Fig. 16.1
Comparison of proteins from the cytosol fractions of E2-treated P. brasiliensis undergoing M to Y transition with M and Y controls. Proteins were resolved through 9 % SDS-PAGE and silver stained. Lane 1, M-cells incubated solely at 25 °C, treated with E2 for 24 h; lanes 2–4, M-cells after 24, 72, and 120 h of E2 treatment at 37 °C, respectively. Lane 5, Y-cells incubated at 37 °C only. Lanes 1–4 represent M cultures treated with E2 (2.6 × 10−7 M), and lane 5 represents the Y control. Note the maintenance of the M-form profile at 24 and 72 h (lanes 2 and 3) and the decreased total number of bands by 120 h (lane 4), which demonstrates little similarity to the Y-form profile (lane 5). For reference, the molecular mass in kDa of the transition bands (between lanes 3 and 4) as well as selected M-specific (near left) and Y-specific (near right) bands are indicated. Molecular masses in kDa of standards are indicated on the far left. Reprinted with permission from the Society for General Microbiology, United Kingdom from reference (Clemons et al. 1989a)
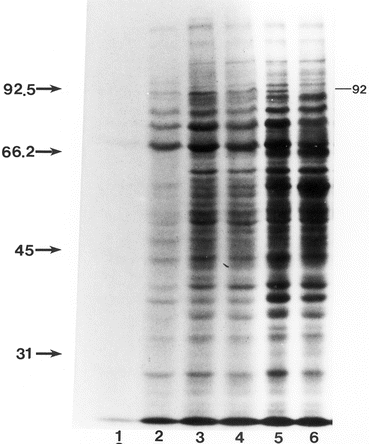
Fig. 16.2
Comparison of [35S]methionine-labeled proteins in cytosol fractions of E2-treated and untreated P. brasiliensis undergoing M to Y transition. Labeling was done for 2 h in the absence of radioinert methionine prior to disruption of organisms and extraction of cellular proteins. Lanes were loaded with equal counts of [35S]-labeled proteins, electrophoresed and processed for fluorography. Lane assignments: lane 1 M control, 2 M E2,-treated, 3 and 5 M controls grown at 37 °C for 24 and 72 h, respectively. Lanes 4 and 6 are M treated with E2 (2.6 × 10−7 M) grown at 37 °C for 24 and 72 h, respectively. Note the effect of E2 on label incorporation of M-form (lane 2) as compared to untreated control (lane 1). Absence of the 92 kDa Y-specific band in lane 6 is indicated. The fluorogram was intentionally over-exposed to enhance the bands in lane 2. Molecular masses in kDa of standards are indicated on the left. Reprinted with permission from the Society for General Microbiology, United Kingdom from reference (Clemons et al. 1989a)
In light of the demonstration of specific E2 binding, and effects on morphological form transition and protein synthesis, we performed in vivo studies in a murine model of pulmonary infection to determine whether E2 could alter the pathogenesis of P. brasiliensis and explain the observed epidemiologic data of resistance of females. In the initial studies we followed morphologic transformation of conidia after pulmonary instillation. Conidial transformation into yeast was not impeded in male mice, with budding yeast forms present as early as 48 h. In contrast, female mice had progressively fewer cells in the bronchoalveolar lavage fluid and no budding yeasts were observed (Aristizabal et al. 1998). In addition, no CFU were recovered from the lungs of female mice after 2–6 weeks of infection, whereas log102 or more CFU were recovered from male mice (Aristizabal et al. 1998). Thus, in female mice transformation of the conidia was severely impeded, with the mice able to clear detectable infection (see Fig. 16.3). Furthermore, we found that castrated male mice reconstituted with high-doses of E2 initially inhibited conidial transformation to yeast and subsequent proliferation. However, these mice had recurrence of disease with progression later in infection, which we speculate may have been due to the immunoregulatory effects of the high-doses of E2 administered to those animals. In contrast, castrated females reconstituted with testosterone were unable to restrict disease (Aristizabal et al. 2002). Taken together, these studies support the hypothesis that the interaction of the organism with E2 contributes to the resistance of females to this infection.
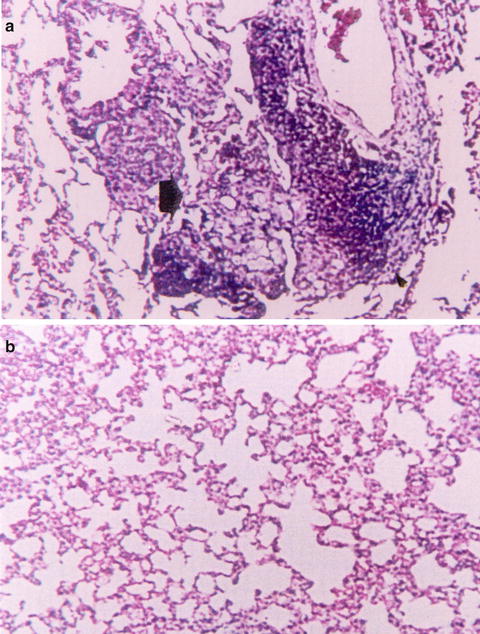

Fig. 16.3
Histopathology of the lungs in normal mice 4 weeks after infection with conidia of P. brasiliensis (H&E, ×100). (a) Normal males (NM) showing an intense chronic inflammatory reaction, also with granuloma formation and presence of yeast cells. (b) Normal females (NF) showing a slight inflammatory reaction, with no yeast cells present. Reprinted with permission of Oxford University Press, publisher for the International Society of Human and Animal Mycoses from the reference Aristizabal, B. H., K. V. Clemons, A. M. Cock, A. Restrepo and D. A. Stevens 2002. Experimental Paracoccidioides brasiliensis infection in mice: influence of the hormonal status of the host on tissue responses. Med Mycol 40(2): 169–178
As noted above, epidemiologic studies indicate a predominance of disease in males, and in vitro and in vivo studies suggestive that the female hormone (17β-estradiol, E2) regulates or inhibits M-or-conidia-to-Y transition (Shankar et al. 2011a). Furthermore, our studies of the effects of E2 on protein expression were suggestive of transcriptional regulation (Clemons et al. 1989a). With the availability of new molecular tools in the form of an 11,000 element random-shear microarray we constructed (Monteiro et al. 2009), we profiled temporal transcript expression to understand the molecular mechanism of how E2 inhibits M-to-Y transition.
We assessed temporal gene expression in strain Pb01 in the presence or absence of E2 at various time points through 9 days of the M-to-Y transition, hybridizing labeled cDNA from representative transcripts to the 11,000 element random-shear genomic DNA microarray. We chose E2-regulated genes, subjected those respective genes present in the cloning vector for DNA sequencing to identify genes and assign biological function.
E2-treatment affected global gene expression of 550 array elements. Of these, 331 elements showed up-regulation and 219 showed down-regulation at one or more time points (P ≤ 0.001). Heat shock genes (hsp90 and hsp70) are induced during M-to-Y transformation but showed low expression after 4 or 12 h exposure to E2, whereas hsp40/Dnaj had higher expression. Genes in pathways involved in energy metabolism, as well as several retrotransposable elements also showed differential regulation in response to E2 exposure. We (Monteiro et al. 2009), and others, have demonstrated mycelial-specific and yeast-specific genes. Interestingly, our temporal study revealed several Y-related genes, including α-1,3-glucan synthase, mannosyl transferase and Y20 that demonstrated low or delayed expression in E2-treated cultures, whereas expression of the mycelial-specific genes, hydrophobin and β-1,3-glucan synthase, were maintained or higher in the presence of E2. Genes potentially involved in signaling, such as palmitoyl transferase (erf2), small GTPase RhoA, phosphatidylinositol-4-kinase, and protein kinase (serine/threonine) showed low expression in the presence of E2, whereas a gene encoding for an arrestin domain-containing protein showed high expression. Genes related to ubiquitin-mediated protein degradation, and oxidative stress response genes were up-regulated by E2. Thus, the effect of E2 at the molecular level on the inhibition of the M-to-Y transition is indicative that the inhibitory actions of E2 may be working through signaling genes that regulate dimorphism (Shankar et al. 2011b).
16.6.4 Aspergillus spp.
In addition to the enhancement of growth by glucocorticoids, several species of Aspergillus respond to serotonin. In vitro, serotonin in concentrations ranging from 14 to 235 mM has antifungal properties for conidia and hyphae (Lass-Florl et al. 2002). Interestingly, serotonin receptor antagonists did not have any effect on growth (Lass-Florl et al. 2002). However, similar to data for C. albicans, sertaline inhibited the growth of Aspergillus, and the authors suggested the mechanism may be that of an interaction with a fungal transporter system (Lass-Florl et al. 2001).
16.6.5 Cryptococcus
Little is known about possible interactions of host hormones with species of Cryptococcus. C. neoformans var grubii and C. neoformans var. neoformans are primary etiologic agents of fungal meningitis in immunocompromised patients (e.g., AIDS), whereas C. gattii causes meningitis largely in immunocompetent individuals (Casadevall and Perfect 1998). Dopamine is a neuroendocrine hormone produced by the tinea nigra of the brain. Cryptococcus is able to utilize dopamine in the production of melanin, which is considered a virulence factor for this organism (Polacheck et al. 1990) and may also be related to the tropism of these fungi for the central nervous system. In recent work, the prevalence of cryptococcal meningitis in males was noted to be greater than in females. Men were more also prone to death despite having significantly higher CD4+ T lymphocyte counts. A laboratory strain and 28 clinical isolates were tested for release of capsular glucoronoxylomannan (a probable immunosuppressant and virulence factor); testosterone but not E2 was associated with higher levels of glucoronoxylomannan release (McClelland et al. 2013).
Tamoxifen has been known to have antifungal activity (Wiseman et al. 1989). A recent study indicated that tamoxifen, which can act as an E2 receptor antagonist, and some analogues, synergize with other antifungals against C. neoformans in vitro and in vivo, inhibit the fungus in macrophages, and appear to act by inhibition of the fungal protein, calmodulin, and related proteins (Butts et al. 2014).
16.7 Effect of Hormonal Interactions on Antifungal Therapy
We have previously discussed the interactions between hormones and drug-resistance genes in Candida and Saccharomyces. Early studies had shown ketoconazole was a competitive stereo-specific binder for the CBP in C. albicans, and acted as an antagonist against mammalian glucocorticoid receptors (Stover et al. 1983). This binding did not appear related to the antifungal activity of the drug, and there appeared no corticoid effect on the activity. Others have reported inhibitory (Hogl and Raab 1980, 1982) or stimulatory (Ramondenc et al. 1998, 2001) interactions of steroids on azoles. It has been speculated that the presence of an estrogen-like 1,2 diarylethane moiety in azoles may relate to their antifungal activity, and synthesis of azole derivatives emphasizing this moiety produced compounds with antifungal activity (Massa et al. 1992).
The observation of gynecomastia in some of our patients led to our discovery that azole drugs block steroidogenesis in mammalian cells and in man, due to interference with specific P450 steroidogenic enzymes (Stevens 1985). This has led to the utility of these agents in the therapy of hypercortisolemic states and in androgen blockade in prostatic cancer.
16.8 Hormonal Effects on Pathogenesis
The direct effects of hormones on the fungi can influence the pathogenesis of the organism, such as those we described for paracoccidioidomycosis. However, there are alternative explanations for fungal pathogenesis that include hormonal effects on host mucosa, as is important in vaginal candidiasis, and possibly oral candidiasis (Junqueira et al. 2005), and hormonal effects on the host immune response (Marriott and Huet-Hudson 2006). There is an extensive literature on hormonal effects on the mucosa and on the immune response, which could profoundly affect the host-fungal pathogen interplay, and we have only cited some key articles here. Perhaps the most well-known effect of hormones on the immune response to fungi relates to the effect of glucocorticoids in promoting invasive mycoses, particularly aspergillosis and mucormycosis. The newest focus of hormone-related effects on host response to infection is on the effect of vitamin D, crucial for the development and maintenance of bones. The vitamin D precursor is created by the human skin through exposure to sunlight; more specifically, ultraviolet B rays convert stored 7-dehydrocholesterol into cholecalciferol (vitamin D3). Vitamin D3 is transferred via the bloodstream to the liver where it is converted to calcidiol. Vitamin D has also been shown to enhance a great variety of immune functions (Baeke et al. 2010). Of particular relevance to fungal infection, vitamin D deficiency increases Th2 responses to A. fumigatus, and vitamin D has been shown to down-regulate Aspergillus-induced Th-2 cytokines, such as IL-5 and IL-13, from cells of allergic bronchopulmonary aspergillosis (ABPA) patients; however, gliotoxin, produced by the fungus, has been shown to overcome the vitamin D/vitamin D receptor down-regulation of IL-5 and IL-13 (Kreindler et al. 2010). We were unable to show any effect of vitamin D on systemic aspergillosis in mice or its treatment (Sirivoranankul et al. 2014).
16.9 Future Directions
As is evident from the information presented in this chapter, the field of fungal endocrinology encompasses a number of different areas and shows that these simple eukaryotic organisms can indeed have true hormone receptor interactions. Additional studies are needed in the search for endogenous hormones that regulate mating of higher fungi, such as the dermatophytes, or Coccidioides. The influence and interactions of mammalian hormones on pathogenic fungi remains an area for study that has thus far given indications that host hormones can positively or negatively influence the pathogenesis of a fungal infection. For example, hormonal influences on C. albicans have been implicated in the pathogenesis of Candida vaginitis (Tarry et al. 2005). The question of whether these interactions occur through a fungal receptor-mediated process has begun to be addressed for C. albicans, where genomic expression studies have demonstrated up-regulation of genes in the organism related to drug resistance, for example. With the advent of genomics tools, such as microarrays and high throughput sequencing technologies, as well as improvements in proteomic technologies, additional studies are now possible to examine whether the hormonal influence on growth of a fungus is receptor-mediated and what cellular pathways are involved. That is to say, how does estrogen stimulate C. albicans or Coccidioides or how does progesterone inhibit the growth of Trichophyton?
Studies such as those we discussed will shed light on not only the hormonal interactions, but on the basic biology of the organism and the mechanisms by which morphologic change might be regulated. There remains a question of whether fungi like C. albicans or P. brasiliensis produce steroid ligands similar to mammalian estrogens. Thus, does an organism like P. brasiliensis make an endogenous hormone that acts as a regulator of morphology?
Lastly are the questions of what other pathogenic fungi show interactions with the host hormonal milieu and what are the responses of the organisms to the hormones? Thus, we believe that fungal endocrinology is still in its infancy as a field of research and that much remains to be done to gain a full understanding of these interactions.
References
Albertson GD, Niimi M, Cannon RD, Jenkinson HF (1996) Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother 40(12):2835–2841PubMedCentralPubMed
Aristizabal BH, Clemons KV, Stevens DA, Restrepo A (1998) Morphological transition of Paracoccidioides brasiliensis conidia to yeast cells: in vivo inhibition in females. Infect Immun 66(11):5587–5591PubMedCentralPubMed
Aristizabal BH, Clemons KV, Cock AM, Restrepo A, Stevens DA (2002) Experimental Paracoccidioides brasiliensis infection in mice: influence of the hormonal status of the host on tissue responses. Med Mycol 40(2):169–178PubMed
Baeke F, Gysemans C, Korf H, Mathieu C (2010) Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol 25(9):1597–1606PubMed
Banerjee D, Pillai B, Karnani N, Mukhopadhyay G, Prasad R (2004) Genome-wide expression profile of steroid response in Saccharomyces cerevisiae. Biochem Biophys Res Commun 317(2):406–413PubMed
Banerjee D, Martin N, Nandi S, Shukla S, Dominguez A, Mukhopadhyay G, Prasad R (2007) A genome-wide steroid response study of the major human fungal pathogen Candida albicans. Mycopathologia 164(1):1–17PubMed
Bardwell L (2005) A walk-through of the yeast mating pheromone response pathway. Peptides 26(2):339–350PubMedCentralPubMed
Beato M, Chavez S, Truss M (1996) Transcriptional regulation by steroid hormones. Steroids 61(4):240–251PubMed
Bennett RJ, Uhl MA, Miller MG, Johnson AD (2003) Identification and characterization of a Candida albicans mating pheromone. Mol Cell Biol 23(22):8189–8201PubMedCentralPubMed
Berdicevesky I, Silbermann M (1982) Effect of glucocorticoid hormones on calcium uptake and morphology of Candida albicans. Cell Biol Int Rep 6:783–790
Bramley TA, Menzies GS, Williams RJ, Adams DJ, Kinsman OS (1990) Specific, high-affinity binding sites for human luteinizing hormone (hLH) and human chorionic gonadotrophin (hCG) in Candida species. Biochem Biophys Res Commun 167(3):1050–1056PubMed
Bramley TA, Menzies GS, Williams RJ, Kinsman OS, Adams DJ (1991) Binding sites for LH in Candida albicans: comparison with the mammalian corpus luteum LH receptor. J Endocrinol 130(2):177–190PubMed
Brasch J, Flader S (1996) Human androgenic steroids affect growth of dermatophytes in vitro. Mycoses 39(9–10):387–392PubMed
Brasch J, Gottkehaskamp D (1992) The effect of selected human steroid hormones upon the growth of dermatophytes with different adaptation to man. Mycopathologia 120(2):87–92PubMed
Brummer E, Castaneda E, Restrepo A (1993) Paracoccidioidomycosis: an update. Clin Microbiol Rev 6(2):89–117PubMedCentralPubMed
Brunt SA, Silver JC (1986a) Steroid hormone-induced changes in secreted proteins in the filamentous fungus Achlya. Exp Cell Res 163(1):22–34PubMed
Brunt SA, Silver JC (1986b) Cellular localization of steroid hormone-regulated proteins during sexual development in Achlya. Exp Cell Res 165(2):306–319PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree