these cells by a putative autocrine loop.
43,47 For instance, adherence of highly purified eosinophils to the extracellular matrix protein, fibronectin, resulted in prolongation of survival of these cells in the absence of exogenous cytokines.
47 Fibronectin-induced eosinophil survival was inhibitable by antibodies against fibronectin and VLA-4 and up-regulated by picogram amounts of IL-3 and GM-CSF derived from eosinophils.
47 Observations on eosinophil cytokine release have been mainly studied in vitro, but a few have been confirmed in vivo.
48,49,50 and 51 A recent study demonstrated that eosinophils elaborate APRIL (a proliferation-inducing ligand) and IL-6 which were essential for maintaining plasma cells in the bone marrow.
52A major distinction in cytokine production between eosinophils and T-cells is that the former store their cytokines intracellularly as pre-formed mediators, and the latter produce and release cytokines only following activation. Although many eosinophil-derived cytokines are elaborated at lower concentrations than other leukocytes, eosinophils possess the ability to release these cytokines immediately (within minutes) following stimulation. Stored cytokines include IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-16, GM-CSF, TNF
α, CC chemokine ligand 11 (CCL11)/eotaxin, IL-8, CCL5/RANTES, NGF, and TGF
α.
45 Studies using immunogold electron microscopic analysis or confocal laser scanning microscopy coupled with double immunofluorescence labeling have indicated that several of these cytokines are found in close association with either the crystalline core or matrix of the crystalloid-specific granules of the cell (
Table 8.2).
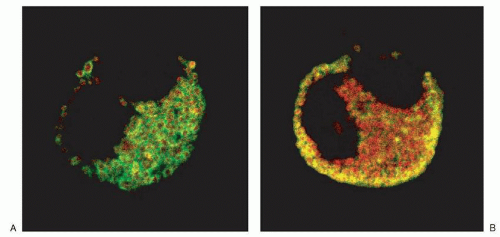
44,53,54,55 and 56,57,58 For example, CCL5/RANTES was found to be associated predominantly with the matrix compartment of the crystalloid granule in eosinophils (
Fig. 8.5).
Developing eosinophils possess the ability to express cytokine message and protein at early stages of maturation. Eosinophils generated from semi-solid culture of cord blood-derived CD34
+ cells in the presence of IL-3 and IL-5 were shown to express IL-5 and GM-CSF mRNA after 10 days of culture.
59 Freshly purified CD34
+ cells expressed IL-4 and CCL5/RANTES mRNA, but not IL-4 and CCL5/RANTES protein. On day 23 of culture, IL-4 and CCL5/RANTES localized to the matrix of MBP
+ crystalloid granules as determined by immunofluorescence.
60 In addition, IL-6 protein expression was found in cells after day 16 of culture.
14Another site of storage of cytokines and chemokines is within the small secretory vesicle. At least two such proteins were shown to be associated with these vesicles, namely CCL5/RANTES and TGF
α immunolabeling.
44,61 These organelles belong to the same group of secretory vesicles identified by electron microscopy analysis as tubulovesicular structures. CCL5/RANTES-positive vesicles are highly sensitive to stimulation by IFN
γ and are rapidly mobilized (within 10 minutes of stimulation) to secrete CCL5/RANTES extracellularly.
44,62 Crystalloid granules, which also contain CCL5/RANTES within their matrix compartment, were found to release this chemokine more slowly in response to IFN
γ (1 hour), whereas the majority of MBP remained associated with the core of these granules. These observations suggest that eosinophils have the ability to “shuttle” CCL5/RANTES from the crystalloid granules to the cell exterior, and may provide an important in vitro model for eosinophil piecemeal degranulation.
Eosinophil Degranulation
Degranulation is defined as the exocytotic fusion of granules with the plasma membrane during receptor-mediated secretion. During exocytosis, the outer leaflet of the lipid bilayer membrane surrounding the granule encounters the inner leaflet of the plasma membrane,
a process known as “docking.” The docking step is hypothesized to be regulated by intracellular membrane-associated proteins that act as receptors directing the specificity of granule targeting. After docking, the granule and plasma membrane fuse together and form a reversible structure called the fusion pore, which is also thought to be regulated by similar, or the same, membrane-associated proteins regulating granule docking. Depending on the intensity of the stimulus, the fusion pore may either retreat, leading to re-separation of the granule from the plasma membrane, or it may expand and allow complete integration of the granule membrane into the plasma membrane as a continuous sheet. The inner leaflet of the granule membrane becomes outwardly exposed, and the granule contents are subsequently expelled to the exterior of the cell.
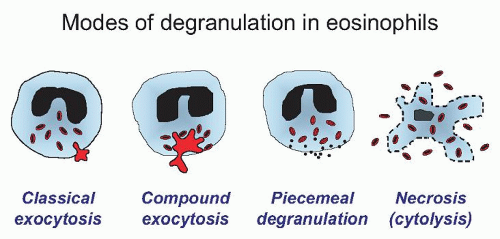
63There are four main forms of eosinophil granule release, which have been observed in vitro and in vivo (
Fig. 8.6). The first is the classical sequential release of single crystalloid granules, which was the original hypothesis suggested for a predominant route of degranulation in eosinophils. This type of release is typically seen in vitro and can be elegantly demonstrated electrophysiologically using patch-clamp procedures that measure changes in membrane capacitance, which are directly proportional to increases in the surface area of the cell membrane. During the
sequential release of individual crystalloid granules, a stepwise increment in capacitance may be observed as their membranes fuse with that of the cell membrane.
64,65,66 The second mode of granule release is compound exocytosis, also demonstrated by patch-clamp analysis in which sudden, very large increments in whole-cell capacitance occur resulting from individual granules fusing with the cell membrane.
67 Ultrastructural studies of guinea pig eosinophils have also demonstrated evidence for compound exocytosis
68 similar to that observed in rat eosinophils adhering to the outer surface of opsonized parasitic larvae.
67,69 Additional evidence for compound exocytosis was suggested in eosinophils stimulated with a cocktail of IL-3, IL-5, and GM-CSF, which were observed to fuse their granules following activation as determined by immunofluorescence for CD63, a marker for crystalloid granules.
70The third manner in which eosinophils degranulate is by piecemeal degranulation (PMD). PMD was first characterized by Dvorak and colleagues for the appearance of numerous small vesicles in the cytoplasm coupled with the apparent loss of crystalloid granule core and matrix components, creating a “mottled” appearance in the crystalloid granules by electron microscopy analysis.
71 This was thought to be due to small vesicles budding off from the larger secondary granules and moving to the plasma membrane for fusion, thereby causing gradual emptying of the crystalloid granules to the outside of the cell. PMD was the most commonly observed pattern of degranulation seen in situ in biopsy samples from the upper airways of allergic individuals,
72 and is likely to be physiologically the most important mechanism for eosinophil mediator release in allergic disease. An in vitro model for PMD has been established using IFN
γ-stimulated eosinophils, in which a piecemeal manner of CCL5/RANTES release was observed.
44,62 More recent studies have demonstrated that PMD occurs through the formation of tubular structures budding off from crystalloid granules.
73,74 Vesicles associated with PMD are also necessary for the transportation of MBP
75 and eosinophil-derived IL-4.
73,76Airway tissue eosinophils in allergic subjects also appear necrotic, which is a fourth pattern of granule release, also termed “cytolysis”.
77 This type of release has been previously observed to occur following in vitro stimulation of human eosinophils with the calcium ionophore A23187,
78 and appears to be a physiologically relevant event in granule release.
Eosinophil degranulation may be induced by immobilized or soluble stimuli. Degranulation responses to immobilized stimuli have been extensively characterized in eosinophils in view of their role in helminth infections. When incubated with opsonized helminths, eosinophils degranulate onto the surface of the parasite.
69,79 A similar phenomenon occurs when eosinophils are incubated with Sepharose beads coated with antibodies, specifically IgG, IgA, and secretory IgA (sIgA), with sIgA being the most potent inducer of degranulation.
80 Cross-linking of immunoglobulin receptors on eosinophils has been shown to be highly effective at inducing respiratory burst and EDN degranulation in eosinophils, with a hierarchy of effectiveness in degranulation demonstrated to be on the order of secretory IgA (sIgA) = IgA > IgG >> IgE.
80 Eosinophil cytokines such as IL-3, IL-5, and GM-CSF were demonstrated to enhance this process.
81Eosinophils express a range of receptors for immunoglobulins that may contribute to chemotactic and activation responses in
tissues. These include receptors for IgA, IgD, IgE, IgG, and IgM, which may possess up to three chains (
α,
β, and
γ). Human eosinophils express Fc
αR, which is enhanced in allergic individuals,
82 as well as Fc
γRII (CD32), but not Fc
γRI (CD64) or Fc
γRIII (CD16). Mucosal tissues are enriched in sIgA, potentially as a mechanism against invasive pathogens. IgA, particularly the secretory isoform, is an important mucosal antibody involved in supporting the body’s first line of defense. Thus, the sensitivity of the eosinophil to IgA is in agreement with its proposed role in protection against invasive organisms in mucosal tissues. Taken together, these findings along with eosinophil localization in mucosal tissues suggest an important role for sIgA and IgG in mediating the effector functions of eosinophils in vivo.
Some controversy has surrounded the existence of the high-affinity receptor for IgE (FcεRI) on eosinophils. Studies have shown that the
α subunit of FcεRI in eosinophils is expressed intracellularly rather than on the cell surface in resting cells, which may be mobilized to the surface and released during activation.
83,84 Interestingly, although mouse FcεRI contains
α,
β, and
γ subunits, the human homologue lacks the
β subunit, suggesting that this subunit is redundant in signaling in cells expressing FcεRI. Eosinophils express an IgE-binding protein, galectin-3 (Mac-2/ε binding protein), as well as the low-affinity FcεRII (CD23), which may have contributed to apparent high-affinity binding for IgE in earlier studies.
Degranulation from eosinophils may also be induced by a range of soluble stimuli. Cytokines and chemokines induce eosinophil degranulation, with IL-5 and GM-CSF having a more potent effect than CCL3/MIP-1
α or CCL5/RANTES.
85,86,87 Eosinophil granule proteins (MBP and EPO) themselves can cause degranulation, suggesting that eosinophil granule proteins may promote degranulation in an autocrine manner.
21 Many other soluble stimuli can also induce degranulation, including complement fragments C3a and C5a, f-Met-Leu-Phe, PAF, and naturally occurring peptides such as substance P and melittin.
88,89 In addition, artificial stimuli potently evoke degranulation such as calcium ionophore (A23187) and phorbol myristate acetate (PMA).
78,90 In particular, PAF is a potent secretagogue for eosinophils, inducing the release of granule proteins, reactive oxygen species, and lipid mediators.
89,91,92 Furthermore, PAF activates at least two distinct effector pathways in eosinophils, one of which is independent of known PAF receptors.
91,93 Eosinophils also respond by degranulation to endogenous molecules released by stressed or damaged tissues. These include uric acid, ATP, high mobility group box (HMGB)-1 protein, and the S100 family of calcium-binding proteins.
94,95 and 96 Recently, eosinophils have been shown to express a newly discovered seven-transmembrane receptor, the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTh2),
97 which responds to prostaglandin D
2, a major prostanoid released by mast cells. PGD
2 induces morphologic changes, chemokinesis, and degranulation in eosinophils through CRTh2.
A role for eosinophils and their degranulation in the maintenance of innate immunity has been shown by their expression of Toll-like receptors (TLRs), which serve a role in recognition of conserved motifs in pathogens.
98 Eosinophils express the lipopolysaccharide (LPS)-binding receptor, TLR4, together with CD14,
99,100 and respond to LPS stimulation. Furthermore, eosinophils constitutively express TLR1, TLR7, TLR9, and TLR10 mRNAs, and are activated by the TLR7 ligand.
101 This may represent an important mechanism for eosinophil-mediated host defense against viral and bacterial infections.
Proteolytic enzymes also have the capacity to induce eosinophil degranulation. These are produced by microbes and are also present in various allergens, including house dust mites, fungi, and cockroaches. These enzymes interact with a family of G protein-coupled protease-activated receptors (PARs), and eosinophils constitutively transcribe mRNA for PAR2 and PAR3, but not PAR1 or PAR4.
102 Exposure of eosinophils to trypsin, a potent serine protease that serves as an agonist for PAR2, induces respiratory burst and degranulation in eosinophils. Eosinophils also degranulate in response to cysteine proteases such as those from cockroaches and
Der f 1 from mite allergens.
103,104 Finally, eosinophils can degranulate in response to aspartate proteases produced by the fungus
Alternaria alternata.105 These findings suggest that eosinophils are able to recognize and respond to proteases in the environment, and that this results in the release of proinflammatory mediators.
Eosinophils also undergo degranulation upon stimulation of a
β2 integrin, Mac-1 (CD11b/CD18,
αMβ2). This molecule has multiple roles in eosinophils; it is not only important in eosinophil adhesion and recruitment but also for activation of eosinophil effector functions. Integrins and especially Mac-1, play a crucial role in eosinophil activation by immobilized stimuli such as IgG.
106 Degranulation responses induced by soluble stimuli are potently enhanced by adhesion of eosinophils through integrins. Moreover, Mac-1 can directly recognize fungal molecules such as
β-glucan, and eosinophils react by degranulation to fungi through this mechanism.
107 Thus, integrins play a major role in recognition of external pathogens and in eosinophil effector functions.
The mechanisms associated with classical exocytosis, compound exocytosis, and PMD, but not cytolysis, are thought to require specific intracellular membrane-associated proteins acting as receptors for granule docking and fusion. These proteins include a family of molecules known as SNAREs (an acronym for
SNAP
receptors). The paradigm associated with the SNARE molecule function predicts that these proteins are essential for exocytosis. SNAREs were originally described in neuronal tissues and were found to group themselves into two distinct locations, the granule-associated SNAREs (the so-called vesicular SNAREs or v-SNAREs) and the plasma membrane-associated SNAREs (target SNAREs or t-SNAREs).
108 In order for a functional SNARE complex to form, allowing the granule to dock with the plasma membrane, one v-SNARE binds to two t-SNARE molecules. In neuronal cells, a commonly observed v-SNARE is vesicle-associated membrane protein (VAMP)-1 or its isoform VAMP-2. In these cells, the t-SNAREs associating with VAMP-2 that were originally described were the synaptosome-associated protein of 25 kDa (SNAP-25) and syntaxin-1A. These three molecules form a stable detergent-resistant four-helix coiled-coil bundle, which may be regulated by protein phosphorylation.
Nonneuronal cells also express SNAREs, although some isoforms have been identified with high sequence homology to the neuronal SNAREs. At least three SNAP-25 and 16 syntaxin isoforms have been characterized based on detection of homologous SNARE motif messenger RNA sequences. Interestingly, most nonneuronal secretory cells appear to require SNAP-23 and syntaxin-3, syntaxin-4, or syntaxin-6
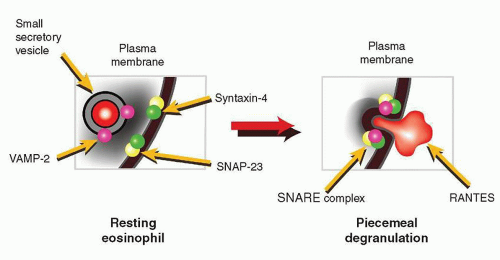
109,110 and 111 for control of exocytosis. Eosinophils have been shown to express the v-SNARE, VAMP-2, in their small secretory vesicles containing CCL5/RANTES, but not their crystalloid granules.
112 Crystalloid granules express mainly VAMP-7 and VAMP-8, the former being important in regulation of crystalloid granule secretion during degranulation responses from permeabilized eosinophils.
113 The t-SNARE isoforms syntaxin-4 and SNAP-23 are expressed in the cell membrane of eosinophils, and these have the potential to act as cognate membrane binding partners for VAMP-2 and VAMP-7 degranulation.
114 The SNARE molecules VAMP-2, SNAP-23, and syntaxin-4 identified in eosinophils are proposed to regulate docking and fusion of CCL5/RANTES-containing small secretory vesicles during piecemeal degranulation (
Fig. 8.7).
A fascinating study has identified a hitherto unrecognized functional capacity for eosinophil granules that have been cytolytically released extracellularly (cell-free), a phenomenon known to occur in vivo.
115 Isolated eosinophil granules were shown to respond to specific cytokine and chemokine stimuli via cognate receptors expressed on granule membranes, in vitro.
116 These cytokine and chemokine receptors were found expressed on the granule membrane, in the same orientation as those on plasma membranes. Stimulating cell-free granule receptors resulted in activation of intragranular signaling pathways, which in turn, elicited secretion of cytokines and cationic proteins from within granules.
116 This study has identified a novel postcytolytic capacity of intracellular organelles to function autonomously outside the eosinophil as ligand-responsive secretion competent structures for regulated secretion of eosinophil granule proteins. Such observations have implications for the notion that tissue-discharged, intact eosinophil granules may continue to contribute to eosinophil-mediated inflammation and immunomodulation.
Mechanisms associated with granule release in eosinophils are critical for effector function of eosinophils. In the absence of degranulation and mediator secretion, the eosinophil is a relatively inert cell, and does not affect surrounding tissues, as seen in cases of idiopathic pulmonary eosinophilia and eosinophilic pneumonia. In these conditions, eosinophil numbers are increased in the capillaries and tissues of the lung, but no cellular or structural damage is evident, likely because of the lack of eosinophil degranulation. In contrast, asthmatic patients show profound eosinophil activation in the airways combined with significant tissue destruction, suggesting that, in addition to eosinophilic infiltration, their undergoing degranulation may contribute to mucosal damage in the airways and related symptoms of asthma. Thus, degranulation is a key event in eosinophil-mediated tissue damage.
Membrane-derived Mediators
Eosinophils produce a wide variety of lipid-derived mediators, which have profound biologic activity. The more important products are eicosanoids, which include leukotrienes (especially LTC
4), prostaglandins (particularly PGE
2), thromboxane, and lipoxins (especially LXA
4) as well as PAF. The main substrate for these mediators is arachidonic acid (AA), which is specifically liberated from membrane phospholipids possessing this fatty acid at the
sn-2 position by phospholipase A
2 (PLA
2) during receptor stimulation. Of the nine known families of PLA
2, two families are expressed in eosinophils, the type IIA and type IV enzymes, which are commonly known as secretory and cytosolic PLA
2, respectively.
117,118 These enzymes are distinguished by their distribution, size, and sensitivity to Ca
2+, where granule-stored sPLA
2 (13 to 15 kDa) requires millimolar amounts of Ca
2+ for activity and cytosolically localized cPLA
2 (85 kDa) is catalytically active in the presence of micromolar amounts of Ca
2+. Interestingly, eosinophils express twenty- to a hundredfold higher levels of secretory PLA
2 in their granules than other circulating leukocytes, suggesting a functional role in inflammatory processes involving eosinophil degranulation.
Eosinophils are a rich source of LTC
4 (5S-hydroxy-6R, S-glutathionyl-7,9,-
trans-11,14-
cis-eicosatetraenoic acid).
119,120 Stimulation with the calcium ionophore A23187 generates up to 40 ng/10
6 cells of LTC
4 from normal density eosinophils, whereas light density eosinophils elaborate 70 ng/10
6 cells. Eosinophils produce negligible amounts (6 ng/10
6 cells) of LTB
4 (5S-12R-dihydroxy-6,14-
cis-8,10-
trans-eicosatetraenoic acid) compared with up to 200 ng/10
6 cells from neutrophils. LTC
4 generation by human eosinophils was also observed after stimulation with both opsonized zymosan and via an Fc
γRII-dependent mechanism using Sepharose beads coated with IgG.
121 Release was maximal at 45 minutes, greater in hypodense eosinophils than normal density eosinophils and was enhanced by fMLF (
see Eosinophil Heterogeneity below for a description of hypodense eosinophils). The production of LTC
4 is critically dependent upon the activation of 5-lipoxygenase, an enzyme that resides in the euchromatin region of the nucleus which translocates to the nuclear membrane upon cell activation, where it activates an 18 kDa protein called FLAP.
122 The substrate for 5-lipoxygenase is AA, which may be released from membrane phospholipids by PLA
2. The first product of this enzyme is an intermediary compound, 5-HPETE, which is transformed into the unstable epoxide LTA
4. At this point, human eosinophils predominantly generate LTC
4 through the action of LTC
4 synthetase.
119,120 Eosinophils are particularly rich in LTC
4 synthetase, and account for 70% of all LTC
4 synthetase-positive cells in the airway mucosa of normal and asthmatic individuals.
123 LTC
4 is generated intracellularly in human eosinophils stimulated with the calcium ionophore A23187. LTC
4 is later exported from the cell in a regulated manner.
124The production of 15-HETE, a lipid mediator generated via the 15-lipoxygenase pathway, occurs in activated eosinophils. The product 15-HETE has proinflammatory actions and can modulate the chemotactic effects of LTB
4 on neutrophils.
125 The enzyme 15-lipoxygenase may be distinguished from 5-lipoxygenase in that it can modify a larger pool of fatty acid substrates than the latter enzyme, and will oxygenate fatty acids that are esterified in phospholipids. Substrates include arachidonic acid, linoleic acid, polyenoic acids, and more complex lipids, such as lipoproteins. Eosinophils are the major cellular source of elevated 15-HETE in asthmatic airways, and are capable of generating 100 to 300 times more 15-HETE than neutrophils, endothelial cells, and fibroblasts.
126 Eosinophils also account for 85% of cells positive for 15-lipoxygenase in the airway submucosa of normal and asthmatic subjects.
127,128Eosinophils generate large amounts of PAF after stimulation with either calcium ionophore, opsonized zymosan, or IgG-coated Sepharose beads.
129,130,131 and 132 PAF (1-
O-alkyl-2-acetyl-
snglycerol-3-phosphocholine) is a potent phospholipid mediator, which causes leukocyte activation. For instance, eosinophils elaborated 25 ng/10
6 cells of PAF after stimulation with calcium ionophore and up to 2 ng/10
6 cells after IgG stimulation. Much of the PAF remained cell-associated, possibly acting as an intracellular messenger, or alternatively binding to PAF receptors on eosinophils thus acting as an autocrine agent. Interestingly, stimulation of eosinophils with fMLF did not augment PAF release and hypodense eosinophils from patients with a marked eosinophilia released less PAF than normal eosinophils. [
3H]PAF added to hypodense eosinophils was more rapidly incorporated into the phospholipid pool than [
3H]PAF with normal density cells.
131 This suggested that hypodense eosinophils were metabolizing the exogenous PAF at a greater rate than normodense cells and may explain why stimulation with fMLF did not result in an increased amount of PAF generation. As with leukotriene synthesis, eosinophil-derived release of PAF was maximal at 45 minutes. Regulated PAF production is controlled by the release of biologically inactive lyso-PAF from membrane phospholipids by PLA
2, which is later acetylated to form PAF by an acetyltransferase.
130The cyclooxygenase pathway is prominent in eosinophils as well, and eosinophils are capable of producing PGE
1 and PGE
2, and thromboxane B
2 from cyclooxygenase acting on free AA. In studies with guinea pig eosinophils, thromboxane B
2 and PGE
2 were shown to be generated following PAF or A23187 stimulation.
133,134Many of the enzymes associated with membrane-derived mediator release from eosinophils, including cyclooxygenase and 5-lipoxygenase, are found stored in association with lipid bodies (see
Table 8.1).
135,136,137
Respiratory Burst
Eosinophils undergo respiratory burst concurrently with the release of other mediators during cell activation. The immune function of respiratory burst is to mediate killing of invasive pathogenic microorganisms; it also has the undesired effect of collateral tissue damage when dysregulated. Respiratory burst is defined as the increase in cell metabolism (measured by the elevated activity of the hexose monophosphate shunt) and oxygen consumption, coupled with the inducible release of reactive oxygen species (ROS) in response to specific stimuli. Many stimuli are capable of inducing respiratory burst in eosinophils, including LTB
4, PAF, fMLF, C5a, opsonized particles, and CCL5/RANTES.
39 The principal product of respiratory burst is superoxide (O
2−•), a potent oxidant with a highly reactive electron in its outer valence, possessing relatively weak intrinsic microbicidal activity and a very brief half-life. The function of O
2−• is thought to reside in its ability to dismutate rapidly into more reactive ROS, including hydrogen peroxide (H
2O
2), the hydroxyl radical (OH
−•), and formation of hypohalous acids (HOBr) upon reaction with EPO produced following eosinophil degranulation. The formation of ROS subsequent to O
2−• generation is dependent upon the presence of a number of catalysts, such as superoxide dismutase (SOD), which accelerates the formation of H
2O
2, and the ferrous ion, which induces OH
−• production from H
2O
2. O
2−•, is also able to react with nitric oxide (NO) produced from nitric oxide synthase enzymes (e.g., iNOS, eNOS) to form the highly reactive peroxynitrite (ONOO-), which alters cell functions and is cytotoxic.
The regulated burst of O
2−• production is mediated through the activation of a membrane-associated enzyme complex, the NADPH oxidase. This enzyme complex is crucial for maintenance of host defense as it is a key mechanism in the destruction of ROSsensitive organisms. In addition, overactivation of the NADPH oxidase is likely to be cytotoxic to tissues, and has been implicated in the pathogenesis of many eosinophil-related disorders including allergic asthma.
138 Interestingly, eosinophils possess the ability to generate up to tenfold more superoxide than other phagocytes, including neutrophils, in which the mechanisms associated with NADPH oxidase activation have been studied in greater detail.
139 The ability of eosinophils to release more O
2−• is thought to be the result of higher levels of expression of the protein components that make up the NADPH oxidase complex.
140,141 and 142,143,144 In addition, preferential assembly of NADPH oxidase occurs at the cell membrane in eosinophils, eliciting a predominantly extracellular form of O
2−• release.
145 This is in contrast to neutrophils, which show predominantly intracellular NADPH oxidase assembly during respiratory burst stimulation and bacterial infection.
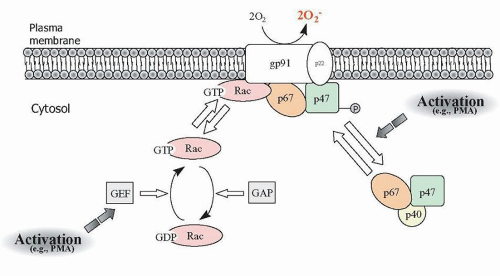
145,146NADPH oxidase is a complex of at least 6 proteins consisting of phagocytic oxidase (
phox) subunits, of which two (p22
phox and gp91
phox) reside in the membrane as part of the cytochrome
β558 protein, and the remaining proteins (p40
phox, p47
phox, p67
phox, and Rac1 or Rac2) are cytosolic in resting cells.
139 The minimal components of the NADPH oxidase complex were determined using cell-free assays,
147 although several other proteins, such as p40
phox and Rap1a, also translocate to the membrane-associated oxidase complex during activation, and may be involved in “finetuning” the activity of the oxidase.
148 Under normal nonstimulated conditions, the phagocytic oxidases p40
phox, p47
phox, and p67
phox are complexed in the cytosol, whereas Rac1 or Rac2 are bound to the cytosolic guanine dissociation inhibitor RhoGDI (
Fig. 8.8). Binding between p47
phox and p67
phox occurs through the C-terminal SH3 domain of p67
phox and a proline-rich region (PRR) in p47
phox. The p67
phox protein also contains a C-terminal Bem1p (PB1) motif that allows a high-affinity interaction with a C-terminal
phox and Cdc motif in p40
phox.
149,150 An SH3 domain also exists in p40
phox that is capable of interacting with the PRR domain in p47
phox,
although in vitro binding studies indicate that the affinity of this interaction is lower than that of p40
phox for p67
phox.
151,152During activation by cell surface receptors engaged by opsonized microbes or inflammatory mediators, p47
phox is phosphorylated by cellular kinases on multiple serine residues, unmasking tandem SH3 domains to allow binding to p22
phox in the membrane. Concurrently, p67
phox and p40
phox, which are still complexed to p47
phox, translocate to the cell membrane, and the “activation domain” in p67
phox promotes electron transfer between NADPH and flavin adenine dinucleotide.
153 Superoxide generation from NADPH oxidase also requires concurrent activation and membrane translocation of Rac1 or Rac2, which binds to tetratricopeptide repeat motifs in the N-terminus of p67
phox and cytochrome b
558 to induce additional conformational changes necessary for efficient electron transport to O
2.
154 The function of p40
phox is still uncertain, although a recent study suggests that it may have a role in activating superoxide production during IgG-mediated phagocytosis.
150Rac1 and Rac2 are monomeric guanine triphosphatases (GTPases), which exhibit 92% homology in their amino acid sequence, and are functionally interchangeable in their ability to activate NADPH oxidase, although they differ in their tissue distribution. Another potential modulator of the oxidase is the monomeric GTPase Rap1a, although its precise role is unknown. Rac1 is ubiquitously expressed throughout the body, whereas neutrophils, eosinophils, and other blood cells predominantly express Rac2, which is mainly expressed in hemopoietic tissues.
145,155,156 and 157 Activation of the respiratory burst in eosinophils leads to translocation of Rac to NADPH complexes predominantly at the cell membrane, in contrast to neutrophils which form NADPH complexes mainly at intracellular sites.
145 These patterns of NADPH oxidase complex formation align with the concept that eosinophils undergo “frustrated phagocytosis” upon encountering large extracellular organisms such as helminthic parasites, whereas neutrophils preferentially phagocytose smaller microbes for intracellular killing. Eosinophils are partially dependent on Rac2 for the activation of respiratory burst responses, based on findings with Rac2
−/− mouse eosinophils, inferring that Rac1 may also be required for NADPH oxidase activation in these cells.
158The pathway leading from receptor stimulation to activation of the oxidase is still poorly understood. Many studies on NADPH oxidase activation use phorbol esters, such as phorbol myristate acetate (PMA), as highly potent artificial stimuli to activate respiratory burst in eosinophils. Phorbol esters are classically known for their ability to activate protein kinase C (PKC) directly.
159,160 and 161 The use of pharmacologic inhibitors of PKC in respiratory burst has generated paradoxical results, in that PKC inhibitors only partially inhibit agonist-induced H
2O
2 release in guinea pig eosinophils.
160,162 However, there is a species difference in sensitivity to PMA, as in human eosinophils, where PKC inhibitors actually augment the rate of oxygen consumption in response to opsonized particles.
163 These findings suggest that PKC is not critical for agonist-induced respiratory burst in eosinophils, although stimulation of PKC appears to be able to induce superoxide release on its own.
Taken together, eosinophils generate substantial amounts of O2−• as part of their role in host defense, and the mechanisms associated with the release of this toxic mediator are under investigation. The release of O2−• from eosinophils is likely to be a crucial component of the pathophysiologic processes underlying eosinophilic inflammation in mucosal tissues.