Fig. 8.1
Catecholamine-induced biofilm formation in Staphylococcus epidermidis. The image panels show scanning electron micrographs of biofilms of S. epidermidis adhering to polystyrene after overnight growth in freshly prepared plasma-SAPI medium in the absence (a) or presence (b–f) of 0.1 mM norepinephrine as described in Lyte et al. (2003). Initial inocula for both S. epidermidis cultures were approximately 102 CFU per ml. Higher magnification scanning electron micrographs showing details of the bacteria-exopolysaccharide clusters are shown in panels (b)–(f). This figure was taken with permission from Freestone and Lyte (2008)
The findings demonstrated in Fig. 8.1 are clinically significant, as bacterial colonization of intravenous catheters, predominantly by the C-NS, is recognized as the most common infection encountered in the intensive care setting. There are also a number of methodological issues in the data shown in Fig. 8.1 that are worth noting. In demonstrating catecholamine inotrope induction of S. epidermidis biofilm, we attempted to make the analytical conditions close to those likely to be occurring in the clinical scenario. We therefore used two methodological aspects that were markedly different from the previous studies of bacterial biofilm formation. Only 10–100 S. epidermidis cells were used to seed the polystyrene plastic section on which the biofilm was to be established, a low inoculum chosen to reflect the number of bacteria likely to be encountered at the beginning of the catheter colonisation. This is in contrast to many prior biofilm studies which have used several log orders higher levels of bacteria to establish biofilms. The second important difference was the use of culture conditions that employed a plasma supplemented minimal media. This again differs from studies which have used rich microbiological media to study biofilm formation, and in so doing, it may be argued, do not realistically reflect in vivo conditions in which host factors present in plasma, which are recognized to play a role in initial bacterial adhesion, are not present.
8.5 Catecholamine Inotropes Can Resuscitate Antibiotic Damaged Staphylococci
Intravenous catheter-related bloodstream infections are invariably associated with increases in length of time in intensive care units and of course hospital costs (Crnich and Maki 2001). In an effort to combat bacterial colonization of catheter lines and any possible subsequent progression to catheter-related bloodstream infections, the use of antimicrobial impregnated catheters, particularly those incorporating antibiotics such as rifampin and minocycline, is becoming increasingly adopted in the clinical care setting, However, there is a measure of doubt concerning the real efficacy of antibiotic-impregnated plastics in the prevention of catheter-related blood stream infections (Crnich and Maki 2004; McConnell et al. 2003). Concern exists as to whether they may also contribute to the emergence of antibiotic-resistant nosocomial pathogens (Sampath et al. 2001). A recent study from our laboratory might explain why antibiotics impregnation of the polymers used to construct intravenous catheter lines has not proven to be as effective as anticipated.
In this investigation (Freestone et al. 2008), we undertook two methodological approaches using norepinephrine and dopamine to investigate whether these inotropes were capable of facilitating the recovery and growth of antibiotic-damaged staphylococci (S. epidermidis, S. haemolyticus and two S. aureus strains, Newman and 832-4). We employed the antibiotics rifampin and minocycline as our test anti-microbials, as they are the two most frequently employed antibiotics used to coat catheters, and carried out the analyses in a serum-based culture medium (serum-SAPI) that more closely approaches the environment the bacteria would have experienced in vivo. The first experimental approach used a minimum inhibitory concentration (MIC) assay, in which the staphylococci were incubated with norepinephrine and dopamine in the presence of increasing concentrations of rifampin or minocycline (up to 100 times the MIC for each antibiotic). Representative data for rifampin is shown in Fig. 8.2 for S. epidermidis (a) and S. aureus strain Newman (b). The responses for S. haemolyticus and S. aureus strain 832-4 were very similar to those shown for S. epidermidis and S. aureus Newman and so are not shown. As can seen, for both strains simultaneous administration of inotrope and rifampin allowed more than 2 logs greater growth in the presence of rifampin concentrations around the MIC (determined to be ~0.1 μg per ml), although the catecholamines did not significantly protect the staphylococci from the inhibitory effects of higher rifampin concentrations. A similar response profile of greater growth in the presence of the inotropes was also obtained when the staphylococci were exposed to minocycline in the presence/absence of catecholamine inotropes (data not shown).
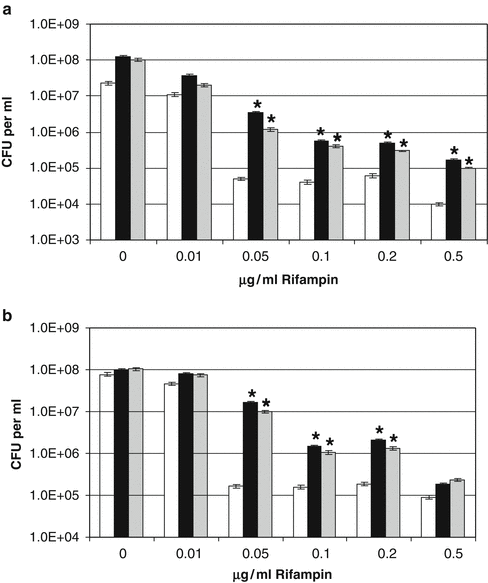

Fig. 8.2
Staphylococcal response to increasing rifampin concentrations in the presence of catecholamine inotropes. Analyses of simultaneous exposure of S. epidermidis and S. aureus to antibiotics and catecholamine inotropes were performed in serum-SAPI medium. Bacteria were incubated for 24 h 37°C in a 5% CO2 humidified incubator and measured for growth and measured for growth using plate analysis. The results shown represent the mean of triplicate cultures; standard error of the mean was not greater than 7% for all cultures shown. The asterisk, *, indicates a statistically significant increase in growth level over the corresponding non-inotrope supplemented control culture (P < 0.0001). (a) Growth response of S. epidermidis (inoculum size 1 × 106 CFU per ml); (b) Growth response of S. aureus Newman (inoculum size 0.92 × 106 CFU per ml). White bar, no additions (control); black bar, 100 μM norepinephrine; gray bar, 100 μM dopamine. This figure was taken with permission from Freestone et al. (2008)
Our second experimental approach involved analyzing whether catecholamine inotropes could rescue the growth of staphylococci pretreated with antibiotics, as it is well known that following antibiotic treatment, some acute bacterial infections can become subclinical, re-activating in response to not always understood changes in the host environment. Figure 8.3 shows the ability of norepinephrine and dopamine to rescue growth of S. epidermidis pre-treated for 4 h with a rifampin concentration of 5 μg per ml (approximately 100 times the MIC) and then serially diluted into serum-SAPI medium containing no additions or the catecholamine inotropes and grown on for 24 h. Figure 8.3a, b show the growth profiles of control and antibiotic treated S. epidermidis, and reveal that the rifampin had reduced the viable count of the treated bacteria by nearly 4 log orders. However, the presence of the inotropes significantly increased the growth of S. epidermidis preexposed to rifampin, even when the antibiotic carryover was near to the MIC (the 10−2 dilution) (P < 0.0001).
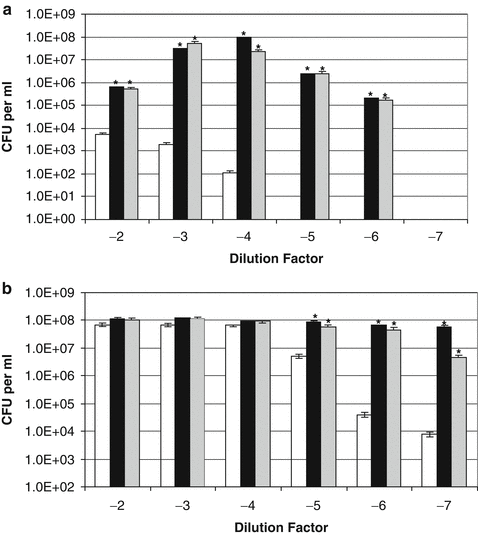

Fig. 8.3
Effect of catecholamine inotropes on coagulase-negative staphylococci pre-exposed to rifampin. Replicates of exponentially growing S. epidermidis cultures grown in serum-SAPI medium were incubated for 4 h at 37°C with 5 μg per ml rifampin (100 times MIC). The antibiotic-treated (inoculum size 5.40 × 104 CFU per ml) (a) and control cultures (inoculum size 1.05 × 108 CFU per ml) (b) were each then serially diluted in tenfold dilution steps into fresh serum-SAPI with no additions (white bar) or supplemented with norepinephrine (black bar) or dopamine (grey bar) each at a concentration of 100 μM. Test and control were incubated at 37°C for 24 h and enumerated for viable cell levels using pour-plate analysis. The asterisk, *, indicates a statistically significant increase in growth level over the corresponding non-catecholamine inotrope supplemented control culture (P < 0.0001). This figure was taken with permission from Freestone et al. (2008)
Earlier work from our laboratories had shown that S. aureus and other coagulase-positive staphylococcal strains showed little growth responsiveness to catecholamines in serum-based medium (Freestone et al. 1999; Neal et al. 2001). This observation was confirmed by the responses of nonantibiotic-treated S. aureus strain Newman to norepinephrine and dopamine (Fig. 8.4b), which showed that growth enhancement by the inotropes was evident at only very low cell densities (~10 CFU per ml). However, antibiotic-treatment of the S. aureus cultures (Fig. 8.4a) caused the bacteria to become significantly more responsive to the inotropes (P < 0.0001). This being so, the growth profile observed over the S. aureus culture dilutions is different to that obtained for S. epidermidis (compare b of Figs. 8.3 and 8.4). This is most likely because unlike the C-NS, the coagulase positive staphylococci possess highly efficient acquisition systems for the scavenging of host sequestered iron stores, which then enables them to overcome the iron-limitation of tissue fluids such as serum and blood without aid of the catecholamines. Indeed, when we analyzed the ability of S. epidermidis and S. aureus to remove iron from 55Fe-labeled transferrin, we found that the coagulase positive staphylococci were able to extract around 10 times more iron than the C-NS. Adding the inotrope enabled the coagulase positive staphylococci to obtain around 2.5-3 times more iron from the transferrin while for the C-NS, the inotrope allowed much more Fe uptake from transferrin – around 30 times more than the noninotrope supplemented controls, such that when the inotrope were present, the final levels of uptake of transferrin-complexed iron was similar between the staphylococci. Figure 8.5 shows the ability of inotropes to rescue growth of staphylococci pretreated with 2 μg per ml minocycline (~100 times MIC). Although both S. epidermidis and S. aureus recovered better from the minocycline assault, than they did from the rifampin, inclusion of norepinephrine and dopamine still induced a much greater rate of recovery.
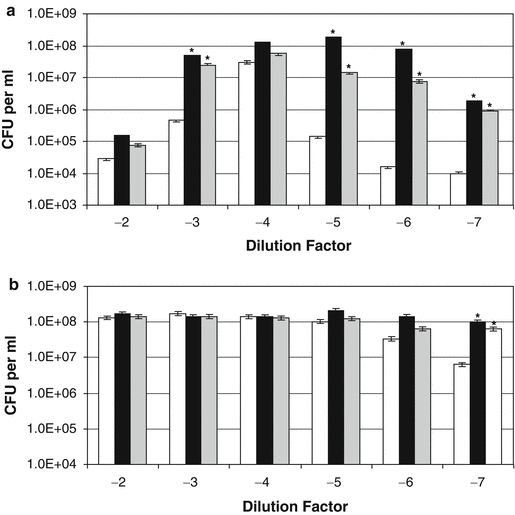
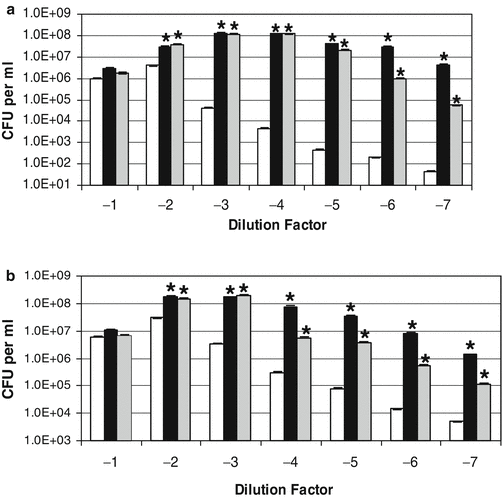

Fig. 8.4
Effect of catecholamine inotropes on coagulase-positive staphylococci pre-exposed to rifampin. Replicates of an exponentially growing S. aureus strain Newman cultures grown in serum-SAPI medium were incubated for 4 h at 37°C with 5 μg per ml rifampin (100 times MIC). The antibiotic-treated (inoculum size 2.21 × 105 CFU per ml) (a) and control cultures (inoculum size 2.22 × 108 CFU per ml) (b) were each then serially diluted in tenfold dilution steps into fresh serum-SAPI with no additions (white bar) or supplemented with norepinephrine (black bar) or dopamine (grey bar) each at a concentration of 100 μM. Test and control were incubated at 37°C for 24 h and enumerated for viable cell levels using pour-plate analysis. The asterisk, *, indicates a statistically significant increase in growth level over the corresponding non-catecholamine inotrope supplemented control culture (P < 0.0001). This figure was taken with permission from Freestone et al. (2008)

Fig. 8.5




Effect of catecholamine inotropes on staphylococci pre-exposed to minocycline. Replicates of an exponentially growing S. epidermidis and S. aureus strain Newman cultures grown in serum-SAPI medium were incubated for 4 h at 37°C with 2 μg per ml minocycline (100 times MIC). The antibiotic treated S. epidermidis culture (inoculum size 5.40 × 104 CFU per ml) (a) and S. aureus Newman culture (inoculum size 8.90 × 104 CFU per ml) (b) were each then serially diluted in tenfold dilution steps into fresh serum-SAPI with no additions (white bar) or supplemented with norepinephrine (black bar) or dopamine (grey bar) each at a concentration of 100 μM. The two sets of cultures were then incubated at 37°C for 24 h and enumerated for viable cell levels using pour-plate analysis. The asterisk, *, indicates a statistically significant increase in growth level over the corresponding non-catecholamine inotrope supplemented control culture (P < 0.0001). This figure was taken with permission from Freestone et al. (2008)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree