Neurotransmitter
In microorganisms
In plants
In animals
Acetylcholine
In 1947 Emmelin and Feldberg found this substance in stinging trichomes and leaves of common nettle by biological method, based on muscle contraction
In 1921–1926 the presence of acetylcholine has been established in animals by Loewi and Navratil. But earlier, in 1906 student Reid Hunt (worked in USA laboratory of John J. Abel) discovered it in adrenal extracts of animals
Dopamine
In 1944, found in Hermidium alipes by Buelow and Gisvold
Discovered in 1950–1952 by pharmacologists Arvid Carlsson, Nils-Åke Hillarp and von Euler in Sweden
Norepinephrine (noradrenaline)
Identified in microorganisms by Tsavkelova et al. (2000)
In 1956–1958 found in banana fruits in Sweden laboratories organized by Waalkes and Udenfriend
Isolated from adrenal gland extracts of animals in 1897–1898 by John J. Abel
Epinephrine (adrenaline)
In 1972 found in leaves of banana Musa by Askar et al. (1972)
Isolated from adrenal gland extracts of animals in 1895 by Polish physiologist Napoleon Cybulski and in 1897 by American John J. Abel
Serotonin
Found in 1986 by Hsu with co-workers in many bacteria
Found in banana fruits (Musa) by Bowden et al. (1954)
Discovered by Erspamer in 1940 and Rapport et al. in 1948
Histamine
Found in ergot fungi Claviceps purpurea in 1910 by Barger, Dale and Kutscher
Observed in higher plants by Werle and Raub in 1948
In 1919 American John Jacob Abel isolated histamine from pituitary extract of animals
As can be seen from Table 2.2, the concentration range of the neurotransmitter compounds is similar for all three kingdoms of living organisms, although some organs and specialized cells of multicellular organisms may be enriched in these compounds. Over 50 years ago, Soviet physiologist Koshtoyantz (1963) presented a hypothesis that the neurotransmitters are unique to all animal cells independent of their position on the evolutionary tree; this view has been confirmed experimentally, and has been described in several monographs (Buznikov 1967, 1987, 1990) and papers (Buznikov et al. 1996; Buznikov 2007). The presence of the neurotransmitters in animals has now been confirmed for all kingdoms—from Protozoa to Mammalia. As for bacterial cells, no more than 10–12 species have so far been characterized as containing acetylcholine, catecholamines, and serotonin, although histamine has been found in most species of prokaryotes. The issue of mammalian-type hormones in microorganisms has also been considered (Lenard 1992).
Table 2.2
Level of neurotransmitters in living organisms
Neurotransmitter | In microorganisms | In plants | In animals |
|---|---|---|---|
μg/g−1 of fresh mass or *μmoles/L or **μg/billion of cells | μg/g−1 of fresh mass | μg/g−1 of fresh mass or *nM/L or **nm/day | |
Acetylcholine | 3.0–6.6 | 0.1–547 | 0.326–65,200 (0.15–0.2 in brain,) |
Dopamine | 0.45–2.13* | 1–4000 | <0. 888* |
1214–2425** | |||
Norepinephrine (noradrenaline) | 0.21–1.87* | 0.1–6760 | 0.615–3.23* |
20–240** | |||
Epinephrine (adrenaline) | No data | 0.22–3833 | 1.9–2.46* |
30–80** | |||
Serotonin | 0.11–50,000** | 0.0017–4000 | 0.21–0.96 |
Histamine | 0.01–3.75 | 1 (1,34—pain reaction for human) | 0.5–100 |
2.2.1.1 Acetylcholine
In animals, acetylcholine and/or the synthesizing enzyme choline acetyltransferase have been demonstrated in epithelial (airways, alimentary tract, urogenital tract, epidermis), mesothelial (pleura, pericardium), endothelial, muscle and immune cells, mainly in granulocytes, lymphocytes, macrophages, and mast cells (see review of Wessler et al. 2001). Classical investigations provided early evidence to the presence of neurotransmitters and related enzymes in both microbial and plant cells.
Acetylcholine has been well-recognized as a component of bacteria (its production was discovered in a strain of Lactobacillus plantarum) (Stephenson et al. 1947; Rowatt 1948; Girvin and Stevenson 1954; Marquardt and Falk 1957; Marquardt and Spitznagel 1959). The first report, showing acetylcholine production in bacterium strains, was from L. plantarum and. L. odontolyticus (Stephenson et al. 1947). Approximately 5 mg acetylcholine/mg dry wt. cells/h was formed if the bacteria were grown both in vegetable juice and washed cells. Cell free enzyme(s) participating in the acetylcholine synthesis were also first found in L. plantarum (Girvin and Stevenson 1954).
Acetylcholine has also been found in Protozoa (Janakidevi et al. 1966a, b). Corrado et al. (2001) showed the synthesis of the molecular acetylcholine during the developmental cycle of Paramecium primaurelia. This neurotransmitter has a negative modulating effect on cellular conjugation. But in these unicellular organisms, the presence of functionally related nicotinic and muscarinic receptors and a lytic enzyme acetylcholinesterase has been established. Moreover, the authors demonstrate (using immunocytochemical and histochemical methods) that the activity of enzyme choline acetyltransferase, which catalyzed acetylcholine synthesis, was located on the surface membrane of mating-competent cells and of mature, but not non-mating-competent P. primaurelia cells.
In the plant kingdom, acetylcholine is found in 65 species belonging to 33 different families (Roshchina 1991, 2001a; Wessler et al. 2001; Murch 2006). Acetylcholine was synthesized not only in a free form, but also in a conjugated form such as, for example, cholinic esters with plant auxins (Fluck et al. 2000). Acetylcholine is particularly abundant in secretory cells of common nettle stinging hairs, where its concentration reaches 10−1 M or 120–180 nmol/g of fresh mass. Together with the histamine contained in the secretion, acetylcholine may provoke a pain response and the formation of blisters when the plant comes into contact with human skin. Kawashima et al. (2007) have attempted to compare the concentration of the neurotransmitter acetylcholine in a wide variety of sources using the same experimental conditions, which involved a radioimmunoassay with high specificity and sensitivity (1 pg/tube). Kawashima et al. (2007) measured the acetylcholine content in samples from the bacteria, archaea, and eukaryote domains of the universal phylogenetic tree. The authors compared the concentrations in different groups of bacteria (Bacillus subtilis), archaea (Thermococcus kodakaraensis KOD1), fungi (shiitake mushroom and yeast), plants (bamboo shoot and fern), and animals (bloodworm and lugworm). The levels of acetylcholine varied considerably, however, the highest acetylcholine content was detected in the top portion of the bamboo shoot (2.9 mmol/g), which contained about 80 times of that found in rat brain. Various levels of acetylcholine-synthesizing activity were also detected in extracts from the cells tested, which contained a choline acetyltransferase-like enzyme (sensitive to bromoacetylcholine, a selective inhibitor of choline acetyltransferase). The enzyme activity was found in T. kodakaraensis KOD1 (15 %), bamboo shoot (91 %), shiitake mushroom (51 %), bloodworm (91 %), and lugworm (81 %). Taken together, these findings demonstrate the ubiquitous expression of acetylcholine and acetylcholine-synthesizing activities among life forms without nervous systems, and support the notion that acetylcholine has been expressed and may be active as a local mediator and modulator of physiological functions since the beginning of life.
2.2.1.2 Catecholamines
In unicellular organisms, biogenic amines are also synthesized. The large amounts of dopamine accumulated by cells of infusoria Tetrahymena pyriformis strain NT-1 and that which was secreted into their growth medium were found to depend primarily upon the extracellular, nonenzymatic conversion of tyrosine to l-dihydroxyphenylalanine (Gundersen and Thompson 1985). Recently, the catecholamines norepinephrine and dopamine have been identified in microorganisms by high-performance liquid chromatography by Tsavkelova et al. (2000). Dopamine in concentrations of 0.45–2.13 mmol/L was found in the biomass of bacteria Bacillus cereus, B. mycoides, B. subtilis, Proteus vulgaris, Serratia marcescens, S. aureus, and E. coli, but was absent in the fungi Saccharomyces cerevisiae, Penicillum chrysogenum, and Zoogloea ramigera. Norepinephrine was found (0.21–1.87 mmol/L) in the bacteria B. mycoides, B. subtilis, P. vulgaris, and S. marcescens as well as in fungi such as S. cerevisiae (0.21 mmol/L) and P. chrysogenum (21.1 mmol/L). It is especially interesting that in many cases, the content of catecholamines in microorganisms is higher than in animals. For example in human, blood norepinephrine is found to be about 0.04 mmol/L (Kruk and Pycock 1990). Moreover, it was demonstrated that bacteria, in particular B. subtilis, may release norepinephrine and dopamine out of the cell and, perhaps participate in intercellular communication both in microorganism–microorganism and bacteria–host contexts.
In plants, catecholamines have been found in 28 species of 18 plant families (Roshchina 1991, 2001a; Kuklin and Conger 1995; Kulma and Szopa 2007). The amount of dopamine found varies during plant development (Kamo and Mahlberg 1984), and sharply increases during stress (Swiedrych et al. 2004). Of particular note is the finding that increased amounts of dopamine (1–4 mg/g fresh mass) are found in flowers and fruits, in particular in Araceae species (Ponchet et al. 1982). Derivatives of dopamine are also known in plants, for example dopamine-betaxanthin in Portulaca oleracea (Gandía-Herrero et al. 2009). This demonstrates the important role of the catecholamines as neurotransmitters in fertilization as well as in fruit and seed development.
High concentrations of the amines reveal the stressed state of the animal organism, including a diseased state (Gruchow 1979). The same approach in the understanding of stress agents may also be applied to plants (Swiedrych et al. 2004). Concentrations of catecholamines differ among plant species, and in some cases increased under unfavorable conditions such as ozone treatment (Roshchina and Yashin 2014; Roshchina et al. 2015a).
2.2.1.3 Serotonin
Some microorganisms living within parasitic nematodes are also able to synthesize serotonin (Hsu et al. 1986). In the bacterial flora of the ascarid Ascaris suum, mainly facultative anaerobes (17 species) produced and excreted serotonin into the culture medium of up to 14.32–500.00 mg/g of fresh mass for Corynebacterium sp. (in the tissues of the helminth itself only 0.25 mg serotonin per g fresh mass). The concentration of serotonin, in terms of mg serotonin/109 cells for different cultures of microorganisms isolated from helminthes is as follows: Klebsiella pneumoniae 8.15, Aeromonas 26.71, Citrobacter 0.58, Corynebacterium sp. 14.32–500.00, Enterobacter agglomerans 2.93, Shigella 1.04, Achromobacter xylosoxidans 1.66, Chromobacterium 3.67, Achromobacter 0.15, Acinetobacter 11.79, Streptococcus 37.52, Listeria monocytogenes 4.71, and E. coli 3.33. Serotonin has also been found in the yeast Candida guilliermondii and bacterium Enterococcus faecalis (Fraikin et al. 1989; Belenikina et al. 1991; Strakhovskaya et al. 1991, 1993). In 1998, Oleskin et al. also established the presence of serotonin in the phototrophic bacterium Rhodospirillum rubrum (1 mg/billion of cells ~3–12,500 mg/g of fresh mass) as well as in non-phototrophic bacteria Streptococcus faecalis and E. coli (50 and 3.3 mg/billion of cells, respectfully). Serotonin was also found in E. coli and Bacillus cereus (Shishov et al. 2009). The inhibitor of tryptophan hydroxylase, N-chlorophenylalanine, affects the growth of the yeast Candida guilliermondii, but not the development of the bacterium E. coli. This suggests that in the latter case, there is an alternative pathway to that found in animals (Oleskin et al. 1998a, b), which is unique (Roshchina 1991, 2001a) to plants: tryptophan → tryptamine → serotonin.
In plants, serotonin is found in 42 species of 20 plant families (Roshchina 1991, 2001a; Ramakrishna et al. 2011). Besides free serotonin, conjugated serotonins such as N-feruloylserotonin, N-(p-coumaroyl) serotonin, and N-(p-coumaroyl) serotonin mono-β-d-glucopyranoside have been isolated from safflower Carthamus tinctorius L. seed. In various plants, serotonin conjugated to form phenolic compounds via thioester linkages during the synthesis of hydroxycinnamic acid amides, including p-coumaroylserotonin, feruloylserotonin, and p-coumaroyltyramine (Ly et al. 2008). It should be noted that serotonin in animals (such as rats) may exist in complexes with heparin that prevents the aggregation of thrombocytes (Kondashevskaya et al. 1996).
2.2.1.4 Histamine
Histamine was initially discovered in the ergot fungus Claviceps purpurea (Table 2.1) and, subsequently, in many bacterial and plant cells by Werle and coauthors (1948, 1949). Since then, it has also been observed in many types of foods as the result of microbial activity. Histamine is one of the biogenic amines formed mainly by microbial decarboxylation of amino acids in numerous foods, including fish, cheese, wine, and fermented products. A number of microorganisms can produce histamine. In particular, bacteria such as Morganella morganii, Proteus sp., and Klebsiella sp. are considered strong histamine formers in fish (Ekici and Coskun 2002; Ekici et al. 2006). Fernández et al. (2006) summarized the data on the toxicity of histamine in food. Histamine poisoning is the most common food borne problem caused by biogenic amines. At non-toxic doses, histamine can cause symptoms such as diarrhoea, hypotension, headache, pruritus, and flushes. Just 75 mg of histamine, a quantity commonly present in normal meals, can induce symptoms in the majority. One separate problem concerns the histamine formed by microorganisms in animal pathogenesis. Gram-negative bacterial species such as Branhamella catarrhalis, Haemophilus parainfluenzae, and Pseudomonas aeruginosa have been demonstrated to synthesize clinically relevant amounts of histamine in vitro that implicate the bacterial production of histamine in situ as an additional aggravating factor in acute chronic bronchitis, cystic fibrosis, and pneumonia. Histamine may also increase the virulence of these bacterial species, unlike some Gram-positive species such as Staphylococcus aureus and Streptococcus pneumoniae (Devalia et al. 1989). Among “non-pathogenic” species, only the Enterobacteriaceae were found to form histamine in significant concentrations.
Significant amounts of histamine have also been observed in higher plants, initially by Werle and Raub in 1948, and subsequently described for 49 plant species belonging to 28 families ranging from basidiomycetes (now taxonomically related to Fungi) to angiosperms (Roshchina 1991, 2001a). Besides histamine itself, its derivatives N-acetylhistamine, N, N-dimethylhistamine, and feruloylhistamine are also found in plants. Especially high levels are observed in species of the family Urticaceae that could be one of the taxonomic classification signs. The Brazilian stinging shrub Jatropha urens (family Euphorbiaceae) contains 1250 mg histamine per 1000 hairs. The presence of histamine in stinging hairs is a protective mechanism that provides order to frighten off predatory animals by inducing burns, pain, and allergic reactions. Under stress conditions, a sharp increase of histamine is observed in plants, as well as in animals. Ekici and Coskun (2002) have determined the histamine content of some commercial vegetable pickles at the range of 16.54 and 74.91 mg/kg (average 30.73 mg/kg). The maximum value (74.91 mg/kg) was obtained from a sample of hot pepper pickles. The amount of histamine varies according to the phase of plant development. For example in the marine red algae Furcellaria lumbricalis (Huds.) Lamour, the occurrence of histamine was from 60 to 500 mg/g fresh mass observed in both non-fertile fronds and sexual-expressed parts, in all regions of the thallus of male, female, and tetrasporophyte (Barwell 1979, 1989). The amount of histamine (in mg/g fresh mass) in the male plant was 90–490 (sometimes up to 1100), in the female plant 60–120, and in asexual tetrasporophyte 100–500. Especially enriched were the neurotransmitter cells of male plants, as the ramuli were approximately five times higher in histamine than female and asexual plants. Additionally, the concentration of histamine at high salt concentration can also be increased (Roshchina 1991; Roshchina and Yashin 2014).
2.2.1.5 Location and Transport of Neurotransmitters Within Cell
According to classical publications based on the data of electron microscopic histochemistry, within the animal cells, neurotransmitters are located mainly in secretory vesicles. In microbial systems localization of catecholamines on the bacterial cell surface was confirmed by Western blot and immunofluorescence microscopy using mussel-inspired catecholamine yielded sticky E. coli as a new type of an engineered microbe for the study of cell-to-cell communication systems (Park et al. 2014). Mechanisms of the intercellular and extracellular transport in non-nervous systems appear to be similar with those known for animal cells. Neurotransmitters are supposed to be released into the extracellular synaptic chink (cleft) with the aid of acidic glycoproteins (~70 kDa) called vesicular monoamine transporters (Wimalasena 2011; Lawal and Krantz 2013). The sequence of neurotransmitter release of out the cell is here briefly described: (1) A hydrogen atom from the inside of the vesicle binds, inducing a conformational change in the transporter. The conformational change induced by the hydrogen atom binding enables the monoamine binding to the active transport site. (2) A second hydrogen atom binds from inside the vesicle to the transporter inducing another change. (3) The monoamine is released inside the vesicle and the two hydrogen atoms are released into the cytosol and the transport process starts over again. Moreover, the transporters are believed to be similar in bacteria to those also found in both plant and human organisms.
Localization of acetylcholine in fungal or plant cells is usually determined based on the presence of cholinesterase, an enzyme that catalyzes the decomposition of the neurotransmitter. As observed in the monograph of Roshchina (2001a), in the above mentioned cells, the enzyme has been found in the cell wall, the exine of spores and plasmalemma as well as in the nucleus and chloroplasts. In maize, acetylcholinesterase is mostly localized to the vascular bundles including the endodermis and epidermis in coleoptile nodes as well as in the mesocotyls of maize seedlings (Yamamoto and Momonoki 2012).
The location and secretions of biogenic amines is difficult to pinpoint in living, individual cells without specialized methods of identification. This difficulty in identification has been solved in animal cells via the use of molecular probes and glyoxylic acid as a reagent for catecholamines (Markova et al. 1985) and ortho-phthalic aldehyde as a reagent for histamine (Cross et al. 1971). Today, such histochemical methods are preferred for the microanalysis of plant single cells, as these techniques do not damage the cells and prevent the premature release of proteins, amino acids and other amines by treating with trichloroacetic acid or hydrochloric acid with or without column chromatography (Fig. 2.1). Glyoxylic acid and ortho-phthalic aldehyde were used to analyze the plant microspores such as vegetative microspores of Equisetum arvense and pollen of 25 plant species using microspectrofluorimetry and laser-scanning confocal microscopy (Roshchina et al. 2012, 2014; Roshchina and Yashin 2014). The presence of catecholamines and histamine has been found not only in secretions, but in DNA-containing organelles—nuclei and chloroplasts. Earlier, Zhirnova et al. (2007) revealed the location of histamine in the nuclei of human skin cells. Exogenous biogenic amines were also demonstrated to interact with isolated nuclei and chloroplasts (Roshchina 1990b, 1991, 2001a; Roshchina et al. 2015b). To study the single intact cells as model systems, it is necessary to use small amounts of material in order to avoid damage to cell structures.
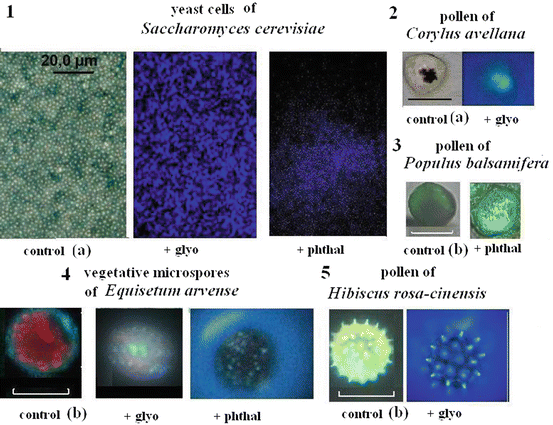

Fig. 2.1
The fluorescence images of yeast cells (1) and plant microspores (2–5) stained with glyoxylic acid (glyo) or o-phthalic aldehyde (phthal). Bars 1–4 = 20 μm, 5–100 μm. Controls 1 and 2 (a)—images in transmitted (transmission) light without treatment (weak fluorescence for photo), controls 3–5 (b)—autofluorescence without treatment. Excitation of emission by UV-light (360–380 nm) of luminescent microscope Leica DM 6000B. Within cells of plant vegetative or generative (pollen) microspores stained the fluorescing DNA-containing organelles such as nuclei (2, 3) or nucleus and chloroplasts (4) are seen while on the surface of 5—small spikes after treatment with o-phthalic aldehyde. The emitted excretions are also observed as holo form
2.2.2 Neurotransmitters as Toxicants: Ecologic, Medical, and Economic Aspects
With the discovery of the presence of neurotransmitter substances not only in animals, but also in plants and microorganisms, medical and economic interest has increased substantially within the twenty-first century. Interest lies in concern of interactions between organisms in biocenosis that includes various non-neuronal functions of neurotransmitters in animals but also concerns the regulation of growth and morphogenesis in microorganisms, animals, and plants. Interest also includes protection in the adaptation to environmental changes as well as other functions. This development contributes benefits to various aspects of medicine insofar that some serotonin and dopamine-containing fruits may protect against Alzheimer’s disease (see Sect. 2.4). An economic standpoint illustrates the economic concern of contamination of food products by stress metabolites produced by plants or animals. Bacterial or fungal microorganisms are known to influence the quality and freshness of food products. Such microorganisms may contaminate such foods through their production and secretion of catecholamines or histamine.
High concentrations of biogenic amines in foodstuffs and beverages can induce a range of toxicological effects (Fernández et al. 2006). Significant attention is needed to control the histamine levels in foods (Bodmer et al. 1999). Histamine poisoning is the most common food-borne problem. Aside from the compounds naturally found in vegetables and fruits as well as those formed as a result of the fermentation of cheese, wine, and sauerkraut, biogenic amines also play an essential role in the metabolism of the histamine-forming bacteria present in foods (Kung et al. 2007). Flushing of the face and neck are symptoms of histamine intoxication, followed by an intense, throbbing headache. Other symptoms include dizziness, itching, faintness, burning of the mouth and throat, and the inability to swallow. Taylor et al. (1978) reported that ingestion of 70–1000 mg of histamine in a single meal is necessary to elicit any symptoms of toxicity. A level of histamine exceeding 10 mg/100 g of fresh weight is associated with poor product quality indicative of microbial spoilage, with levels of 200 mg histamine per kg of food product accepted as a toxic indicator for fish, and 10 mg/kg for wines, whereas for hot pepper pickles values are generally below the level of 1000 mg/kg.
On average, histamine content in food of approximately 30 mg/kg can be considered the minimal level for clinical symptoms of toxicity (Ekici and Coskun 2002). These toxicological problems are particularly severe in individuals who, for whatever reason, are deficient in diamine oxidase, the histamine-degrading enzyme. At non-toxic doses, histamine can cause intolerance symptoms such as diarrhea, hypotension, headache, and flushing. Just 75 mg of histamine, a quantity commonly present in normal meals, can induce symptoms in the majority of healthy persons with no history of histamine intolerance. Serotonin also may interact with Candida albicans and regulate the fungal organism’s virulence (Mayr et al. 2005)
The amount of neurotransmitters in cellular secretions can be increased following stressful stimuli, including certain interactions with other organisms. Large amounts of dopamine are usually secreted by cells of infusoria Tetrahymena pyriformis into their growth medium (Gundersen and Thompson 1985). This release of dopamine is especially important during infection, when the animal or plant accumulates some of the neurotransmitters, while pathogens release neurotransmitters (Romanovskaya and Popenenkova 1971). On north-eastern Pacific coasts, the alga Ulvaria obscura produces large amounts of dopamine (van Alstyne et al. 2006). This organism, dominant in subtidal “green tide” blooms due to this anti-herbivore defense, can be harmful to marine communities, fisheries, and aquaculture facilities because the alga presence is the cause of reduced feeding by echinoderms, molluscs, and arthropods.
Dopamine constituted an average of 4.4 % of the alga’s dry mass, and was responsible for the decreased feeding by sea urchins (Strongylocentrotus droebachiensis). Subsequent experiments demonstrated that dopamine also reduced the feeding rates of snails (Littorina sitkana) and isopods (Idotea wosnesenskii). This is the first experimental demonstration of a plant (algal) catecholamine functioning as a feeding deterrent.
Five histamine-producing bacterial strains isolated by Jaw et al. (2012) from fish meal samples producing 1.31–6.21 ppm of histamine in trypticase soy broth supplemented with 1.0 % histidine were identified as Bacillus licheniformis (three strains), B. amyloliquefaciens (one strain), and B. subtilis (one strain). Legal upper limit of biogenic amines as toxicants (Silla Santos 1996): histamine: 100 mg/kg food. 1000 mg/kg amine (based on histamine intoxication & amine concentration in food) is dangerous for human health. The toxic dose of biological amines depends on the individual’s own sensitivity and other unique characteristics. Individuals may influence cell proliferation, regulate nucleic acid function, and protein synthesis. Products such as wine, beer, and other foods that are prepared with the yeast S. cerevisiae, require attention be given to microorganismal biogenic amines. Histamine intoxication can cause nausea, respiratory distress, hot flushing, heart palpitation, headache, hypertension and hypotension.
Current rapid detection methods for neurotransmitters in food and environmental samples have proved invaluable. The use of gas and liquid chromatographic methods in the detection of biogenic amines in vegetables (Ly et al. 2008), is complemented by the histochemical fluorescent method of staining with glyoxylic acid or o-phthalic aldehyde, for example, in the analysis of algae (Barwell 1979, 1989) and allergenic pollen (Roshchina et al. 2014; Roshchina and Yashin 2014). High concentrations of catecholamines or histamine released by algae may be hazardous for the development of surrounding species, including sea animals (Barwell 1979; van Alstyne et al. 2006, 2011, 2013, 2014). Catecholamine and histamine release are also of concern in eliciting allergic reaction in humans following contact with pollen (Roshchina et al. 2014).
The influence of neurotransmitters in aquatic biocenosis is both relevant and useful in marine ecology. Instances of usefulness to marine ecology include bacterial response-reaction to some diatoms (Kaeppel et al. 2012), such as the marine Marinobacter adhaerens, which aggregates with the diatom Thalassiosira weissflogii sp. nov.
2.2.3 Components of Cholinergic and Aminergic Systems
In microbial cells, components of cholinergic and aminergic systems are similar to those found in mammalian cells, including the complete biosynthetic pathway required for their synthesis (synthetases) and their catabolism (cholinesterases, aminooxidases, and others), as well as functional analogues of cholino- and amino-receptors have been demonstrated to be present.
2.2.3.1 Choline Acetyltransferase
The enzymes choline acetyltransferases or choline acetylases (EC 2.3.1.6) participate in the synthesis of acetylcholine from choline and acetic acid (Nachmansohn and Machado 1943). A cell-free enzyme preparation with “choline acetylase” activity was present in Lactobacillus plantarum (Girvin and Stevenson 1954). This type of enzyme activity has also been found in many plant species (Roshchina 1991, 2001a).
2.2.3.2 Cholinesterase
Enzymes which degrade acetylcholine to choline and acetic acid are named cholinesterases and were first found in 1937 by Loewi in the hearts of amphibia. The function of acetylcholinesterase at cholinergic synapses of animals is to terminate cholinergic neurotransmission (Augustinsson 1949). However, the enzyme is expressed in tissues that are not directly innervated by cholinergic nerves. Moreover, transient expression in the brain during embryogenesis suggests that acetylcholinesterase may function in the regulation of neurite outgrowth. Overexpression of cholinesterases has also been correlated with tumorigenesis and abnormal mega-karyocytopoiesis (Small et al. 1996). Cholinesterase is also found in unicellular animals such as Paramecium (Corrado et al. 1999). An immunoblot analysis of the Paramecium enzyme revealed that the acetylcholinesterase had a molecular mass from 42 to 133 kDa, as reported for an analogous enzyme isolated from higher organisms. Structural homologies between cholinesterases and the adhesion proteins indicate that cholinesterases could also function as cell–cell or cell–substrate adhesion molecules. Abnormal expression of cholinesterases of both types has been detected around the amyloid plaques and neurofibrillary tangles in the brains of patients with Alzheimer’s disease (Small et al. 1996).
As for microorganisms, Goldstein and Goldstein (1953) first described the production of cholinesterase by a strain of bacterium Pseudomonas fluorescens after the culture was grown with acetylcholine as the sole source of carbon. The P. fluorescens enzyme was inducible, mainly, by choline (not as a carbon substrate, but, perhaps, as a source of nitrogen) or by two- to threefold less by some choline esters: acetylcholine > propionylcholine = benzoylcholine > butyrylcholine > acetyl-β-methylcholine. Addition of glucose completely prevented induction of the P. fluorescens enzyme. The pH optimum for growth of the culture and cholinesterase activity was 7.0, although the culture growth was higher in alkaline medium, where spontaneous hydrolysis of acetylcholine is also maximal. Choline oxidase synthesis in P. fluorescens has also been induced by choline. The cholinesterase of the bacterium may hydrolyze acetylcholine or propionylcholine, but to a lesser degree butyrylcholine, benzoylcholine, or acetyl-β-methylcholine. Like cholinesterase in animals, the enzyme activity in P. fluorescens was inhibited by neostigmine, with complete inactivation observed at high concentrations (10−3–10−2 M) and only partly at the lower levels of 10−6 M. These levels of inhibition are similar to that observed in mammalian organ systems. The P. fluorescens protein was isolated and characterized (Goldstein 1959; Searle and Goldstein 1957, 1962; Fitch 1963a, b). Moreover, the strains of P. fluorescens tested exhibited preference for acetylcholine for growth promotion over choline, glycerol, glucose, succinate, betaine, and serine (Fitch 1963a). The isolated cholinesterase was inhibited by neostigmine in smaller (1000 times) concentration, than by physostigmine, but was not inhibited by diisopropylfluorophosphate (Fitch 1963b). A bell-shaped substrate saturation curve was observed, and specific activity of the 115-times purified cholinesterase was 10.5 mmol/mg protein/min. The enzyme had features both of true cholinesterase and acetylcholinesterase (Laing et al. 1967, 1969). Specific activity of the cholinesterase from P. fluorescens purified 40-fold by CM-50 Sephadex was up to 70 mmol/mg protein/min. The values of Km at pH 7.4 and 37 °C were 1.4 × 10−5 M for acetyl-choline and 2.0 × 10−5 M for propionylcholine, respectively, while butyrylcholine and benzoylcholine were not hydrolysable at all. The purified enzyme was inhibited by organophosphorus compounds and neostigmine, but not by physostigmine.
Imshenetskii et al. (1974) showed that a large variety of microorganisms may decompose acetylcholine including 31 strains of bacteria (genera Arthrobacter and Pseudomonas) and two strains of fungi (from 194 strains studied) that live in soil. Around 100–200 mg of wet biomass of active microbial strains were able to decompose 15–30 mmol of acetylcholine during a 2 h incubation, with the most active strains (50 mg of wet biomass) able to degrade up to 10 mmol/min. This active soil strain was identified as Arthrobacter simplex var. cholinesterasus var. nov. The amount of the decomposed acetylcholine by this microbe was 30 times higher than in other strains (Pseudomonas fluorescens—4 mM/h, P. aeruginosa—1 mM/h), while Arthrobacter simplex var. cholinesterasus var. nov had an activity of 300 mM/h. Actinomycetes (except two strains) and yeast had no significant cholinesterase activity. Our experiments showed a small rate of hydrolysis of acetylthiocholine and butyrylthiocholine in extracts of Saccharomyces cerevisiae where only 20–30 % inhibited by neostigmine or physostigmine, inhibitors of animal cholinesterase. Roshchina and Alexandrova (1991) isolated cholinesterase from the mycelium of the fungus Aspergillus niger. The enzyme hydrolyzed cholinic esters at rates similar with animal enzyme (KM 7 × 10−4 M). Moreover, the enzyme has a molecular mass about 600 kDa determined by gel-filtration on Sephadex G-200 and after gel-electrophoresis with sodium dodecyl sulphate—two subunits 63 and 44 kDa. The enzyme activity was higher with acetylthiocholine, than butyrylthiocholine, and neostigmine inhibited the substrates’ hydrolysis, unlike physostigmine.
Cholinesterase activity has also been found in lower groups of the plant kingdom: in extracts of Characeae algae Nitella by Dettbarn, in 1962 and mycelium of fungi Physarum polycephalum by Nakajima and Hatano in 1962, and then a series of classical papers of Jaffe and Fluck with coworkers in 1970–1975 were devoted to the observation of the enzyme in many plant species: ~118 terrestrial species and ten marine algae were identified as having cholinesterase activity (for more details see the relevant references in monographs Roshchina 1991, 2001a). The values of the enzyme activity (the substrate hydrolysis rate) in higher plants were an average of 1–900 mmol/h/g fresh weight, depending on the plant species. It was shown that Bryophytes (mosses, liverworts and hornworts) demonstrate maximal cholinesterase activity of up to 0.360 mmol/h/g fresh weight (Gupta et al. 2001). Pollen also contain the enzyme (Bednarska and Tretyn 1989; Bednarska 1992; Roshchina et al. 1994; Rejon et al. 2012). Thus, detection of cholinesterase activity could serve as an additional indicator of the presence of acetylcholine.
Recently, the identification, purification, and cloning of maize acetylcholinesterase provided the first direct evidence of the enzyme formation in plants (Sagane et al. 2005). An especially important fact is that the acetylcholinesterase distribution in seedlings is sensitive to gravity, leading to asymmetry of the enzyme distribution (Momonoki 1997). Maize acetylcholinesterase activity localized in vascular bundles the coleoptile node and mesophyll seedlings is enhanced through a post-translational modification response to heat stress (Yamamoto and Momonoki 2012). The gene purportedly encoding for acetylcholinesterase was cloned from maize (Muralidharan et al. 2013). The Arabidopsis protein encoded by the gene exhibited lipase activity with preference for long chain substrates but did not hydrolyze choline esters. In several edible fruits and vegetables, members of the Solanaceae family (potato, eggplant and tomato), acetylcholinesterase inhibitors have been detected; these were mainly alkaloids which induced gastrointestinal and neurological symptoms in mammalian.
Plant cholinesterases are included in several processes (Roshchina and Semenova 1990; Roshchina 2001a, b), including but not limited to: pollen germination release just after moistening of the pollen grains (Roshchina et al. 1994; Roshchina 2007), as well as within the intercellular interactions in the pollen-pistil system (Kovaleva and Roshchina 1997). Cholinesterase may prevent the binding of dopamine with its receptor and decrease the toxic effect of d-tubocurarine (antagonist of nicotinic type of cholinoreceptor) on cells of horsetail vegetative microspores (Roshchina et al. 2012). Yamamoto and co-workers (2011) postulated that acetylcholinesterase is a positive regulator of heat tolerance in plants.
2.2.3.3 Enzymes of Biogenic Amine Metabolism
The biosynthetic pathway of biogenic amines includes the decarboxylation and hydroxylation of corresponding amino acids, in particular phenylalanine for the catecholamines, tryptophan for serotonin, and histidine for histamine (Lawrence 2004). Phenylalanine, precursor of dopamine, norepinephrine and epinephrine, is first hydroxylated, transforming to tyrosine and then to dihydroxyphenylalanine (DOPA). These processes are catalyzed by phenylalanine hydroxylase or phenylalanine monoxidase and tyrosine hydroxylase or tyrosine-3-monoxidase. Dopamine, an immediate precursor of norepinephrine and epinephrine, arises from DOPA through decarboxylation by means of the enzyme decarboxylase dioxyphenylalanine and the decarboxylase of aromatic amino acids (EC 4.1.1.26). Another route of tyrosine transformation is via decarboxylation, when it transforms to tyramine, and then by hydroxylation with the participation of tyramine hydroxylase into dopamine, which is then oxidized to norepinephrine by the copper-containing enzyme- β-hydroxylase 3,4-dioxyphenylethylamine. Then, under the influence of the transmethylase of phenylethanolamines, the formation of epinephrine takes place.
In the catabolism of catecholamines, aminooxidases participate as in the oxidative deamination of the catecholamines to metanephrine, normetanephrine, vanillic aldehyde, dehydroxymandelic and vanillic acids. For microorganisms, this metabolic process has not yet been studied. In plants, diamineoxidases play the main role in catecholamine metabolism, unlike animals that use monoaminooxidases for this purpose (Roshchina 2001a). As for catecholamine-O-methyltransferases, they are present in all animal tissues, and especially active in nervous cells. In plants, the catecholamine-O-methyltransferases pathway is also possible because the last three compounds are ordinary products of plant metabolism (Kuklin and Conger 1995; Roshchina 2001a; Kulma and Szopa 2007). Besides the abovementioned pathways of metabolism, catecholamines are oxidized by oxygen in air, forming oxidized products—red pigments aminochromes and black-brown pigments melanines which are polymers of indole (found both in plant and animals). The mechanism of oxidation is connected with the generation of superoxide radicals. Prevention of the oxidation of dopamine by superoxide dismutase confirms this mechanism. Enzymatic oxidation of catecholamines to melanines by polyphenol oxidase has been demonstrated (Roshchina 2001a). The abovementioned enzymes are found only in animals and plants. There is little data for catecholamine oxidation in microorganisms, although monoaminooxidase activity in mycobacteria (Pershin and Nesvadba 1963) and E. coli (Takenaka et al. 1997) has been found.
Serotonin is synthesized in plants and animals from tryptophan formed by the shikimate pathway, which has also been proposed for microorganisms (Oleskin et al. 1998a, b; Oleskin 2007). This process proceeds by two pathways: either via 5-hydroxytryptophan or tryptamine formation, or the first step of serotonin biosynthesis via decarboxylation of tryptophan, which then transforms in plants to tryptamine by action of the enzyme tryptophan decarboxylase (EC 4.1.1.27), or by the decarboxylation of aromatic amino acids (EC 4.1.1.26/27). Then, tryptamine is transformed to serotonin by hydroxylation with participation of the enzymes tryptamine-5-hydroxylase or 1-tryptophan-5-hydroxylase (EC 1.14.16.4). Hydroxylation of tryptophan leads to the formation of 5-oxytryptophan in the presence of tryptophan-5-hydroxylase (EC1.14.16.4). At the next stage, 5-oxytryptophan is decarboxylated by the decarboxylation of aromatic acids to yield serotonin. Tryptamine 5-hydroxylase, which converts tryptamine into serotonin and is common in animals, was also found as a soluble enzyme that had maximal activity in rice roots (Kang et al. 2007). The tissues of rice seedlings grown in the presence of tryptamine exhibited a dose-dependent increase in serotonin in parallel with enhanced enzyme activity. However, no significant increase in serotonin was observed in rice tissues grown in the presence of tryptophan, suggesting that tryptamine is a bottleneck intermediate substrate for serotonin synthesis. If we compare the enzymes from the different kingdoms, we can see more similarity. In particular, in the plant genus Arabidopsis, there is a homolog to part of a DNA binding complex corresponding to the animal tyrosine and tryptophan hydroxylases (Lu et al. 1992). Fujiwara et al. (2010) have found that unlike tryptophan hydroxylase of animals, an analogous enzyme in plants is a cytochrome P450 located in the membranes of the endoplasmic reticulum. The enzyme was not expressed in the bacteria E. coli, but may be expressed here via special genetic construction with recombinant genes (Park et al. 2011). Aminooxidases of biogenic amines may differ in microorganisms in relation to substrate specificity, in particular for the bacterium Methanosarcina barkeri and infusoria Tetrahymena pyriformis (Yagodina et al. 2000). Both studied enzymes can deaminate serotonin, but not histamine. The existence of one active centre for substrate binding is postulated as the aminooxidase of the bacterium, while several centres are thought to exist in the Infusorians.
For all living organisms, the biosynthesis pathway of histamine includes histidine decarboxylase which participates in the decarboxylation of histidine (Roshchina 2001a; Boron and Boulpaep 2005; Martín et al. 2005). The gene encoding histidine decarboxylase (hdcA) has been identified in different Gram-positive bacteria (Martín et al. 2005). Histidine decarboxylase used to be part of a cluster that included a gene of unknown function (hdcB) and a histidine–histamine antiporter gene (hdcC) in Pediococcus parvulus 276 and Lactobacillus hilgardii 321 has been identified (Landete et al. 2005). Catabolism of histamine occurs also via methylation or acetylation in the presence of histamine-N-methyltransferase, or histamine-N-acetyltransferase, and genes coding of the enzymes have been found in bacteria, plants, and animals (Iyer et al. 2004).
2.2.3.4 Recognition of Neurotransmitters
Appreciation of the presence and role of neurotransmitters in microbial cells was previously considered mainly in the context of receptors specific to the neurotransmitter compounds (Adler 1969), with particular understanding gained from concepts of neurotransmitter reception in animals. A major method by which organisms may be studied is the use of pharmacological assays wherein the neurotransmitter of a cellular reaction is analyzed via the use of agonists and antagonists to that neurotransmitter. All investigations concerning non-neuronal (non-synaptic) systems have a fundamental similarity to traditional studies undertaken for the nerve cell.
For acetylcholine, there are two types of acetylcholine receptors: nicotinic (receptors that respond to nicotine) and muscarinic (receptors that are sensitive to muscarine). Corrado et al. (2001) showed the presence of functionally related nicotinic and muscarinic receptors and the lytic enzyme acetylcholinesterase in the unicellular animal Paramecium primaurelia. In plants, the presence of similar receptors has also been shown (Roshchina 2001a). Recently, it was established that muscarinic and nicotinic acetylcholine receptors are involved in the regulation of stomata function—the opening and closing movement—in the plants Vicia faba and Pisum sativum (Wang et al. 1998, 1999a, 2000). Leng et al. (2000) showed the regulation role of acetylcholine and its antagonists in inward rectified K+ channels from Vicia faba guard cells. Location of the muscarinic receptor was shown in plasmatic membrane and chloroplast membranes (Meng et al. 2001), and cholinesterase activity was found in the cells (Wang et al. 1999b). The germination of plant microspores such as vegetative microspores of horsetail Equisetum arvense or pollen (generative microspores) of knight’s star Hippeastrum hybridum was blocked by the antagonists of acetylcholine, which are linked with nicotinic cholinoreceptors and Na+/K+ ion channels (Roshchina and Vikhlyantsev 2009). The nicotinic cholinoreceptors were cytochemically identified in the single-cell amoebae Dictyostelium discoideum, slugs, and spores, however, proteins immunologically-related to the muscarinic receptors were not present in the spores (Amaroli et al. 2003). Interestingly, nicotine and acetylcholine as the ligands of human nicotinic cholinoreceptors in culture of epithelial cells HEp-2 may stimulate the growth of Chlamydia pneumoniae (Yamaguchi et al. 2003).
The receptors for biogenic amines which are unique to animals are known as dopamine receptors, adrenoreceptors, and serotonin and histamine receptors. Similar receptors were observed in bacteria (Lyte and Ernst 1993; Lyte 2004; Freestone et al. 2007) and in plant cells (see monographs Roshchina 1991, 2001a). Alpha and beta adrenergic-like receptors may be involved in catecholamine-induced growth of Gram-negative bacteria (Lyte and Ernst 1993). In particular, Freestone et al. (2007) showed the blockade of catecholamine-induced growth of E. coli, Salmonella enterica, and Yersinia enterocolitica by adrenergic and dopaminergic receptor antagonists. In plants, adrenoreceptors participate in cytoplasm movement, ion permeability, and membrane potential, in flowering of Lemna paucicostata, photophosphorylation, as well as seeds, vegetative microspores’ and pollen germination (Roshchina 1991, 2001a; Baburina et al. 2000; Kulma and Szopa 2007). Serotonin- and histamine-sensitive receptors in plants regulate the seed, pollen, and vegetative microspores’ germination (Roshchina 1991, 2001a, 2004, 2005a). Shmukler et al. (2007) discussed earlier hypotheses of protosynapse for low-complexity organized animals and embryos of high-complexity organized animals, where the distribution of membrane serotonin receptors is restricted to the period of blastomere formation during cleavage and localized within the area of interblastomere contact. The hypothesis was based on their experiments where the membrane currents of the Paracentrotus lividus early embryos were registered after local application of serotonin drugs via special micropipette. Receptors of neurotransmitters may be linked with ion channels. Moreover, some domains of the ion channels appear connected to the cytoskeleton, in particular with actin (Cantiello 1997), and so the received chemosignal is likely to spread to the organelles via actomyosin filaments (Roshchina 2005a, 2006a, b).
Aspects of newer investigations of receptors are concerned with recombinant systems. For example, the recombinant mouse brain serotonin receptor (5HT1c) was used to study the response of plant cells and oocytes to a stress signal activated by the serotonin–serotonin receptor interaction and associated Ca2+ flux (Beljelarskaya and Sutton 2003). Based on plant expression vectors, recombinant constructs were obtained to direct production of 5HT1c fused with the green fluorescent protein in plant cells. The mRNAs for hybrid proteins were synthesized in an in vitro transcription system. The expression and function of the hybrid protein and the function of the associated ion channels were electrophysiologically studied in Xenopus laevis oocytes injected with the hybrid mRNA. The hybrid protein was functional and changed the operation of the Ca2+ channel in oocytes. To study the expression of the hybrid constructs in plant cells, the in vitro transcription product was inoculated in tobacco leaves, which thereupon fluoresced.
2.3 Perspectives on the Universal Functions of Neurotransmitters (Biomediators)
The presence of neurotransmitters in organisms in general leads us to the problem of information transmission within and between living cells. Like the genetic code, a common class of mediator-type molecules within all living organisms indicates that the mechanism of reaction-response (communication) appears to have a common foundation in the form of universal, or universally-recognized, chemical signals. The compounds acetylcholine and biogenic amines categorized as neurotransmitters, besides having specialized mediator function in organisms with nervous systems, also play other roles, not only in animals, but also in microorganisms and plants. As such, one could call the compounds “biomediators,” rather than “neurotransmitters” or “neuromediators” (Roshchina 1989, 1991).
2.3.1 Functions of Neurotransmitters on Different Evolutionary Steps
The function of compounds named neurotransmitters originates from simple chemotaxis and chemosignaling of microbial cells and leads to intercellular communication (Fig. 2.2). The so-called neurotransmitters may regulate (as hormones) growth and development of other unicellular organisms, and be attractants or repellents for them. In higher concentrations the same substances also play a role in defense (i.e. such as in the fight or flight response) or, in some cases, contribute to creating unique cultural foundations for food and cuisine. The following step in evolution includes the development of interactive relationships (parasitic, symbiotic, or otherwise) as well as the formation of multicellular organisms that may further specialize and attenuate the function of biomediators to serve unique requirements of communicative reaction-response within a larger, more complex multicellular system. This evolutionary pathway leads us to the concept of neurology as applicable to both animals and plants (Baluska et al. 2005; Brenner et al. 2006; Murch 2006; Ramakrishna et al. 2011).
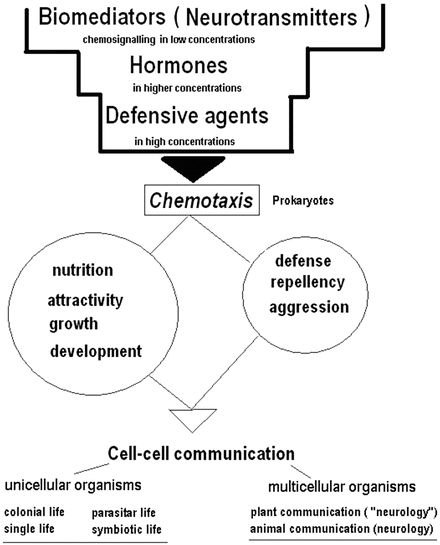

Fig. 2.2
Evolutionary development of neurotransmitter (biomediator) function
In Table 2.3, the main functions of the neurotransmitters in all kingdoms are compared. The overall effect of a communication signal into a cell from an extracellular surface site or even intracellularly between cellular compartments, may occur via neurotransmitters. At the cellular level, the compounds may induce different reactions. Neurotransmitters are stored in secretory vesicles, and can be released inside or outside of the cell. Primary reaction to acetylcholine is often a change in membrane permeability for ions, while other reactions for both the neurotransmitter and biogenic amines are connected with the systems of secondary messengers—cyclic nucleotides, Ca2+, inositol-3-phosphate, and others.
Table 2.3
The established functions of neurotransmitters in living organisms
Neurotransmitter | Microorganisms | Plants | Animals |
|---|---|---|---|
Acetylcholine | Regulation of motility | Regulation of membrane permeability and other cellular reactions up to growth and development in many plant species | Regulation of cell proliferation, growth and morphogenesis. The carriage of nerve impulses across the synaptic chink, from one neuron to another of impulses across the “motor plate”, from a neuron to a muscle cell, where it generates muscle contractions |
Substrate and nitrogen origin | Regulation of contractile proteins functioning | ||
Action potential production | |||
Dopamine | Stimulation the cultural growth of E. coli, Y. enterocolitica, S. enterica, S. epidermidis etc., and the cellular aggregation and formation of colonies of E coli | Regulation of many cellular processes from growth and development to defence reactions | Decreases peripheral vascular resistance, increases pulse pressure and mean arterial pressure. The positive chronotropic effect produces a small increase in heart rate as well. Important for forming memories. In embryos of Vertebrata and lower animals may regulate development |
Regulation of production of some metabolites, virulence processes, bacterial motility and formation of biofilms | Regulation of contractile proteins functioning | ||
Norepinephrine (noradrenaline) | The similar effects for Gram-negative bacteria E. coli, S. enterica, and Y. enterocolitica | Regulation of many cellular processes from growth and development to defence reactions | Increases peripheral vascular resistance, pulse pressure and mean arterial pressure as well as stimulates of the thrombocytes’ aggregation |
Regulation of contractile proteins functioning | |||
Epinephrine (adrenaline) | Similar effects on bacteria to those described for norepinephrine | Regulation of many cellular processes from ion permeability, growth and development to defence reactions | Induced vasodilation (mainly in skeletal muscle) and vasoconstriction (especially skin and viscera) |
Regulation of contractile proteins functioning | |||
Serotonin | Stimulation of growth of culture and cellular aggregation bacteria Streptococcus faecalis, yeast Candida guilliermondii, E. coli K–12 and Rhodospirillum rubrum | Regulation of growth and development of many plant cells | Control of appetite, sleep, memory and learning, temperature regulation, mood, behaviour (including sexual and hallucinogenic), vascular function, muscle contraction, endocrine regulation, and depression. In embryos of Vertebrata and lower animals may regulate development |
Regulation membrane potential | |||
Histamine | Stimulation of cultural growth and cellular aggregation of E. coli K-12 | Regulation of the growth and development at stress | Involves in many allergic reactions and increases permeability of capillaries, arterial pressure is decreased, but increases intracranial pressure that causes headache, smooth musculature of lungs is reduced, causing suffocation, causes the expansion of vessels and the reddening of the skin, the swelling of cloth |
Stimulation of the secretion of gastric juice, saliva (digestive hormone) |
A neurotransmitter functions as a chemosignal (i.e. the neurotransmitter is released from a cell and elicits a reaction in another cell) in the context of certain structures, including intercellular connections as well as intracellular environment such as between organelles within a cell. We can usually see the intercellular structural connection between plasma membranes of any cells that are in contact with one another, such as at the membranes of unicellular organisms or synaptic membranes of cells in organisms with a nervous system (Roshchina 1991, 2001a, b; Buznikov et al. 1996; Shmukler et al. 2007). As seen in Fig. 2.3, at any membrane point-of-contact either transitory or permanent, structural connections may be formed. There are junctions between endoplasmic reticulum and organelles within cells, or between different cells. Today, permanent or transitory structural-links between cells or within the cell are considered a necessary structural component in the transfer of the chemosignal.


Fig. 2.3
Site, or structure, where the action of neurotransmitters may take place
As can be seen in Table 2.3, common cellular effects of neurotransmitters in living cells of all kingdoms include changes in membrane permeability (short-term effects) as well as the regulation of growth and development (long-term effects). The regulatory functions of neurotransmitters appear to have been conserved through evolution, effectively relating intracellular processes with unicellular populations within the environment. In this respect, the primary role of such compounds appear to be as a substrate in the acquisition of nitrogen by microorganisms (Imshenetskii et al. 1974), as well as serving as regulators in the microorganismal growth and development (Oleskin et al. 2010; Oleskin 2012).
2.3.1.1 Functions in Microorganisms
The ability of bacteria and fungi to both produce and respond to the panoply of neuroendocrine hormones that are more commonly associated with mammalian organisms is becoming increasingly recognized as playing a pivotal role in both disease pathogenesis as well as the maintenance of homeostasis (Lyte 2013). The beneficial role of probiotics enriched in such compounds has also been investigated (Lyte 2011; Freestone 2013), as well as has their possible role in soil nitrogen fixation by bacteria living in symbiosis with plants.
Acetylcholine. Early function of acetylcholine was demonstrated in its regulation of motility of the photosynthetic bacteria Rhodospirillum rubrum and Thiospirillum jenense (Faust and Doetsch 1971) as well as in non-photosynthetic bacteria, such as Pseudomonas fluorescens (Chet et al. 1973). Acetylcholine can be also used as substrate for microorganisms, and regulates their development in special conditions (Imshenetskii et al. 1974).
Catecholamines. Early publications showed that the chemotactic response of Pseudomonas fluorescens was significantly enhanced by epinephrine, but acetylcholine, a physiological antagonist of epinephrine, inhibited bacterial chemotaxis (Chet et al. 1973). Unlike acetylcholine, catecholamines act as regulators of growth and development of many microbial cultures.
Catecholamines can regulate the growth of Gram-negative bacteria, including E. coli (where concentration dependent specificity was observed with response to norepinephrine > epinephrine > dopamine), Y. enterocolitica and P. aeruginosa (Lyte and Ernst 1992; Freestone et al. 1999). Dopamine also stimulates the cultural growth of E. coli, Y. enterocolitica, S. enterica, S. epidermidis, etc., and the cellular aggregation and formation of colonies of E. coli and S. epidermidis (Lyte and Ernst 1993; Neal et al. 2001; Freestone et al. 2007; Anuchin et al. 2007, 2008). Similar effects on Gram-negative bacteria E. coli, S. enterica and Y. enterocolitica were observed for norepinephrine (Lyte and Ernst 1992, 1993; Lyte et al. 1997; Freestone et al. 1999, 2007; Burton et al. 2002) and on E. coli for epinephrine (Anuchin et al. 2007; Freestone et al. 2007). Freestone et al. (2008a, b) showed that catecholamine stress hormones can significantly increase the growth of a wide range of gram negative and gram positive bacteria. Various effects of catecholamines and their agonist isoproterenol added in pathogenic bacterial cultures were observed, although all of the compounds markedly increased during bacterial growth compared to controls (Belay and Sonnenfeld 2002). Norepinephrine and dopamine had the greatest enhancing effects on growth of cultures of Pseudomonas aeruginosa and Klebsiella pneumoniae, while epinephrine and isoproterenol also enhanced growth to a lesser extent. The growth of Escherichia coli in the presence of norepinephrine was greater than growth in the presence of the three other neurochemicals used in the study. Growth of Staphylococcus aureus was also enhanced in the presence of norepinephrine, but not to the same degree as was the growth of Gram-negative bacteria.
Besides growth reactions, catecholamines may regulate production of some metabolites, virulence processes, bacterial motility and formation of biofilms. Stress catecholamines (50 μM adrenaline and noradrenaline) were shown to stimulate the production of volatile sulfur compounds (mainly hydrogen sulfide H2S) by periodontopathogenic bacteria Fusobacterium nucleatum, Porphyromonas endodontalis, Prevotella intermedia and Porphyromonas that produce volatile sulfur compounds, the major gases responsible for bad breath (Calil et al. 2014). Bacteria can use mammalian hormones to modulate pathogenic processes that play essential roles. Catecholamines from pathogenic microflora are capable of regulating gene expression in disease development, for example it is known that the bacterium Actinobacillus pleuropneumoniae, an important porcine respiratory pathogen, causes significant economic loss in the global pig industry (Li et al. 2012). 158 and 105 genes were differentially expressed in the presence of epinephrine and norepinephrine, respectively. Only 18 genes were regulated by both hormones. Adhesion of the bacterium Actinobacillus pleuropneumoniae to host cells was induced by norepinephrine but not by epinephrine (Li et al. 2012). This study revealed A. pleuropneumoniae gene expression, including those encoding virulence factors, was altered in response to both catecholamines. The differential regulation of A. pleuropneumoniae gene expression by the two hormones suggests that this pathogen may have multiple responsive systems for the two catecholamines. Porphyromonas gingivalis, a gram-negative oral anaerobic bacterium known as an important pathogen in chronic periodontitis has been shown to respond to catecholamines released during stress processes by modifying their growth and virulence (Graziano et al. 2014). It occurs due to the increased expression of virulence and oxidative stress genes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree